Calcination Improves the In Vivo Efficacy of a Montmorillonite Clay to Bind Aflatoxin G1 in Broiler Chickens: A Toxicokinetic Approach
Abstract
:1. Introduction
2. Results
2.1. UHPLC-MS/MS Plasma AFG1 Method Validation
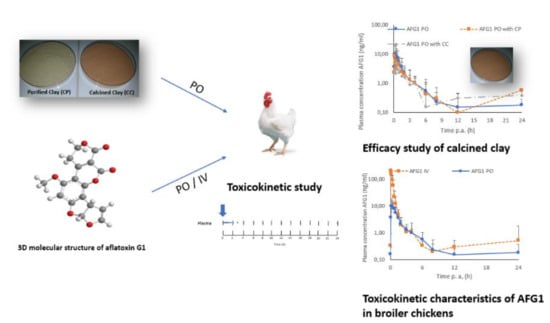
2.2. Toxicokinetic Characteristics Of Afg1
2.3. UHPLC-HRMS Analysis
2.4. Efficacy of the Calcinated Clay
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Standard, Reagents, and Solutions
5.2. Preparation of Standard Solutions
5.3. Animals and Toxicokinetic Study Design
5.4. Plasma AFG1 Analysis
5.4.1. Sample Pre-Treatment
5.4.2. UHPLC-MS/MS Analysis
5.4.3. In-House Method Validation
5.5. Plasma Phase I and Phase II Metabolites of AFG1
5.6. Toxicokinetic and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medina, Á.; Rodríguez, A.; Sultan, Y.; Magan, N. Climate change factors and Aspergillus flavus: Effects on gene expression, growth and aflatoxin production. World Mycotoxin J. 2015, 8, 171–179. [Google Scholar] [CrossRef]
- Kamika, I.; Takoy, L.L. Natural occurrence of Aflatoxin B1 in peanut collected from Kinshasa, Democratic Republic of Congo. Food Control 2011, 22, 1760–1764. [Google Scholar] [CrossRef]
- Karami-Osboo, R.; Mirabolfathy, M.; Kamran, R.; Shetab-Boushehri, M.; Sarkari, S. Aflatoxin B1 in maize harvested over 3 years in Iran. Food Control 2012, 23, 271–274. [Google Scholar] [CrossRef]
- Kang’ethe, E.K.; Lang’a, K.A. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr. Health Sci. 2009, 9, 218–226. [Google Scholar] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, S.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Aoyama, K.; Fujita, K.; Kai, S.; et al. Aflatoxin and ochratoxin A contamination of retail foods and intake of these mycotoxins in Japan. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M. Determination of aflatoxins in imported rice to Iran. Food Chem. Toxicol. 2009, 47, 2064–2066. [Google Scholar] [CrossRef]
- Shen, H.; Lv, P.; Xing, X.; Xing, L.; Yan, X.; Wang, J.; Zhang, X. Impairment of alveolar type-II cells involved in the toxicity of Aflatoxin G1 in rat lung. Food Chem. Toxicol. 2012, 50, 3222–3228. [Google Scholar] [CrossRef]
- Anttila, A.; Bhat, R.V.; Bond, J.A.; Borghoff, S.J.; Bosch, F.X.; Carlson, G.P.; Castegnaro, M.; Cruzan, G.; Gelderblom, W.C.A.; Hass, U.; et al. IARC Monographs on the evaluation of carcinogenic risks to humans, some traditional herbal medicins, some mycotoxins, naphtalene and styrene. In Proceedings of the International Agency for Reasearch on Cancer, Lyon, France, 12–19 February 2002; Volume 82. [Google Scholar]
- IARC. Aflatoxins. In Chemical Agents and Related Occupations; IARC: Lyon, France, 2002; Volume 100F, pp. 225–248. [Google Scholar]
- World Health Organization. Aflatoxins “Aflatoxins Pose a Serious Health Risk to Human and Livestock.”; REF. No. WHO/NHM/FOS/RAM/18.1; WHO: Geneva, Switzerland, 2018; pp. 1–5. [Google Scholar]
- Chibanga, J.F.; Banda-nyirenda, D.; Simbaya, J. Effects of Supplementing Different Levels of Vitamin A to Aflatoxin B 1 Contaminated Diets on the Performance of Broiler Chickens. Asian J. Nat. Appl. Sci. 2014, 3, 35–46. [Google Scholar]
- Basavanta Kumar, C.; Venkatarami Reddy, B.S.; Gideon Gloridoss, R.; Prabhu, T.M.; Suresh, B.N.; Naveen Kumar, S. Amelioration of Aflatoxicosis through a Bio-Technologically Derived Aflatoxin Degrading Commercial Product in Broilers. Pak. Vet. J. 2015, 35, 217–221. [Google Scholar]
- Manafi, M. Counteracting Effect of High Grade Sodium Bentonite during Aflatoxicosis in Counteracting Effect of High Grade Sodium Bentonite during Aflatoxicosis in Broilers. J. Agric. Sci. Technol. 2012, 14, 539–547. [Google Scholar]
- Manafi, M.; Hedayati, M.; Yari, M. Application of Rosemary (Rosmarinus officinalis L.) Essence on Chicks Fed Aflatoxin B 1: Impacts on Internal Organ Weights, Biochemical Traits and Mortality. Res. Zool. 2014, 4, 13–19. [Google Scholar]
- Sodhi, S.; Brar, A.P.S.; Grewal, G.S. Biochemical studies on combined immunomodulatory effects of aflatoxin and malathion in broiler chicks. Indian J. Poult. Sci. 1996, 31, 88–91. [Google Scholar]
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.C.; Fremy, J.M.; Massimi, C.; Prigent, P.; Debongnie, P.; Pussemier, L.; Boudra, H.; et al. Review of Mycotoxin-Detoxifying Agents Used as Feed Additives: Mode of Action, Efficacy and Feed/Food Safety; CFP/EFSA/EEDAP/2009/01; EFSA: Parma, Italy, 2009.
- Huang, X.H.; Zhang, X.H.; Li, Y.H.; Wang, J.L.; Yan, X.; Xing, L.X.; Wang, F.R. Experimental lung carcinogenic in vivo study of aflatoxin G1 in NIH mice. Zhonghua Bing Li Xue Za Zhi 2004, 33, 260–263. [Google Scholar] [PubMed]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, J.; Zhang, Y. Probing the characterization of the interaction of aflatoxins B1 and G1 with calf thymus DNA in vitro. Toxins 2017, 9, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocarov-Stancic, A.; Adamovic, M.; Salma, N.; Bodroza-Solarov, M.; Vuckovic, J.; Pantic, V. In vitro efficacy of mycotoxins adsorption by natural mineral adsorbents. Biotechnol. Anim. Husb. 2011, 27, 1241–1251. [Google Scholar] [CrossRef]
- De Mil, T.; Devreese, M.; Van Ranst, E.; Eeckhout, M.; De Backer, P.; Croubels, S. Characterization of 27 Mycotoxin Binders and the Relation with in Vitro Zearalenone Adsorption at a Single Concentration. Toxins 2015, 7, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Huwig, A.; Freimund, S.; Käppeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Prapapanpong, J.; Udomkusonsri, P.; Mahavorasirikul, W.; Choochuay, S.; Tansakul, N. In Vitro Studies on Gastrointestinal Monogastric and Avian Models to Evaluate the Binding Efficacy of Mycotoxin adsorbents by Liquid Chromatography-Tandem Mass Spectrometry. J. Adv. Vet. Anim. Res. 2019, 6, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mutua, F.; Lindahl, J.; Grace, D. Availability and use of mycotoxin binders in selected urban and Peri-urban areas of Kenya. Food Secur. 2019, 11, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.H.; Li, Z.; Jiang, W.T.; Jean, J.S. Adsorption and intercalation of tetracycline by swelling clay minerals. Appl. Clay Sci. 2009, 46, 27–36. [Google Scholar] [CrossRef]
- Papaioannou, D.; Katsoulos, P.D.; Panousis, N.; Karatzias, H. The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatment of certain farm animal diseases: A review. Microporous Mesoporous Mater. 2005, 84, 161–170. [Google Scholar] [CrossRef]
- Bhatti, S.A.; Khan, M.Z.; Saleemi, M.K.; Saqib, M. Aflatoxicosis and ochratoxicosis in broiler chicks and their amelioration with locally available bentonite clay. Pak. Vet. J. 2016, 36, 68–72. [Google Scholar]
- Indresh, H.C.; Devegowda, G.; Ruban, S.W.; Shivakumar, M.C. Effects of high grade bentonite on performance, organ weights and serum biochemistry during aflatoxicosis in broilers. Vet. World 2013, 6, 313–317. [Google Scholar] [CrossRef]
- Imani, A.; Bani, M.S.; Noori, F.; Farzaneh, M.; Moghanlou, K.S. The effect of bentonite and yeast cell wall along with cinnamon oil on aflatoxicosis in rainbow trout (Oncorhynchus mykiss)_ Digestive enzymes, growth indices, nutritional performance and proximate body composition. Aquaculture 2017, 476, 160–167. [Google Scholar] [CrossRef]
- Nones, J.; Nones, J.; Gracher, H.; Poli, A.; Gonçalves, A.; Cabral, N. Thermal treatment of bentonite reduces aflatoxin b1 adsorption and affects stem cell death. Mater. Sci. Eng. C 2015, 55, 530–537. [Google Scholar] [CrossRef]
- Mao, J.; Lv, G.; Zhou, R. Effect of acid-treated and hexadecyltrimethylammonium bromide–modified montmorillonites on adsorption performance of mycotoxins. Environ. Sci. Pollut. Res. 2020, 27, 4284–4293. [Google Scholar] [CrossRef]
- Tenorio Arvide, M.G.; Mulder, I.; Barrientos Velazquez, A.L.; Dixon, J.B. Smectite clay adsorption of Aflatoxin vs. octahedral composition as indicated by FTIR. Clays Clay Miner. 2008, 56, 571–578. [Google Scholar] [CrossRef]
- Nones, J.; Riella, H.G.; Trentin, A.G.; Nones, J. Effects of bentonite on different cell types: A brief review. Appl. Clay Sci. 2015, 105–106, 225–230. [Google Scholar] [CrossRef]
- Abbès, S.; Ben Salah-abbès, J.; Hetta, M.M.; Ibrahim, M.; Abdel-wahhab, M.A.; Bacha, H.; Oueslati, R. Efficacy of Tunisian montmorillonite for in vitro aflatoxin binding and in vivo amelioration of physiological alterations. Appl. Clay Sci. 2008, 42, 151–157. [Google Scholar] [CrossRef]
- Bojemueller, E.; Nennemann, A.; Lagaly, G. Enhanced pesticide adsorption by thermally modified bentonites. Appl. Clay Sci. 2001, 18, 277–284. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Neto, A.F.A.; Gimenes, M.L.; da Silva, M.G.C. Removal of nickel on Bofe bentonite calcined clay in porous bed. J. Hazard. Mater. 2010, 176, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, R.; Antonissen, G.; De Boevre, M.; Detavernier, C.; Van De Velde, M.; De Saeger, S.; Ducatelle, R.; Ayed, M.H.; Ghorbal, A. Calcination enhances the aflatoxin and zearalenone binding efficiency of a Tunisian clay. Toxins 2019, 11, 602. [Google Scholar] [CrossRef] [Green Version]
- Cheknane, B.; Bouras, O.; Baudu, M.; Basly, J.P.; Cherguielaine, A. Granular inorgano-organo pillared clays (GIOCs): Preparation by wet granulation, characterization and application to the removal of a Basic dye (BY28) from aqueous solutions. Chem. Eng. J. 2010, 158, 528–534. [Google Scholar] [CrossRef]
- Jaynes, W.F.; Zartman, R.E. Aflatoxin Toxicity Reduction in Feed by Enhanced Binding to Surface-Modified Clay Additives. Toxin 2011, 3, 551–565. [Google Scholar] [CrossRef]
- EFSA. Statement on the establishment of guidelines for the assessment of additives from the functional group ‘substances for reduction of the contamination of feed by mycotoxins’. EFSA J. 2010, 8, 1–8. [Google Scholar]
- Devreese, M.; Girgis, G.N.; Tran, S.T.; De Baere, S.; De Backer, P.; Croubels, S.; Smith, T.K. The effects of feed-borne Fusarium mycotoxins and glucomannan in turkey poults based on specific and non-specific parameters. Food Chem. Toxicol. 2014, 63, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Pasha, T.N.; Farooq, M.U.; Khattak, F.M.; Jabbar, M.A.; Khan, A.D. Effectiveness of sodium bentonite and two commercial products as aflatoxin absorbents in diets for broiler chickens. Anim. Feed Sci. Technol. 2007, 132, 103–110. [Google Scholar] [CrossRef]
- Zervas, G. The role of bentonite binders in single or concomitant mycotoxin contamination of chicken diets. Br. Poult. Sci. 2016, 57, 551–558. [Google Scholar]
- Devreese, M.; Osselaere, A.; Goossens, J.; Vandenbroucke, V.; De Baere, S.; Eeckhout, M.; De Backer, P.; Croubels, S. New bolus models for in vivo efficacy testing of mycotoxin-detoxifying agents in relation to EFSA guidelines, assessed using deoxynivalenol in broiler chickens. Food Addit. Contam. 2012, 29, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.J.; Hernàndez, E. In situ absorption of aflatoxins in rat small intestine. Mycopathologia 1996, 134, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, L.A.; Vettorazzi, A.; Arbillaga, L.; González-Peñas, E.; López de Cerain, A. An approach to the toxicity and toxicokinetics of aflatoxin B1 and ochratoxin A after simultaneous oral administration to fasted F344 rats. Food Chem. Toxicol. J. 2012, 50, 3440–3446. [Google Scholar] [CrossRef]
- Wong, Z.A.; Hsieh, D.P. The Comparative Metabolism and Toxicokinetics in the Monkey, Rat, and Mouse. Toxicol. Appl. Pharmacol. 1980, 55, 115–125. [Google Scholar] [CrossRef]
- Sawhney, D.S.; Vadehea, D.V.; Baker, R.C. The Metabolism of 14 C Aflatoxins in Laying Hens. Poult. Sci. 1972, 52, 1302–1309. [Google Scholar] [CrossRef]
- Hussain, Z.; Zargham, M.; Khan, A.; Javed, I.; Kashif, M.; Mahmood, S.; Rafique, M. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010, 48, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- EMA/CMVP/VICH Steering Committee. VICH topic GL49: Studies to evaluate the metabolism and residues kinetics of veterinary drugs in human food-producing animals: Validation of analytical methods used in residue depletion studies. Eur. Med. Agency 2016, 44, 1–21. [Google Scholar]
- Hsieh, D.P.H.; Wong, J.J. Pharmacokinetics and Excretion of Aflatoxins. The Toxicology of Aflatoxins: Human Health, Veterinary and Agricultural Significance; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Coulombe, R.A., Jr.; Sharma, R.P. Clearance and excretion of intracheally and orally administred aflatoxin B1 in the rat. Food Chem. Toxicol. 1985, 23, 827–830. [Google Scholar] [CrossRef]
- Gallo, A.; Moschini, M.; Masoero, F. Aflatoxins absorption in the gastro-intestinal tract and in the vaginal mucosa in lactating dairy cows. Ital. J. Anim. Sci. 2008, 7, 53–63. [Google Scholar] [CrossRef]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef] [Green Version]
- Slobodchikova, I.; Sivakumar, R.; Rahman, S.; Vuckovic, D. Characterization of Phase I and Glucuronide Phase II Metabolites of 17 Mycotoxins Using Liquid Chromatography-High-Resolution Mass Spectrometry. Toxins 2019, 11, 433. [Google Scholar] [CrossRef] [Green Version]
- Lauwers, M.; Croubels, S.; Letor, B.; Gougoulias, C.; Devreese, M. Biomarkers for Exposure as a Tool for Efficacy Testing of a Mycotoxin Detoxifier in Broiler Chickens and Pigs. Toxins 2019, 11, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolosova, A.; Stroka, J. Substances for reduction of the contamination of feed by mycotoxins: A review. World Mycotoxin J. 2011, 4, 225–256. [Google Scholar] [CrossRef]
- Barrientos-velázquez, A.L.; Arteaga, S.; Dixon, J.B.; Deng, Y. The effects of pH, pepsin, exchange cation, and vitamins on aflatoxin adsorption on smectite in simulated gastric fluids. Appl. Clay Sci. 2016, 120, 17–23. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- De Baere, S.; Goossens, J.; Osselaere, A.; Devreese, M.; Vandenbroucke, V.; De Backer, P.; Croubels, S. Quantitative determination of T-2 toxin, HT-2 toxin, deoxynivalenol and deepoxy-deoxynivalenol in animal body fluids using LC–MS / MS detection. J. Chromatogr. B. 2011, 879, 2403–2415. [Google Scholar] [CrossRef]
- Almeida, A.M.; Castel-Branco, M.M.; Falcão, A.C. Linear regression for calibration lines revisited: Weighting schemes for bioanalytical methods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 774, 215–222. [Google Scholar] [CrossRef]
- Heitzman, R.J. Veterinary Drug Residues “Residues in Food Producing Animals and Their Products: Reference Materials and Methods”, 2nd ed.; Heitzman, R.J., Ed.; Commission of the European Communities: Brussels, Belgium, 1994; Volume 636, ISBN 0-8412-3419-1. [Google Scholar]
- Knecht, J.; Stork, G. Prozentuales und logarithmisches Verfahren zur Berechnung yon Eichkurven. Z. Anal. Chem. 1974, 99, 97–99. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]


| Matrix | Calibration Range (ng/mL) | g (%) a | ra | LOQ (ng/mL) | LOD (ng/mL) |
|---|---|---|---|---|---|
| Broiler Chicken plasma | 0.50–200 | 4.50 ± 1.48 | 0.9987 ± 0.0008 | 0.50 | 0.16 |
| Toxicokinetic Parameters | Route of Administration | ||||
|---|---|---|---|---|---|
| IV | PO | ||||
| PO | PO + CP | PO + CC | p-Value | ||
| AUC0-t (h*ng/mL) | 89.06 ± 36.94 | 12.83 ± 4.19 a | 11.36 ± 5.10 a,b | 6.78 ± 4.24 b | 0.026 |
| AUC0-∞ (h*ng/mL) | 89.86 ± 36.73 | 15.10 ± 4.82 a | 14.73 ± 5.08 a,b | 9.06 ± 5.03 b | 0.041 |
| Cmax (ng/mL) | _ | 11.01 ± 5.32 | 10.41 ± 7.50 | 13.59 ± 14.95 | 0.760 |
| C0 (ng/mL) | 274.62 ± 144.49 | _ | _ | _ | _ |
| Tmax (h) | _ | 0.50 ± 0.30 | 0.48 ± 0.36 | 0.48 ± 0.34 | 0.987 |
| kel (h-1) | 1.53 ± 0.42 | 0.57 ± 0.17 | 0.35 ± 0.26 | 0.55 ± 0.36 | 0.158 |
| T1/2el (h) | 0.50 ± 0.20 | 1.36 ± 0.55 | 2.15 ± 1.61 | 2.49 ± 0.30 | 0.498 |
| Cl (L/h/kg) | 28.50 ± 18.22 | _ | _ | _ | _ |
| Vd (L/kg) | 22.52 ± 20.32 | _ | _ | _ | _ |
| F (%) | 100 | 14.40 ± 4.70 a | 12.75 ± 5.73 a,b | 7.61 ± 4.76 b | 0.026 |
| Time (min) | % Mobile Phase A | % Mobile Phase B |
|---|---|---|
| 0–1.0 | 80 | 20 |
| 3.0 | - | 90 |
| 3.0–7.0 | 10 | 90 |
| 7.3 | - | 90 |
| 7.3–10.0 | 80 | 20 |
| Time (min) | % Mobile Phase A | % Mobile Phase B |
|---|---|---|
| 0–2.0 | 90 | 10 |
| 4.0 | - | 30 |
| 4.0–6.0 | 70 | 30 |
| 8 | - | 50 |
| 8.0–10.0 | 50 | 50 |
| 12.0 | - | 70 |
| 12.0–14.0 | 30 | 70 |
| 14.2 | - | 90 |
| 14.2–16.5 | 10 | 90 |
| 17.0 | - | 10 |
| 17.0–20.0 | 90 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rejeb, R.; Baere, S.D.; Devreese, M.; Ducatelle, R.; Croubels, S.; Ayed, M.H.; Ghorbal, A.; Antonissen, G. Calcination Improves the In Vivo Efficacy of a Montmorillonite Clay to Bind Aflatoxin G1 in Broiler Chickens: A Toxicokinetic Approach. Toxins 2020, 12, 660. https://doi.org/10.3390/toxins12100660
Rejeb R, Baere SD, Devreese M, Ducatelle R, Croubels S, Ayed MH, Ghorbal A, Antonissen G. Calcination Improves the In Vivo Efficacy of a Montmorillonite Clay to Bind Aflatoxin G1 in Broiler Chickens: A Toxicokinetic Approach. Toxins. 2020; 12(10):660. https://doi.org/10.3390/toxins12100660
Chicago/Turabian StyleRejeb, Roua, Siegrid De Baere, Mathias Devreese, Richard Ducatelle, Siska Croubels, Madiha Hadj Ayed, Achraf Ghorbal, and Gunther Antonissen. 2020. "Calcination Improves the In Vivo Efficacy of a Montmorillonite Clay to Bind Aflatoxin G1 in Broiler Chickens: A Toxicokinetic Approach" Toxins 12, no. 10: 660. https://doi.org/10.3390/toxins12100660
APA StyleRejeb, R., Baere, S. D., Devreese, M., Ducatelle, R., Croubels, S., Ayed, M. H., Ghorbal, A., & Antonissen, G. (2020). Calcination Improves the In Vivo Efficacy of a Montmorillonite Clay to Bind Aflatoxin G1 in Broiler Chickens: A Toxicokinetic Approach. Toxins, 12(10), 660. https://doi.org/10.3390/toxins12100660