A Sensitive Two-Analyte Immunochromatographic Strip for Simultaneously Detecting Aflatoxin M1 and Chloramphenicol in Milk
Abstract
:1. Introduction
2. Results
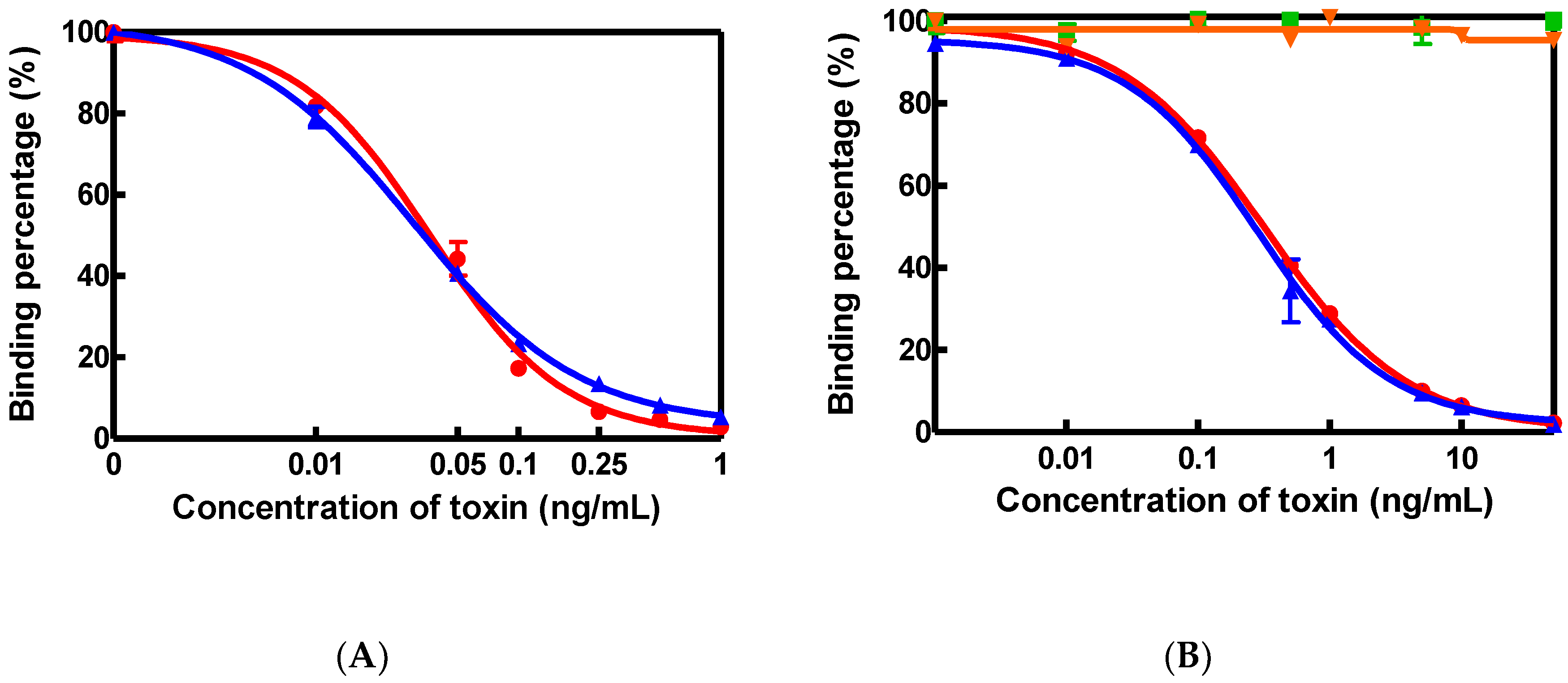
2.1. Characterization of AFM1 and CAP Antibodies
2.2. Recovery Test of AFM1- or CAP-Spiked Milk Samples with dcELISA
2.3. Determination of AFM1 and CAP Levels in Samples Using dcELISA
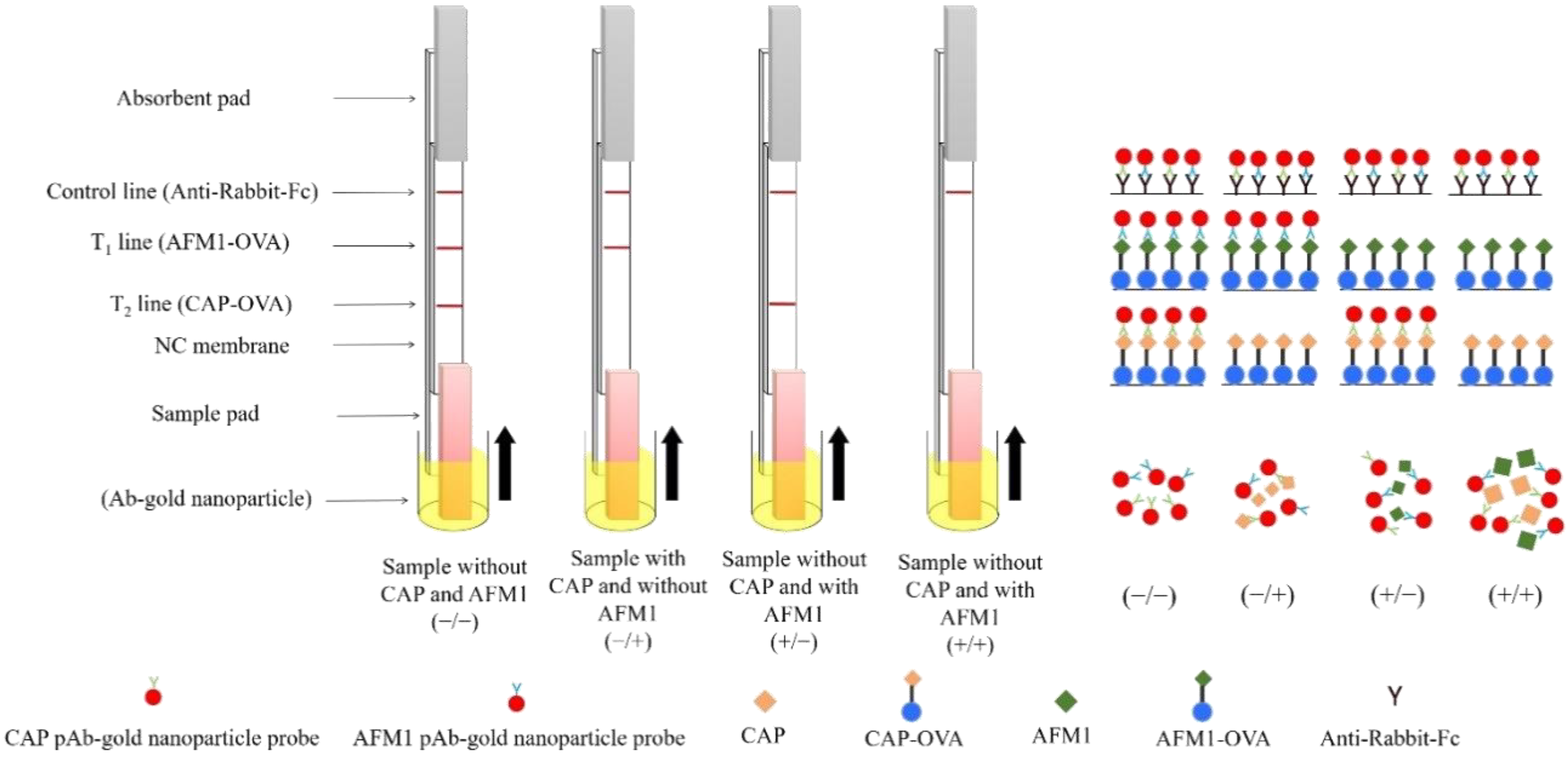
2.4. Construction of a Two-Analyte Immunostrip
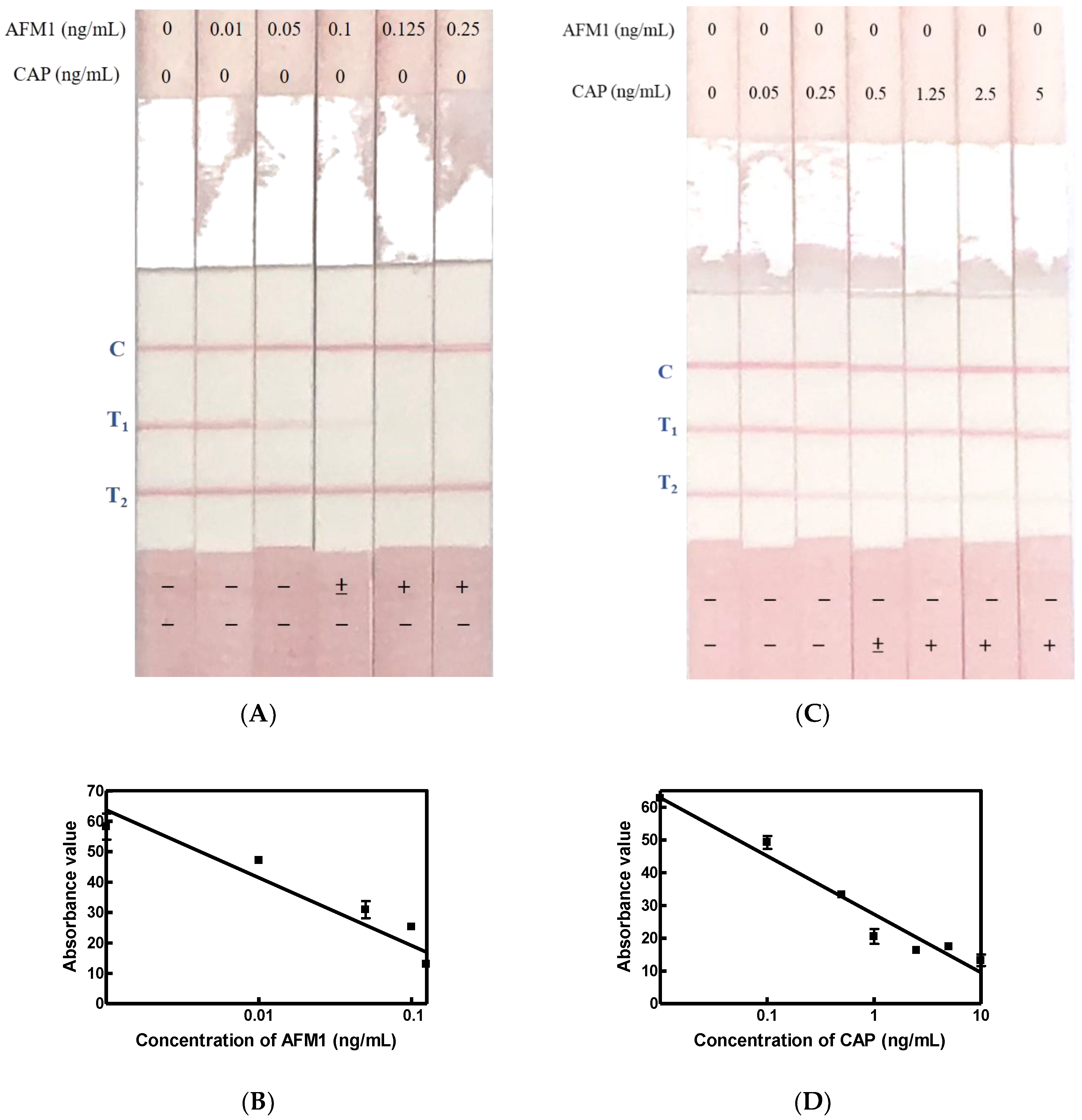
2.5. Limit of Detection for AFM1 and CAP Immunostrip Assay
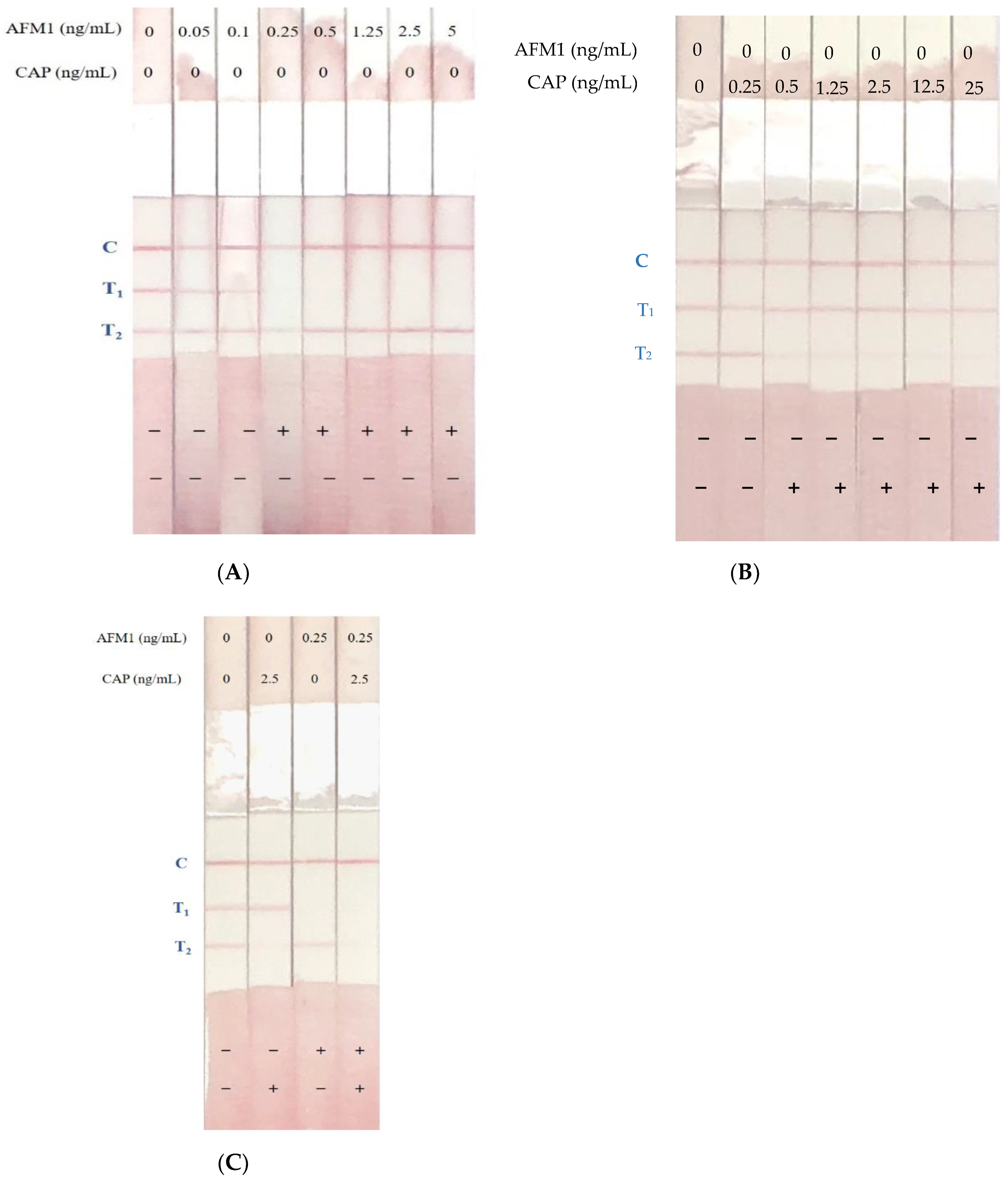
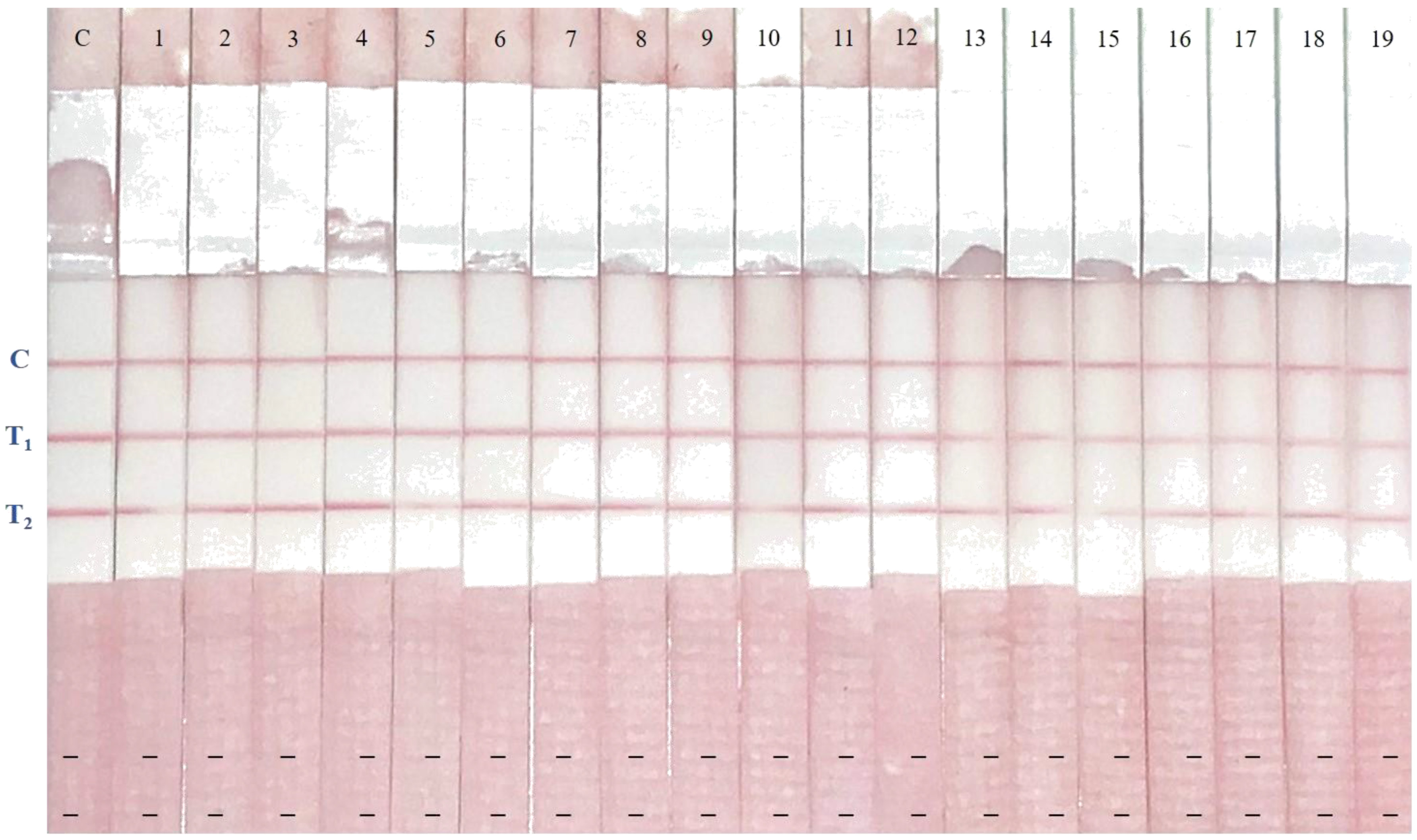
2.6. Determination of AFM1 and CAP Levels in Spiked Samples Using Immunostrips
2.7. Results of Naturally Contaminated Samples
3. Discussion and Conclusions
4. Materials and Methods
4.1. Preparation of Various AFM1 Conjugates and CAP Conjugates
4.1.1. Conjugation of AFM1 to OVA or HRP
4.1.2. Conjugation of CAP-SH to OVA or HRP
4.2. Generation of Polyclonal Antibody
4.3. dcELISA
4.4. Milk Sample Preparation
4.5. Coupling the Antibody-Gold Nanoparticle (Ab-Gold)
4.6. Immunostrip Assembly
4.7. Determining AFM1 and CAP Levels in Milk Samples with Immunostrip
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Battacone, G.; Nudda, A.; Palomba, M.; Mazzette, A.; Pulina, G. The transfer of aflatoxin M1 in milk of ewes fed diet naturally contaminated by aflatoxins and effect of inclusion of dried yeast culture in the diet. J. Dairy Sci. 2009, 9, 4997–5004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.M. Methods for determination of aflatoxin M1 in milk and milk productsd a review of performance characteristics. Food Addit. Contam. 1989, 6, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Galofaro, V.; Galvano, G. Occurrence and stability of aflatoxin M1 in milk and milk products: A worldwide review. J. Food Prot. 1996, 59, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Govaris, A.; Roussi, V.; Koidis, P.A.; Botsoglou, N.A. Distribution and stability of aflatoxin M1 during production and storage of yoghurt. Food Addit. Contam. 2002, 19, 1043–1050. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 683/2004 of 13 April 2004 as regards aflatoxins and ochratoxin A in foods for infants and young children. Off. J. Eur. Union 2004, L106, 3–5. [Google Scholar]
- Commission Regulation (EC). No 519/2014 of 16 May 2014 amending regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off. J. Eur. Union 2014, L147, 29–43. [Google Scholar]
- Wood, G.E. Mycotoxins in foods and feeds in the United States. J. Anim. Sci. 1992, 70, 3941–3949. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, J.; Bartz, Q.R.; Smith, R.M.; Joslyn, D.A. Chloromyetin, a new antibiotic from a soil actinomycete. Science 1947, 106, 417. [Google Scholar] [CrossRef]
- Ambekar, C.S.; Cheung, B.; Lee, J.; Chan, L.C.; Liang, R.; Kumana, C.R. Metabolism of chloramphenicol succinate in human bone marrow. Eur. J. Clin. Pharmacol. 2000, 56, 405–409. [Google Scholar] [CrossRef]
- Kong, C.T.; Holt, D.E.; Ma, S.K.; Lie, A.K.; Chan, L.C. Effects of antioxidants and a caspase inhibitor on chloramphenicol-induced toxicity of human bone marrow and HL-60 cells. Hum. Exp. Toxicol. 2000, 19, 503–510. [Google Scholar] [CrossRef] [PubMed]
- EFSA contam panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on Chloramphenicol in food and feed. EFSA J. 2017, 12, 3907. [Google Scholar]
- Braun, D.; Ezekiel, C.N.; Abia, W.A.; Wisgrill, L.; Degen, G.H.; Turner, P.C.; Marko, D.; Warth, B. Monitoring Early Life Mycotoxin Exposures via LC-MS/MS Breast Milk Analysis. Anal. Chem. 2018, 90, 14569–14577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chughtai, M.I.; Maqbool, U.; Iqbal, M.; Shah, M.S.; Fodey, T. Development of in-house ELISA for detection of chloramphenicol in bovine milk with subsequent confirmatory analysis by LC-MS/MS. J. Environ. Sci. Health B 2017, 52, 871–879. [Google Scholar] [CrossRef]
- Khaneghahi Abyaneh, H.; Bahonar, A.; Noori, N.; Yazdanpanah, H.; Shojaee AliAbadi, M.H. The overall and variations of Aflatoxin M1 contamination of milk in Iran: A systematic review and meta-analysis study. Food Chem. 2020, 310, 125848. [Google Scholar] [CrossRef]
- Liu, B.H.; Chu, K.C.; Yu, F.Y. Novel monoclonal antibody-based sensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Wu, S.W.; Wang, M.Y.; Liu, B.H.; Yu, F.Y. Sensitive enzyme-linked immunosorbent assay and gold nanoparticle immunochromatocgraphic strip for rapid detecting chloramphenicol in food. J. Food Saf. 2020, 40, 12759. [Google Scholar] [CrossRef]
- Khodadadi, M.; Malekpour, A.; Mehrgardi, M.A. Aptamer functionalized magnetic nanoparticles for effective extraction of ultratrace amounts of aflatoxin M1 prior its determination by HPLC. J. Chromatogr. A 2018, 1564, 85–93. [Google Scholar] [CrossRef]
- Guo, L.; Song, S.; Liu, L.; Peng, J.; Kuang, H.; Xu, C. Comparison of an immunochromatographic strip with ELISA for simultaneous detection of thiamphenicol, florfenicol and chloramphenicol in food samples. Biomed. Chromatogr. 2015, 29, 1432–1439. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chen, X.; Kuang, H.; Song, S.; Xu, C. Immunochromatographic strip development for ultrasensitive analysis of aflatoxin M1. Anal. Methods 2013, 23, 6567–6571. [Google Scholar] [CrossRef]
- Pei, S.C.; Zhang, Y.Y.; Eremin, S.A.; Lee, W.J. Detection of aflatoxin M1 in milk products from China by ELISA using monoclonal antibodies. Food Control 2009, 20, 1080–1085. [Google Scholar] [CrossRef]
- Fodey, T.; Murilla, G.; Cannavan, A.; Elliott, C. Characterisation of antibodies to chloramphenicol, produced in different species by enzyme-linked immunosorbent assay and biosensor technologies. Anal. Chim. Acta 2007, 592, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liu, B.H.; Hsu, Y.T.; Yu, F.Y. Sensitive competitive direct enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2011, 22, 964–969. [Google Scholar] [CrossRef]
- Qie, Z.; Yan, W.; Gao, Z.; Meng, W.; Xiao, R.; Wang, S. An anti-BSA antibody-based immunochromatographic assay for chloramphenicol and aflatoxin M1 by using carboxy-modified CdSe/ZnS core–shell nanoparticles as label. Microchim. Acta 2020, 187, 10–17. [Google Scholar] [CrossRef]
- Wu, X.; Tian, X.; Xu, L.; Li, J.; Li, X.; Wang, Y. Determination of aflatoxin M1 and chloramphenicol in milk based on background fluorescence quenching immunochromatographic assay. BioMed Res. Int. 2017, 2017, 8649314. [Google Scholar] [CrossRef]
- Wang, C.; Peng, J.; Liu, D.F.; Xing, K.Y.; Zhang, G.G.; Huang, Z.; Cheng, S.; Zhu, F.F.; Duan, M.L.; Zhang, K.Y.; et al. Lateral flow immunoassay integrated with competitive and sandwich models for the detection of aflatoxin M1 and Escherichia coli O157:H7 in milk. J. Dairy Sci. 2018, 101, 8767–8777. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.H.; Hung, C.T.; Lu, C.C.; Chou, H.N.; Yu, F.Y. Production of monoclonal antibody for okadaic acid and its utilization in an ultrasensitive enzyme-linked immunosorbent assay and one-step immunochromatographic strip. J. Agric. Food Chem. 2014, 62, 1254–1260. [Google Scholar] [CrossRef]
- Yu, F.Y.; Chu, F.S. Production and characterization of antibodies against fumonisin B1. J. Food Prot. 1996, 59, 992–997. [Google Scholar] [CrossRef]
- Wu, S.W.; Yu, Y.A.; Liu, B.H.; Yu, F.Y. Development of a sensitive enzyme-linked immunosorbent assay and rapid gold nanoparticle immunochromatographic strip for detecting citrinin in monascus fermented food. Toxins 2018, 10, 354. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.H.; Tsao, Z.J.; Wang, J.J.; Yu, F.Y. Development of a monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Anal. Chem. 2008, 80, 7029–7035. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-W.; Ko, J.-L.; Liu, B.-H.; Yu, F.-Y. A Sensitive Two-Analyte Immunochromatographic Strip for Simultaneously Detecting Aflatoxin M1 and Chloramphenicol in Milk. Toxins 2020, 12, 637. https://doi.org/10.3390/toxins12100637
Wu S-W, Ko J-L, Liu B-H, Yu F-Y. A Sensitive Two-Analyte Immunochromatographic Strip for Simultaneously Detecting Aflatoxin M1 and Chloramphenicol in Milk. Toxins. 2020; 12(10):637. https://doi.org/10.3390/toxins12100637
Chicago/Turabian StyleWu, Shih-Wei, Jiunn-Liang Ko, Biing-Hui Liu, and Feng-Yih Yu. 2020. "A Sensitive Two-Analyte Immunochromatographic Strip for Simultaneously Detecting Aflatoxin M1 and Chloramphenicol in Milk" Toxins 12, no. 10: 637. https://doi.org/10.3390/toxins12100637





