Venom Function of a New Species of Megalomyrmex Forel, 1885 (Hymenoptera: Formicidae)
Abstract
1. Introduction
2. Results
2.1. Taxonomic Account
2.1.1. Repositories
2.1.2. Type Material
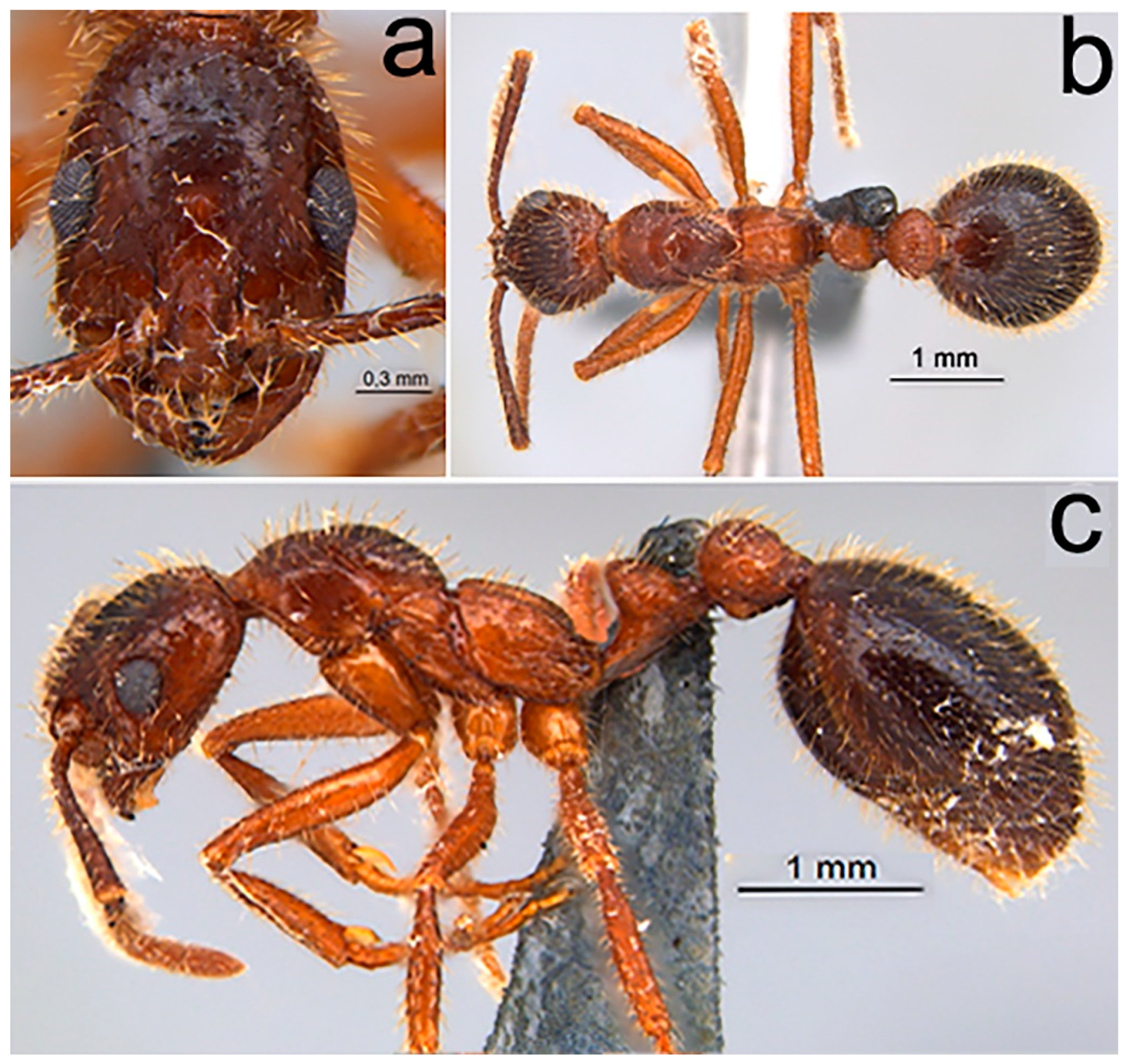
2.1.3. Diagnosis (Worker)
- Piligerous punctures on head surface slightly raised and coarser than mesosoma (Figure 1a)
- Dental formula 1 + 4 equally spaced, with the first tooth slightly smaller and the apical tooth slightly larger (Figure 1b)
- Antennal clava three segmented comparatively enlarged (Figure 1c)
- Promesonotal suture distinct and well impressed (Figure 1d)
- Metanotal sulcus deeply impressed and wide
- Absence of a sulcus between the anepisternum and katepisternum
- Ventral portion of the petiole with a translucent flange
- In lateral view, ventral region of postpetiole with globose shape (Figure 1e)
2.1.4. Worker Description
2.1.5. Ergatoid Queen
2.1.6. Male
2.1.7. Distribution and Nesting Biology
2.1.8. Additional Information
Etymology
Comments
Additional Material Examined
2.2. Chemical Analysis
2.3. MIC Assays
2.4. Insecticidal Assays
2.5. LD50 Assays of Synthetic Alkaloid
3. Discussion
4. Materials and Methods
4.1. Insect Colonies
4.2. Taxonomic Account
4.2.1. Terminology
4.2.2. Measurements
- HW. Head width. Maximum width of head in full-face view (excluding the eyes).
- HL. Head length. Maximum length of head in full-face view from anterior margin of clypeus to posterior margin of head, including occipital carina [27].
- ML. Mandibular length. Straight length of mandible, from the mandibular apex to the anterior clypeal margin.
- EL. Eye length. Maximum length of compound eye in lateral view [27].
- SL. Scape length. Maximum length of scape in dorsal view from apex to basal flange, not including basal condyle and neck [27].
- WL. Weber’s length. Diagonal length of mesosoma, from the anterior pronotal slope to the distal edge of the metapleura [44].
- PrW. Pronotum width. Maximum width of the pronotum in dorsal view [44].
- MFL. Metafemur length (most suitable view). Maximum length of the metafemur, measured from the distal margin of the trochanter to the metafemur apex [45].
- PL. Petiole length. Maximum length of the petiole in lateral view [44].
- PH. Petiole height. Maximum height of the petiole in lateral view [44].
- PPL. Postpetiole length. Maximum length of the postpetiole in lateral view.
- PPH. Postpetiole height. Maximum height of the postpetiole in lateral view.
- ATW. Abdominal tergum IV width. Maximum width of the fourth abdominal tergum with anterior, posterior, and lateral borders in the same plane of focus [46].
4.2.3. Automontage Images
4.2.4. Distribution Map
4.3. Chemical Analysis
4.4. Artificial Preparation of Alkaloid
4.5. Raw Venom Extraction
4.6. Minimum Inhibitory Concentration (MIC) Assays
4.6.1. Strains
4.6.2. Determination of MIC
4.7. Insecticidal Function of Raw Venom
4.8. LD50 Assays of Synthetic Alkaloid
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology, and Applications, 2nd ed.; Elsevier: Oxford, UK, 2015; pp. 1–496. ISBN 9780444594624. [Google Scholar]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Ujváry, I. Nicotine and other insecticidal alkaloids. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Yamamoto, I., Casida, J.E., Eds.; Springer: Tokyo, Japan, 1999; pp. 29–69. [Google Scholar]
- Zhang, Y.; Han, T.; Ming, Q.; Wu, L.; Rahman, K.; Qin, L. Alkaloids produced by endophytic fungi: A review. Nat. Prod. Commun. 2012, 7, 963–968. [Google Scholar] [CrossRef]
- Riedell, W.E.; Kieckhefer, R.E.; Petroski, R.J.; Powell, R.G. Naturally-occurring and synthetic loline alkaloid derivatives: Insect feeding behavior modification and toxicity. J. Entomol. Sci. 1991, 26, 122–129. [Google Scholar] [CrossRef]
- Hartmann, T.; Ober, D. Biosynthesis and metabolism of pyrrolizidine alkaloids in plants and specialized insect herbivores. In Biosynthesis; Leeper, F.J., Vederas, J.C., Eds.; Springer: Berlin, Germany, 2000; pp. 207–243. [Google Scholar]
- Boppré, M. Insects pharmacophagously utilizing defensive plant chemicals (pyrrolizidine alkaloids). Naturwissenschaften 1986, 73, 17–26. [Google Scholar] [CrossRef]
- Hartmann, T. Chemical ecology of pyrrolizidine alkaloids. Planta 1999, 207, 483–495. [Google Scholar] [CrossRef]
- Dussourd, D.E.; Ubik, K.; Harvis, C.; Resch, J.; Meinwald, J.; Eisner, T. Biparental defensive endowment of eggs with acquired plant alkaloid in the moth Utetheisa ornatrix. Proc. Natl. Acad. Sci. USA 1988, 85, 5992–5996. [Google Scholar] [CrossRef]
- Dumbacher, J.P.; Wako, A.; Derrickson, S.R.; Samuelson, A.; Spande, T.F.; Daly, J.W. Melyrid beetles (Choresine): A putative source for the batrachotoxin alkaloids found in poison-dart frogs and toxic passerine birds. Proc. Natl. Acad. Sci. USA 2004, 101, 15857–15860. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990; pp. 1–732. ISBN 9780674040755. [Google Scholar]
- Bolton, B. A review of the Solenopsis genus-group and revision of Afrotropical Monomorium Mayr (Hymenoptera: Formicidae). Bull. Br. Museum Nat. Hist. 1987, 54, 263–452. [Google Scholar]
- Touchard, A.; Aili, S.; Fox, E.; Escoubas, P.; Orivel, J.; Nicholson, G.; Dejean, A. The biochemical toxin arsenal from ant venoms. Toxins 2016, 8, 30. [Google Scholar] [CrossRef]
- Andersen, A.N.; Blum, M.S.; Jones, T.H. Venom alkaloids in Monomorium “rothsteini” Forel repel other ants: Is this the secret to success by Monomorium in Australian ant communities? Oecologia 1991, 88, 157–160. [Google Scholar] [CrossRef]
- Blum, M.S.; Jones, T.H.; Hölldobler, B.; Fales, H.M.; Jaouni, T. Alkaloidal venom mace: Offensive use by a thief ant. Naturwissenschaften 1980, 67, 144–145. [Google Scholar] [CrossRef]
- Numata, A.; Ibuka, T. Alkaloids from ants and other insects. Alkaloids Chem. Pharmacol. 1987, 31, 193–315. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Wu, X.; Wang, L.; Chen, L.; Lu, Y.; Xu, Y. Queen venom isosolenopsin A delivers rapid incapacitation of fire ant competitors. Toxicon 2019, 158, 77–83. [Google Scholar] [CrossRef]
- Jouvenaz, D.P.; Blum, M.S.; MacConnell, J.G. Antibacterial activity of venom alkaloids from the imported fire ant, Solenopsis invicta Buren. Antimicrob. Agents Chemother. 1972, 2, 291–293. [Google Scholar] [CrossRef]
- Vander Meer, R.K.; Morel, L. Ant queens deposit pheromones and antimicrobial agents on eggs. Naturwissenschaften 1995, 82, 93–95. [Google Scholar] [CrossRef]
- Obin, M.S.; Vander Meer, R.K. Gaster flagging by fire ants (Solenopsis spp.): Functional significance of venom dispersal behavior. J. Chem. Ecol. 1985, 11, 1757–1768. [Google Scholar] [CrossRef]
- Adams, R.M.M.; Jones, T.H. The evolution of venom alkaloids in Megalomyrmex ants, from predators to social parasites. In Proceedings of the International Society of Chemical Ecology 26th Annual Meeting, Tours, France, 31 July–4 August 2010. [Google Scholar]
- Adams, R.M.M.; Liberti, J.; Illum, A.A.; Jones, T.H.; Nash, D.R.; Boomsma, J.J. Chemically armed mercenary ants protect fungus-farming societies. Proc. Natl. Acad. Sci. USA 2013, 110, 15752–15757. [Google Scholar] [CrossRef]
- Adams, R.M.M.; Jones, T.H.; Longino, J.T.; Weatherford, R.G.; Mueller, U.G. Alkaloid venom weaponry of three Megalomyrmex thief ants and the behavioral response of Cyphomyrmex costatus host ants. J. Chem. Ecol. 2015, 41, 373–385. [Google Scholar] [CrossRef]
- Jones, T.H.; DeVries, P.J.; Escoubas, P. Chemistry of venom alkaloids in the ant Megalomyrmex foreli (Myrmicinae) from Costa Rica. J. Chem. Ecol. 1991, 17, 2507–2518. [Google Scholar] [CrossRef]
- Jones, T.H.; Blum, M.S.; Fales, H.M.; Brandão, C.R.F.; Lattke, J. Chemistry of venom alkaloids in the ant genus Megalomyrmex. J. Chem. Ecol. 1991, 17, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.M.M.; Shah, K.; Antonov, L.D.; Mueller, U.G. Fitness consequences of nest infiltration by the mutualist-exploiter Megalomyrmex adamsae. Ecol. Entomol. 2012, 37, 453–462. [Google Scholar] [CrossRef]
- Boudinot, B.E.; Sumnicht, T.P.; Adams, R.M.M. Central American ants of the genus Megalomyrmex Forel (Hymenoptera: Formicidae): Six new species and keys to workers and males. Zootaxa 2013, 3732, 1–82. [Google Scholar] [CrossRef]
- Westermann, F.L.; Mcpherson, I.S.; Jones, T.H.; Milicich, L.; Lester, P.J. Toxicity and utilization of chemical weapons: Does toxicity and venom utilization contribute to the formation of species communities? Ecol. Evol. 2015, 5, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.H.; Zottig, V.E.; Robertson, H.G.; Snelling, R.R. The venom alkaloids from some African Monomorium species. J. Chem. Ecol. 2003, 29, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Pedder, D.J.; Fales, H.M.; Jaouni, T.; Blum, M.; MacConnell, J.; Crewe, R.M. Constituents of the venom of a South African fire ant (Solenopsis punctaticeps). 2,5-dialkylpyrrolidines and -pyrrolines, identification and synthesis. Tetrahedron 1976, 32, 2275–2279. [Google Scholar] [CrossRef]
- Adams, R.M.M. Unraveling the Origins of Social Parasitism in Megalomyrmex Ants. Ph.D. Thesis, The University of Texas, Austin, TX, USA, 2008. [Google Scholar]
- Vander Meer, R.K. Ant interactions with soil organisms and associated semiochemicals. J. Chem. Ecol. 2012, 38, 728–745. [Google Scholar] [CrossRef]
- Peeters, C.; Adams, R.M.M. Uncoupling flight and reproduction in ants: Evolution of ergatoid queens in two lineages of Megalomyrmex (Hymenoptera: Formicidae). J. Insect Sci. 2016, 16, 85. [Google Scholar] [CrossRef]
- Brandão, C.R.F. Further revisionary studies on the ant genus Megalomyrmex Forel (Hymenoptera: Formicidae: Myrmicinae: Solenopsidini). Pap. Avulsos Zool. 2003, 43, 145–159. [Google Scholar] [CrossRef]
- Longino, J.T. A taxonomic review of the ant genus Megalomyrmex Forel (Hymenoptera: Formicidae) in Central America. Zootaxa 2010, 2720, 35–58. [Google Scholar] [CrossRef]
- Prado, L.P. Do Taxonomic revision of Megalomyrmex. 2020. Unpublished work. [Google Scholar]
- Melhaoui, A.; Mallear, M.; Jossang, A.; Bodo, B. Antibiotic and antifungal pyrrolidine alkaloids, from Arisarum vulgare. Nat. Prod. Lett. 1993, 2, 237–242. [Google Scholar] [CrossRef]
- Arslan, S.; Loǧoǧlu, E.; Öktemer, A. Antimicrobial activity studies on some piperidine and pyrrolidine substituted halogenobenzene derivatives. J. Enzyme Inhib. Med. Chem. 2006, 21, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; Blum, M. The biological activities of ant-derived alkaloids. Appl. Myrmecol. 1990, 482–489. [Google Scholar]
- Wilson, E.O. A monographic revision of the ant genus Lasius. Bull. Mus. Comp. Zool. 1955, 113, 59–77. [Google Scholar]
- Esteves, F.A.; Fisher, B.L. Taxonomic revision of Stigmatomma Roger (Hymenoptera: Formicidae) in the Malagasy region. Biodivers. Data J. 2016, 4, e8032. [Google Scholar] [CrossRef]
- Harris, R.A. A glossary of surface sculpturing. Occas. Pap. Entomol. 1979, 28, 1–31. [Google Scholar]
- Bolton, B. Identification Guide to the Ant genera of the World; Harvard University Press: Cambridge, MA, USA, 1994; pp. 1–232. [Google Scholar]
- Silva, R.R.; Brandão, C.R.F. Morphological patterns and community organization in leaf-litter ant assemblages. Ecol. Monogr. 2010, 80, 107–124. [Google Scholar] [CrossRef]
- Branstetter, M.G. Revision of the Middle American clade of the ant genus Stenamma Westwood (Hymenoptera, Formicidae, Myrmicinae). Zookeys 2013, 295, 1–277. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.R.; Aldawood, A.S. Review of the ant genus Meranoplus Smith, 1853 (Hymenoptera: Formicidae) in the Arabian Peninsula with description of a new species M. mosalahi sp. n. from Oman. PeerJ 2019, 7, e6287. [Google Scholar] [CrossRef]
- Romano, G.M. A high resolution shapefile of the Andean biogeographical region. Data Br. 2017, 13, 230–232. [Google Scholar] [CrossRef]
- Jones, T.H.; Franko, J.B.; Blum, M.S.; Fales, H.M. Unsymmetrical 2,5-dialkylpyrrolidines via reductive amination of 1,4-diketones. Tetrahedron Lett. 1980, 21, 789–792. [Google Scholar] [CrossRef]
- Storey, G.K.; Vander Meer, R.K.; Boucias, D.G.; McCoy, C.W. Effect of fire ant (Solenopsis invicta) venom alkaloids on the in vitro germination and development of selected entomogenous fungi. J. Invert. Path. 1991, 58, 88–95. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; National Committee for Clinical Laboratory Standards: Annapolis Junction, MD, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Quigley, T. Monitoring the Growth of E. coli with Light Scattering Using the SynergyTM 4 Multi-Mode Microplate Reader with Hybrid TechnologyTM; BioTek: Winooski, VT, USA, 2008. [Google Scholar]
- R Core Team. A Language and Environment of Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Sprouffske, K.; Wagner, A. Growthcurver: An R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinform. 2016, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Package ‘survminer’. In Drawing Survival Curves Using ‘ggplot2’.(R package version 0.3. 1.); Microsoft R Application Network: Redmond, WA, USA, 2017. [Google Scholar]
- Ritz, C.; Strebig, J.C.; Ritz, M.C. Package ‘drc.’; Creative Commons: Mountain View, CA, USA, 2016. [Google Scholar]





| Bacterial Strain | MIC (μg/mL) |
|---|---|
| Escherichia coli | 62.5 |
| Staphylococcus saprophyticus | 62.5 |
| Bacillus subtilis | 62.5 |
| Aquaspirillum serpens | 62.5 |
| Corynebacterium stationis | 31.25 |
| Ralstonia pickettii | 62.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozanski, K.; Prado, L.P.d.; Mularo, A.J.; Sadowski, V.A.; Jones, T.H.; Adams, R.M.M. Venom Function of a New Species of Megalomyrmex Forel, 1885 (Hymenoptera: Formicidae). Toxins 2020, 12, 679. https://doi.org/10.3390/toxins12110679
Sozanski K, Prado LPd, Mularo AJ, Sadowski VA, Jones TH, Adams RMM. Venom Function of a New Species of Megalomyrmex Forel, 1885 (Hymenoptera: Formicidae). Toxins. 2020; 12(11):679. https://doi.org/10.3390/toxins12110679
Chicago/Turabian StyleSozanski, Kyle, Lívia Pires do Prado, Andrew J. Mularo, Victoria A. Sadowski, Tappey H. Jones, and Rachelle M. M. Adams. 2020. "Venom Function of a New Species of Megalomyrmex Forel, 1885 (Hymenoptera: Formicidae)" Toxins 12, no. 11: 679. https://doi.org/10.3390/toxins12110679
APA StyleSozanski, K., Prado, L. P. d., Mularo, A. J., Sadowski, V. A., Jones, T. H., & Adams, R. M. M. (2020). Venom Function of a New Species of Megalomyrmex Forel, 1885 (Hymenoptera: Formicidae). Toxins, 12(11), 679. https://doi.org/10.3390/toxins12110679






