Covariation of Ergot Severity and Alkaloid Content Measured by HPLC and One ELISA Method in Inoculated Winter Rye across Three Isolates and Three European Countries
Abstract
:1. Introduction
2. Results
2.1. Influence of Genotype, Environment, Isolate, and Interaction on Ergot Severity and EA Content
2.2. Covariation of Ergot Severity and Alkaloid Content Measured by HPLC
2.3. Covariations with ErgoREAD ELISA Values
2.4. Effect of the Isolates on Ergot Infection and EA Content
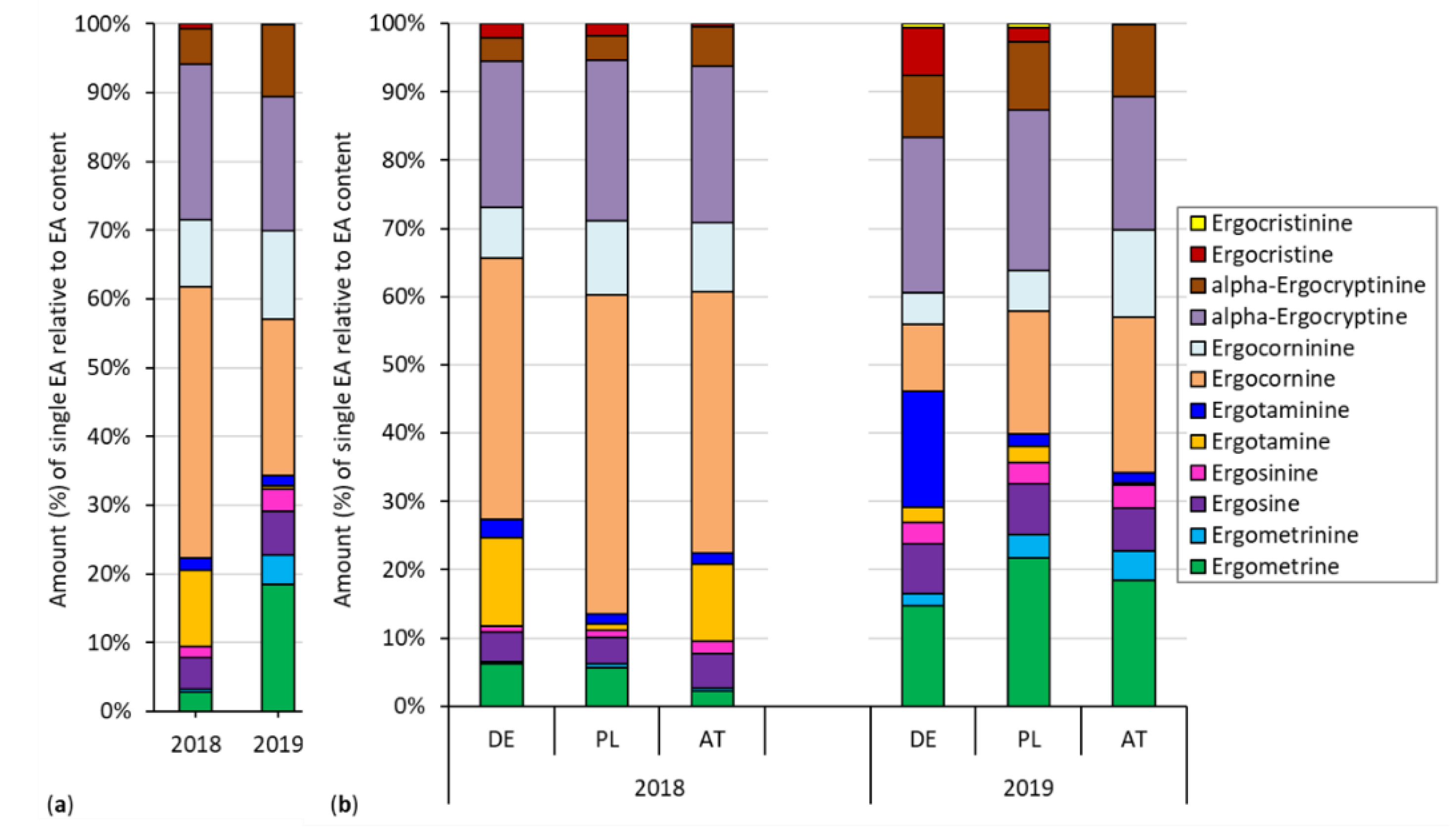
2.5. Composition of the EA Spectrum
3. Discussion
3.1. Environment (Location × Year Combination) Has a High Impact
3.2. Isolates Affect Ergot Severity and EA Formation in a Host-Unspecific Way
3.3. EA Spectrum Was Rather Stable across Years and Isolates
3.4. Covariation of Ergot Severity and EA Content is Moderate
4. Conclusions
5. Materials and Methods
5.1. Samples, Field Trials, and Inoculation Procedure
5.2. Sample Preparation
5.3. High Performance Liquid Chromatography (HPLC) Analysis of EAs
5.4. Enzyme-Linked Immunosorbent Assay (ELISA) of EAs
5.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wegulo, S.N.; Carlson, M.P. Ergot of Small Grain Cereals and Grasses and its Health Effects on Humans and Livestock. 2011. University of Nebraska, Extension, EC1880. Available online: http://ianrpubs.unl.edu/live/ec1880/build/ec1880.pdf (accessed on 9 March 2020).
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 12 March 2020).
- Money, N.P. Fungi and biotechnology. In The Fungi, 3rd ed.; Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780123820341. [Google Scholar] [CrossRef]
- Florea, S.; Panaccione, D.G.; Schardl, C.L. Ergot alkaloids of the family Clavicipitaceae. Phytopathology 2017, 107, 504–518. [Google Scholar] [CrossRef] [Green Version]
- Schiff, P.L. Ergot and its alkaloids. Am. J. Pharm. Educ. 2006, 70, 98. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.A.; Stokes, J.T.; Redmond, C.T.; Schardl, C.L.; Panaccione, D.G. Contribution of ergot alkaloids to suppression of a grass-feeding caterpillar assessed with gene knockout endophytes in perennial ryegrass. Entomol. Exp. Appl. 2008, 126, 138–147. [Google Scholar] [CrossRef]
- Panaccione, D.G.; Arnold, S.L. Ergot alkaloids contribute to virulence in an insect model of invasive aspergillosis. Sci. Rep. 2017, 7, 8930. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, D.G.; Cipoletti, J.R.; Sedlock, A.B.; Blemings, K.P.; Schardl, C.L.; Machado, C.; Seidel, G.E. Effects of ergot alkaloids on food preference and satiety in Rabbits, as assessed with gene-knockout endophytes in perennial ryegrass (Lolium perenne). J. Agric. Food Chem. 2006, 54, 4582–4587. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, N.; Keller, N.P. Mycotoxins in conversation with bacteria and fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef]
- Křen, V.; Cvak, L. Ergot: The Genus Claviceps; Harwood Academic Publishers: Amsterdam, The Netherlands, 1999; pp. 173–200. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Cheng, J.Z.; O’Connor, S.E. Biosynthesis of the ergot alkaloids. Nat. Prod. Rep. 2014, 31, 1328. [Google Scholar] [CrossRef]
- Schardl, C.L.; Panaccione, D.G.; Tudzynski, P. Ergot alkaloids—Biology and molecular biology. Alkaloids. Chem. Biol. 2006, 63, 45–86. [Google Scholar] [CrossRef]
- Young, C.A.; Schardl, C.L.; Panaccione, D.G.; Florea, S.; Takach, J.E.; Charlton, N.D.; Moore, N.; Webb, J.S.; Jaromczyk, J. Genetics, genomics and evolution of ergot alkaloid diversity. Toxins 2015, 7, 1273–1302. [Google Scholar] [CrossRef] [Green Version]
- Battilani, P.; Costa, L.G.; Dossena, A.; Gullino, M.L.; Marchelli, R.; Galaverna, G.; Pietri, A.; Dall’Asta, C.; Giorni, P.; Spadaro, D.; et al. Scientific Information on Mycotoxins and Natural Plant Toxicants. 2009. Scientific/Technical Report Submitted to EFSA. Available online: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2009.EN-24/pdf (accessed on 12 March 2020).
- European Food Safety Authority (EFSA). Scientific Opinion on Ergot Alkaloids in Food and Feed. EFSA Panel on Contaminants in the Food Chain (CONTAM). 2012. EFSA 10: 2798. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2012.2798/pdf (accessed on 12 March 2020).
- Krska, R.; Crews, C. Significance, chemistry and determination of ergot alkaloids: A review. J. Food Contam. 2008, 25, 722–731. [Google Scholar] [CrossRef]
- Malysheva, S.V.; Larionova, D.A.; Di Mavungu, J.D.; De Saeger, S. Pattern and distribution of ergot alkaloids in cereals and cereal products from European countries. World Mycotoxin J. 2014, 7, 217–230. [Google Scholar] [CrossRef]
- Lin, W.; Kuang, Y.; Wang, J.; Duan, D.; Xu, W.; Tian, P.; Nzabanita, C.; Wang, M.; Li, M.; Ma, B. Effects of seasonal variation on the alkaloids of different ecotypes of Epichloë endophyte-Festuca sinensis associations. Front. Microbiol. 2019, 10, 1695. [Google Scholar] [CrossRef] [Green Version]
- Menzies, J.G.; Klein-Gebbinck, H.W.; Gordon, A.; O’Sullivan, D. Evaluation of Claviceps purpurea isolates on wheat reveals complex virulence and host susceptibility relationships. Can. J. Plant Pathol. 2017, 39, 307–317. [Google Scholar] [CrossRef]
- Kodisch, A.; Wilde, P.; Schmiedchen, B.; Fromme, F.J.; Rodemann, B.; Tratwal, A.; Oberforster, M.; Wieser, F.; Schiemann, A.; Jørgensen, L.N.; et al. Ergot infection in winter rye hybrids shows differential contribution of male and female genotypes and environment. Eupyhtica 2020, 216, 65. [Google Scholar] [CrossRef] [Green Version]
- Crews, C. Analysis of ergot alkaloids. Toxins 2015, 7, 2024–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flieger, M.; Wurst, M.; Shelby, R. Ergot alkaloids—Sources, structures and analytical methods. Folia Microbiol. 1997, 42, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M. Analysis of ergot alkaloids—A review. Mycotoxin Res. 2007, 23, 113–121. [Google Scholar] [CrossRef]
- Schardl, C.L. Introduction to the toxins special issue on ergot alkaloids. Toxins 2015, 7, 4232–4237. [Google Scholar] [CrossRef] [Green Version]
- Debegnach, F.; Patriarca, S.; Brera, C.; Gregori, E.; Sonego, E.; Moracci, G.; De Santis, B. Ergot alkaloids in wheat and rye derived products in Italy. Foods 2019, 8, 150. [Google Scholar] [CrossRef] [Green Version]
- Meister, U.; Batt, N. Fusarium Toxins and Ergot Alkaloids in Rye of the Federal State Brandenburg Harvested 2013. In Proceedings of the 36th Mycotoxin Workshop, Göttingen, Germany, 16 June 2014; p. 131. Available online: http://www.mycotoxin-workshop.de/36th_Mycotoxin_Workshop_2014_-Proceedings.pdf (accessed on 12 March 2020).
- Mulder, P.P.J.; van Raamsdonk, L.W.D.; van Egmond, H.J.; Voogt, J.; van Brakel, M.W.; van der Horst, G.M.; de Jong, J. Dutch Survey Ergot Alkaloids and Sclerotia in Animal Feeds. Report 2012.005. RIKILT. 2012. Available online: http://edepot.wur.nl/234699 (accessed on 12 March 2020).
- Müller, C.; Kemmlein, S.; Klaffke, H.; Krauthaus, W.; Preiß-Weigert, A.; Wittkowski, R. A basic tool for risk assessment: A new method for the analysis of ergot alkaloids in rye and selected rye products. Mol. Nutr. Food Res. 2009, 53, 500–550. [Google Scholar] [CrossRef]
- Ruhland, M.; Tischler, J. Determination of ergot alkaloids in feed by HPLC. Mycotoxin Res. 2008, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Veršilovskis, A.; Pereboom-de Fauw, D.P.K.H.; Smits, N.; Mulder, P.P.J.; Mol, H.; de Nijs, M. EURL-MP-report_001. Screening of Ergot Alkaloids by ELISA Test Kits Available on the Market. 2019. EURL Mycotoxins and Plant Toxins, Wageningen Food Safety Research, Part of Wageningen University & Research. Available online: https://www.wur.nl/en/show/EURL-MP-report_001-Screening-of-ergot-alkaloids-using-ELISA-kits.htm (accessed on 12 October 2020).
- Cell Signaling Technology (CST). Overview of Enzyme-Linked Immunosorbent Assay (ELISA). 2020. Available online: https://en.cellsignal.de/contents/_/overview-of-enzyme-linked-immunosorbent-assay-(elisa)/elisa-educational (accessed on 12 March 2020).
- LCTech. ELISA Rapid Test ErgoREAD. 2020. Available online: https://www.lctech.de/en/products/elisa-rapid-test-ergoread.html (accessed on 11 March 2020).
- Miedaner, T.; Geiger, H.H. Biology, genetics, and management of ergot (Claviceps spp.) in rye, sorghum, and pearl millet. Toxins 2015, 7, 659–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miedaner, T.; Mirdita, V.; Rodemann, B.; Drobeck, T.; Rentel, D. Genetic variation of winter rye cultivars for their ergot (Claviceps purpurea) reaction tested in a field design with minimized interplot interference. Plant Breed. 2010, 129, 58–62. [Google Scholar] [CrossRef]
- Hulvová, H.; Galuszka, P.; Frébortová, J.; Frébort, I. Parasitic fungus Claviceps as a source for biotechnological production of ergot alkaloids. Biotechnol. Adv. 2013, 31, 79–89. [Google Scholar] [CrossRef]
- Van Dongen, P.W.; de Groot, A.N. History of ergot alkaloids from ergotism to ergometrine. Eur. J. Obs. Gynecol. Reprod. Biol. 1995, 60, 109–116. [Google Scholar] [CrossRef]
- Coufal-Majewski, S.; Stanford, K.; McAllister, T.; Blakley, B.; McKinnon, J.; Chaves, A.V.; Wang, Y. Impacts of cereal ergot in food animal production. Front. Vet. Sci. 2016, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- European Communities. Directive 2002/32/EC of the European parliament and of the council of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Communities 2002, L140, 10–22. [Google Scholar]
- European Union. Commission Regulation (EU) 2015/1940 of 28 October 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Ergot Sclerotia in Certain Unprocessed Cereals and the Provisions on Monitoring and Reporting. Off. J. Eur. Union 2015, L283, 3. [Google Scholar]
- Beuerle, T.; Benford, D.; Brimer, L.; Cottrill, B.; Doerge, D.; Dusemund, B.; Farmer, P.; Fürst, P.; Humpf, H.; Mulder, P.P.J. Scientific opinion on ergot alkaloids in food and feed. EFSA J. 2012, 10, 2798–2956. [Google Scholar] [CrossRef]
- Byrd, N.; Slaiding, I.R. Final Project Report: Monitoring of Mycotoxins and Other Contaminants in UK Cereals Used in Malting, Milling and Animal Feed. AHDB PR578. 2017. Available online: https://ahdb.org.uk/final-project-report-contaminants-monitoring-150517 (accessed on 12 March 2020).
- MacDonald, S.J.; Anderson, W.A.C. Final Project Report: A Desk Study to Review Current Knowledge on Ergot Alkaloids and Their Potential for Contamination to Cereal Grains. AHDB PR575. 2017. Available online: https://ahdb.org.uk/a-desk-study-to-review-current-knowledge-on-ergot-alkaloids-and-their-potential-for-contamination-to-cereal-grains (accessed on 12 March 2020).
- Gordon, A.; Delamare, G.; Tente, E.; Boyd, L. Final Project Report: Determining the Routes of Transmission of Ergot Alkaloids in Cereal Grains. AHDB PR603. 2019. Available online: https://ahdb.org.uk/determining-the-routes-of-transmission-of-ergot-alkaloids-in-cereal-grains (accessed on 12 March 2020).
- Topi, D.; Jakovac-Strajn, B.; Pavšič-Vrtač, K.; Tavčar-Kalcher, G. Occurrence of ergot alkaloids in wheat from Albania. Food Addit. Contam. Part A 2017, 34, 1333–1343. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K. Stability of ergot alkaloids during the process of baking rye bread. LWT 2019, 110, 269–274. [Google Scholar] [CrossRef]
- Meleard, B. Degradation and Epimerization of Wheat Ergot Alkaloids during French Baking Test. 2016. Available online: https://www.english.arvalisinstitutduvegetal.fr/file/galleryelement/pj/84/76/8a/c1/meleardalkaloids_and_bread_mytox739905815900991417.pdf (accessed on 12 March 2020).
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; Turnock, D.; Taylor, D.; Fu, B.X. Fate of ergot alkaloids during laboratory scale durum processing and pasta production. Toxins 2019, 11, 195. [Google Scholar] [CrossRef] [Green Version]
- Canadian Grain Commission. Harvest Survey of Canadian Grain Quality. 2013. Available online: http://www.grainscanada.gc.ca/quality-qualite/hsp-per/hs-er-eng.html (accessed on 12 March 2020).
- Alderman, S. Ergot: Biology and Control. 2006. Available online: http://www.ars.usda.gov/SP2UserFiles/person/81/ErgotDVDtranscript.pdf (accessed on 4 September 2020).
- Gordon, A.; Basler, R.; Bansept-Basler, P.; Fanstone, V.; Harinarayan, L.; Grant, P.K.; Birchmore, R.; Bayles, R.A.; Boys, L.A.; O’Sullivan, D.O. The identification of QTL controlling ergot sclerotia size in hexaploid wheat implicates a role for the Rht dwarfing alleles. Theor. Appl. Genet. 2015, 128, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; McCartney, C.; Knox, R.E.; Ereful, N.; Hiebert, C.W.; Konkin, D.J.; Hsueh, Y.C.; Bhadauria, V.; Sgroi, M.; O’Sullivan, D.M.; et al. Genetic and transcriptional dissection of resistance to Claviceps purpurea in the durum wheat cultivar Greenshank. Theor. Appl. Genet. 2020, 133, 1873–1886. [Google Scholar] [CrossRef] [Green Version]
- Dung, J.K.S.; Cheng, Q.; Kaur, N.; Walenta, D.L.; Cating, R.A.; Rondon, S.I.; Frost, K.E.; Alderman, S.C.; Hamm, P.B. Population biology and epidemiology of Claviceps purpurea in cool-season grass seed crops. In Proceedings of the 10th International Seed Conference, Corvallis, OR, USA, 12–19 May 2019; p. 78. [Google Scholar]
- Menzies, J.G.; Turkington, T.K. An overview of the ergot (Claviceps purpurea) issue in western Canada: Challenges and solutions. Can. J. Plant Pathol. 2015, 37, 40–51. [Google Scholar] [CrossRef]
- Wang, E.; Meinke, H.; Ryley, M.; Herde, D.; Henzell, B. On the relation between weather variables and sorghum ergot infection. Aust. J. Agric. Res. 2000, 51, 313–324. [Google Scholar] [CrossRef]
- Workneh, F.; Rush, C.M. Evaluation of relationships between weather patterns and prevalence of sorghum ergot in the Texas panhandle. Phytopathology 2002, 92, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Workneh, F.; Rush, C.M. Weather factors associated with development of sorghum ergot in the Texas panhandle. Plant Dis. 2006, 90, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, B.S.; Mirdita, V.; Miedaner, T. Preliminary evaluation of locations for conducting selection for resistance to ergot (Claviceps purpurea) in rye. Indian J. Genet. Pl. Br. 2010, 23, 265–268. [Google Scholar]
- Mirdita, V.; Miedaner, T. Resistance to ergot in self-incompatible germplasm resources of winter rye. J. Phytopathol. 2009, 157, 350–355. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; Menzies, J.G. The effect of selected factors on measured ergot alkaloid content in Claviceps purpurea-infected hexaploid and durum wheat. World Mycotoxin J. 2016, 9, 555–564. [Google Scholar] [CrossRef]
- Cagaš, B.; Macháč, R. Different pathogenicity of ergot isolates (Claviceps purpurea [Fr.] Tul.) on Kentucky bluegrass (Poa pratensis L.). Plant Prot. Sci. 2002, 38, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Franzmann, C.; Wächter, J.; Dittmer, N.; Humpf, H.S. Ricinoleic acid as a marker for ergot impurities in rye and rye products. J. Agric. Food Chem. 2010, 58, 4223–4229. [Google Scholar] [CrossRef] [PubMed]
- Di Mavungu, D.J.; Malysheva, S.V.; Sanders, M.; Larionova, D.; Robbens, J.; Dubruel, P.; Peteghem, C.V.; De Saeger, S. Development and validation of a new LC–MS/MS method for the simultaneous determination of six major ergot alkaloids and their corresponding epimers. Application to some food and feed commodities. Food Chem. 2012, 135, 292–303. [Google Scholar] [CrossRef]
- Schummer, C.; Zandonella, I.; van Nieuwenhuyse, A.; Moris, G. Epimerization of ergot alkaloids in feed. Helyion 2020, 6. [Google Scholar] [CrossRef]
- Kniel, B.; Meißner, M.; Koehler, P.; Schwake-Anduschus, C. Studies on the applicability of HPLC-FLD and HPLC–MS/MS for the determination of ergot alkaloids in rye-containing breads. J. Consum. Prot. Food S. 2018, 13, 69–78. [Google Scholar] [CrossRef]
- Blaney, B.J.; Molloy, J.B.; Brock, I.J. Alkaloids in Australian rye ergot (Claviceps purpurea) sclerotia: Implications for food and stockfeed regulations. Anim. Prod. Sci. 2009, 49, 975–982. [Google Scholar] [CrossRef]
- Shi, H.; Yu, P. Exploring the potential of applying infrared vibrational (micro)spectroscopy in ergot alkaloids determination: Techniques, current status, and challenges. Appl. Spectrosc. Rev. 2018, 53, 395–419. [Google Scholar] [CrossRef]
- Shelby, R.A.; Kelley, V.C. Detection of ergot alkaloids from Claviceps species in agricultural products by competitive ELISA using a monoclonal antibody. J. Agric. Food Chem. 1992, 40, 1090–1092. [Google Scholar] [CrossRef]
- Tunali, B.; Shelby, R.A.; Morgan-Jones, G.; Kodan, M. Endophytic fungi and ergot alkaloids in native Turkish grasses. Phytoparasitica 2000, 28, 375–377. [Google Scholar] [CrossRef]
- Kenyon, S.A.; Roberts, C.A.; Kallenbach, R.L.; Lory, J.O.; Kerley, M.S.; Rottinghaus, G.E.; Hill, N.S.; Ellersieck, M.R. Vertical distribution of ergot alkaloids in the vegetative canopy of tall fescue. Crop Sci. 2018, 58, 925–931. [Google Scholar] [CrossRef]
- Roberts, C.A.; Davis, D.K.; Looper, M.L.; Kallenbach, R.L.; Rottinghaus, G.E.; Hill, N.S. Ergot alkaloid concentrations in high- and low-moisture tall fescue silage. Crop Sci. 2014, 54, 1887–1892. [Google Scholar] [CrossRef]
- Schnitzius, J.M.; Hill, N.S.; Thompson, C.S.; Craig, A.M. Semiquantitative determination of ergot alkaloids in seed, straw, and digesta samples using a competitive enzyme-linked immunosorbent assay. J. Vet. Diagn. Invest. 2001, 13, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Ao, D.; Mahadevan, S. Calibration experimental design considering field response and model uncertainty. Comput. Methods Appl. 2017, 318, 92–119. [Google Scholar] [CrossRef] [Green Version]
- Raposo, F. Evaluation of analytical calibration based on least-squares linear regression for instrumental techniques: A tutorial review. Trends Anal. Chem. 2006, 77, 167–185. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Podolska, G.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Occurrence of ergot and its alkaloids in winter rye harvested in Poland. World Mycotoxin J. 2018, 11, 635–646. [Google Scholar] [CrossRef]
- Grusie, T.; Cowan, V.; Singh, J.; McKinnon, J.; Blakley, B. Correlation and variability between weighing, counting and analytical methods to determine ergot (Claviceps purpurea) contamination of grain. World Mycotoxin J. 2017, 10, 209–218. [Google Scholar] [CrossRef]
- Orlando, B.; Maumené, C.; Piraux, F. Ergot and ergot alkaloids in French cereals: Occurrence, pattern and agronomic practices for managing the risk. World Mycotoxin J. 2017, 10, 327–338. [Google Scholar] [CrossRef]
- Byrd, N.; De Alwis, J.; Booth, M.; Jewell, K. Final Project Report: Monitoring the Presence of Ergot Alkaloids in Cereals and a Study of a Possible Relationship between Occurrence of Sclerotia Content and Levels of Ergot Alkaloids. Project Number FS516009.2014. Available online: https://www.food.gov.uk/sites/default/files/media/document/FS516009%20Final%20Ergot%20Alkaloid%20report%20(3).pdf (accessed on 18 June 2020).
- Schummer, C.; Brune, L.; Moris, G. Development of a UHPLC-FLD method for the analysis of ergot alkaloids and application to different types of cereals from Luxembourg. Mycotoxin Res. 2018, 34, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Dänicke, S.; Schmiedchen, B.; Wilde, P.; Wortmann, H.; Dhillon, B.S.; Mirdita, V. Genetic variation for ergot (Claviceps purpurea) resistance and alkaloid concentrations in cytoplasmic-male sterile winter rye under pollen isolation. Euphytica 2010, 173, 299–306. [Google Scholar] [CrossRef]
- Kirchhoff, H. Beiträge zur Biologie und Physiologie des Mutterkornpilzes. Centralbl. Bakteriol. Parasitenk. Abt. II 1929, 77, 310–369. [Google Scholar]
- Mielke, H. Untersuchungen zur Bekämpfung des Mutterkorns. Nachr. Deut. Pflanzenschutzd. 1993, 45, 97–102. [Google Scholar]
- Engelke, T. Ansätze für eine integrierte Bekämpfung des Mutterkorns (Claviceps purpurea [Fr.] Tul.) im Roggen. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2002. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH Monograph. 2001. Available online: https://www.julius-kuehn.de/media/Veroeffentlichungen/bbch%20epaper%20en/page.pdf (accessed on 4 April 2020).
- BVL L 15.01/02–5:2012–01. Untersuchung von Lebensmitteln—Bestimmung von Ergotalkaloiden in Roggen und Weizen—HPLC-Verfahren mit Reinigung an einer basischen Aluminiumoxid-Festphase. 2012. Available online: https://www.beuth.de/de/technische-regel/bvl-l-15–01–02–5/150736503 (accessed on 4 April 2020).
- Bernal-Vasquez, A.M.; Utz, H.F.; Piepho, H.P. Outlier detection methods for generalized lattices: A case study on the transition from ANOVA to REML. Theor. Appl. Genet. 2016, 129, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G.; Cox, G.M. Experimental Designs; Wiley: New York, NY, USA, 1957; ISBN 978-0-471-54567-5. [Google Scholar]
- Fehr, W.R. Principles of Cultivar Development, Theory and Technique; Macmillan: New York, NY, USA, 1987; Volume 1, ISBN 0029499208. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3–900051–07–0. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio, Inc., Boston. 2016. Available online: https://www.rstudio.com/ (accessed on 4 March 2020).



| Trait | 2018 | 2019 | 2018 + 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Isolate | DE | PL | AT | DE | PL | AT | DE | PL | AT |
| Ergot Severity (%) | 2.04a 1 | 2.07a | 1.53a | 0.67a | 1.13b | 1.72c | 1.36a | 1.60a | 1.62a |
| EAs, HPLC (mg/kg) | 1.10a | 6.21a | 37.64b | 8.41b | 0.80a | 16.75c | 5.00a | 3.32a | 26.50b |
| EAs, ELISA (mg/kg) | 7.5a | 26.8b | 23.5b | 862.9ab | 1700.7a | 254.8b | 387.7a | 824.8b | 120.7a |
| Genotype | Breeding Company | Test Environments | Isolate(s) | No. of Samples | |
|---|---|---|---|---|---|
| Number | Name | ||||
| D.Amber 1 | “DANKO” Hodowla Roslin Sp. z o.o. | 16 | All 2 | DE, PL, AT | 96 |
| Elias 1 | Saatzucht LFS Edelhof | 16 | All 2 | DE, PL, AT | 96 |
| H_Hyb5 1 | HYBRO Saatzucht GmbH & Co. KG | 16 | All 2 | DE, PL, AT | 96 |
| D.Amber | “DANKO” Hodowla Roslin Sp. z o.o. | 2 | OLI | DE | 4 |
| Elias | Saatzucht LFS Edelhof | 2 | OLI | DE | 4 |
| H_Hyb5 | HYBRO Saatzucht GmbH & Co. KG | 2 | OLI | DE | 4 |
| Conduct | KWS LOCHOW GmbH | 2 | OLI | DE | 4 |
| K_Hyb2 | KWS LOCHOW GmbH | 2 | OLI | DE | 4 |
| H_Pop | HYBRO Saatzucht GmbH & Co. KG | 2 | OLI | DE | 4 |
| FC(15) | KWS LOCHOW GmbH | 2 | OLI | Mix | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodisch, A.; Oberforster, M.; Raditschnig, A.; Rodemann, B.; Tratwal, A.; Danielewicz, J.; Korbas, M.; Schmiedchen, B.; Eifler, J.; Gordillo, A.; et al. Covariation of Ergot Severity and Alkaloid Content Measured by HPLC and One ELISA Method in Inoculated Winter Rye across Three Isolates and Three European Countries. Toxins 2020, 12, 676. https://doi.org/10.3390/toxins12110676
Kodisch A, Oberforster M, Raditschnig A, Rodemann B, Tratwal A, Danielewicz J, Korbas M, Schmiedchen B, Eifler J, Gordillo A, et al. Covariation of Ergot Severity and Alkaloid Content Measured by HPLC and One ELISA Method in Inoculated Winter Rye across Three Isolates and Three European Countries. Toxins. 2020; 12(11):676. https://doi.org/10.3390/toxins12110676
Chicago/Turabian StyleKodisch, Anna, Michael Oberforster, Armin Raditschnig, Bernd Rodemann, Anna Tratwal, Jakub Danielewicz, Marek Korbas, Brigitta Schmiedchen, Jakob Eifler, Andres Gordillo, and et al. 2020. "Covariation of Ergot Severity and Alkaloid Content Measured by HPLC and One ELISA Method in Inoculated Winter Rye across Three Isolates and Three European Countries" Toxins 12, no. 11: 676. https://doi.org/10.3390/toxins12110676






