Overview of Pharmacokinetics and Liver Toxicities of Radix Polygoni Multiflori
Abstract
:1. Introduction
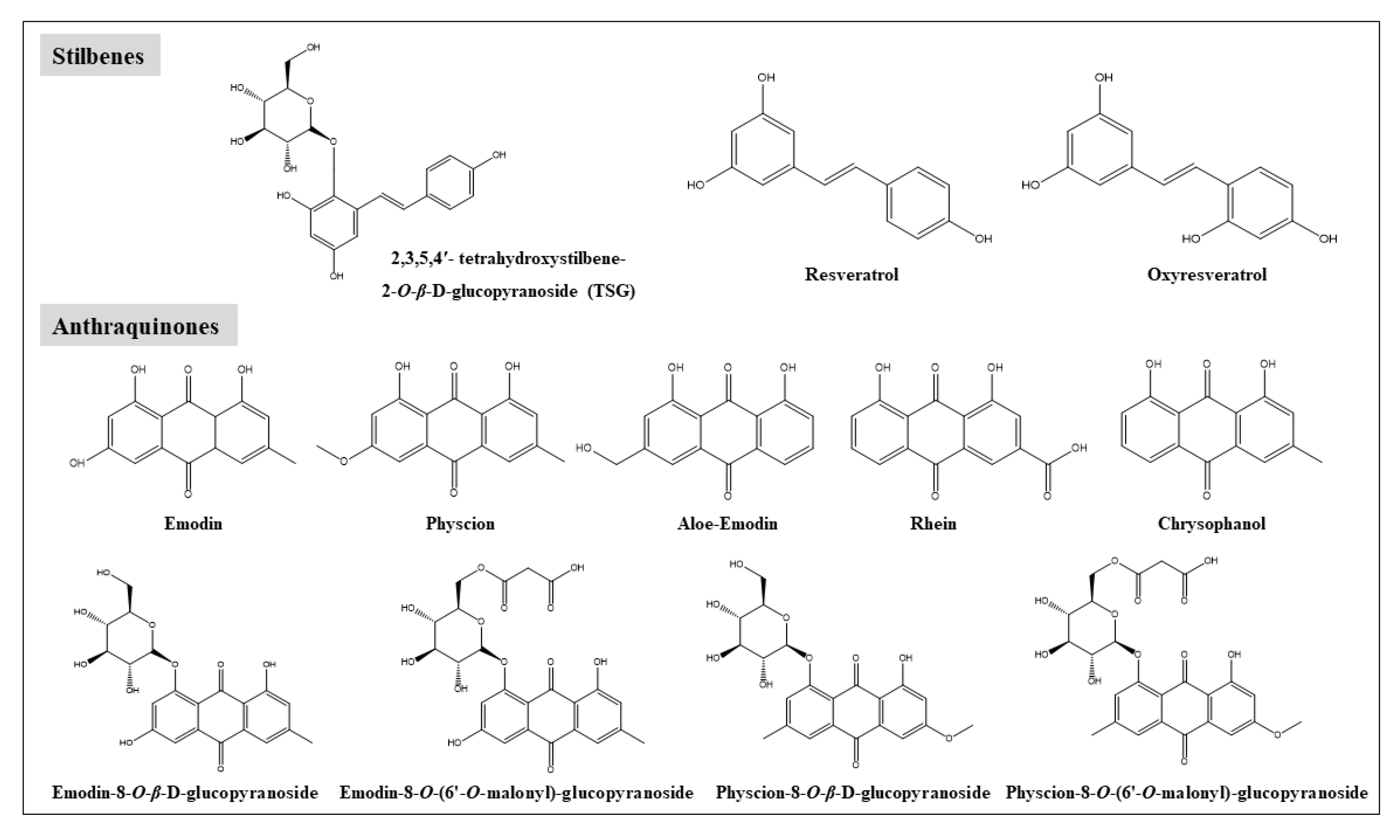
2. Chemical Constituents in RPM
3. Pharmacokinetics of RPM
3.1. Pharmacokinetic Studies of RPM Extract
3.2. Pharmacokinetic Properties of TSG, Emodin, and Physcion
3.2.1. Absorption
3.2.2. Distribution
3.2.3. Metabolism
3.2.4. Elimination
3.2.5. Effect of Co-Occurring Ingredients in RPM on the Pharmacokinetics of Emodin
4. Hepatotoxicity
4.1. Case Reports on Liver Injury of RPM
4.2. Mechanistic Studies on Liver Injury Induced by RPM Extract and Its Major Components
4.2.1. Metabolic Enzymes Alteration and Genetic Polymorphism
4.2.2. Hepatocytes Apoptosis
4.2.3. Disruption of Bile Acids Homeostasis
4.2.4. Inflammatory Damage
4.3. Correlations between Pharmacokinetics of RPM and Its Induced Liver Injury
4.4. Role of Herb–Drug/Herb Interactions in RPM Induced Liver Injury
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | Area under the concentration-time curve |
| ADME | Absorption, distribution, metabolism and elimination |
| BSEP | Bile salt export pump |
| Cmax | Peak concentration |
| CLint | Intrinsic clearance |
| CYP | Cytochrome P450 |
| Cyt c | Cytochrome c |
| EMG | Emodin-8-O-β-D-glucopyranoside |
| FXR | Farnesoid X receptor |
| GCDCA | Glycochenodeoxycholic acid |
| HLA | Human leukocyte antigen |
| HDCA | Hyodeoxycholic acid |
| Ka | Absorption rate constant |
| LPS | Lipopolysaccharide |
| MRP | Multidrug resistance-associated protein |
| NTCP | Sodium taurocholate co-transporting polypeptide |
| Papp | Apparent permeability coefficient |
| Peff | Effective intestinal permeability |
| RPM | Radix Polygoni Multiflori |
| SGLT1 | Na+/glucose cotransporter |
| TSG | 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucopyranoside |
| Tmax | Time to maximum plasma concentration |
| TβMCA | Tauro-β-muricholic acid |
| UGT | UDP glycosyltransferase |
References
- Chinese Pharmacopeia Commission. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2009; Volume 75, pp. 175–177. [Google Scholar] [CrossRef]
- Lin, H.; Ho, M.T.; Lau, L.S.; Wong, K.K.; Shaw, P.; Wan, D.C. Anti-acetylcholinesterase activities of traditional Chinese medicine for treating Alzheimer’s disease. Chem. Interact. 2008, 175, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Xu, J.P.; Li, L.; Qiao, C. Studies on nootropic activity and mechanism of emodin-8-O-β-d-glucopyranoside. Chin. Tradit. Herb. Drugs 2001, 32, 39–41. [Google Scholar]
- Zhang, Q.; Xu, Y.; Zou, S.; Zhang, X.; Cao, K.; Fan, Q. Novel functional polysaccharides from Radix Polygoni Multiflori water extracted residue: Preliminary characterization and immunomodulatory activity. Carbohydr. Polym. 2016, 137, 625–631. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, S.-Z.; Ying, H.-Z.; Dai, X.-Y.; Li, X.-X.; Yu, C.; Ye, H.-C. Chemical characterization and immunostimulatory effects of a polysaccharide from Polygoni Multiflori Radix Praeparata in cyclophosphamide-induced anemic mice. Carbohydr. Polym. 2012, 88, 1476–1482. [Google Scholar] [CrossRef]
- Lou, Z.; Xia, B.; Su, J.; Yuefang, H.; Yan, M.; Huang, Y.; Lv, G. Effect of a stilbene glycoside-rich extract from Polygoni Multiflori Radix on experimental non-alcoholic fatty liver disease based on principal component and orthogonal partial least squares discriminant analysis. Exp. Ther. Med. 2017, 14, 4958–4966. [Google Scholar] [CrossRef]
- Huang, C.-H.; Horng, L.-Y.; Chen, C.-F.; Wu, R.-T. Chinese herb Radix Polygoni Multiflori as a therapeutic drug for liver cirrhosis in mice. J. Ethnopharmacol. 2007, 114, 199–206. [Google Scholar] [CrossRef]
- Zhu, W.; Xue, X.; Zhang, Z. Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 2016, 91, 132–142. [Google Scholar] [CrossRef]
- Cha, D.S.; Jeon, H. Anti-inflammatory effect of MeOH extracts of the stem of Polygonum multiflorum in LPS-stimulated mouse peritoneal macrophages. Nat. Prod. Sci. 2009, 15, 83–89. [Google Scholar]
- But, P.P.; Tomlinson, B.; Lee, K.L. Hepatitis related to the Chinese medicine Shou-wu-pian manufactured from Polygonum multiflorum. Vet. Hum. Toxicol. 1996, 38, 280–282. [Google Scholar]
- Park, G.J.-H.; Mann, S.P.; Ngu, M.C. Acute hepatitis induced by Shou-Wu-Pian, a herbal product derived from Polygonum multiflorum. J. Gastroenterol. Hepatol. 2001, 16, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.; Restrepo, J.C.; Sierra, F.; Correa, G. Acute Hepatitis Due to Shen-Min. J. Clin. Gastroenterol. 2006, 40, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Laird, A.R.; Ramchandani, N.; Degoma, E.M.; Avula, B.; Khan, I.A.; Gesundheit, N. Acute Hepatitis Associated With the Use of an Herbal Supplement (Polygonum Multiflorum) Mimicking Iron-overload Syndrome. J. Clin. Gastroenterol. 2008, 42, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.C.; Min, H.J.; Ha, C.Y.; Kim, H.J.; Kim, T.H.; Jung, W.-T.; Lee, O.J.; Bae, I.-G. Reactivation of Pulmonary Tuberculosis in a Patient with Polygonum multiflorum Thunb-Induced Hepatitis. Gut Liver 2009, 3, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Min, H.J.; Yoo, S.S.; Kim, H.J.; Choi, S.N.; Ha, C.Y.; Kim, H.J.; Kim, T.H.; Jung, W.T.; Lee, O.J.; et al. Drug-Induced Liver Injury: Twenty Five Cases of Acute Hepatitis Following Ingestion of Polygonum multiflorum Thunb. Gut Liver 2011, 5, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Slain, D.; Cheng, J.; Ma, W.; Liang, W. Eighteen cases of liver injury following ingestion of Polygonum multiflorum. Complement. Ther. Med. 2014, 22, 70–74. [Google Scholar] [CrossRef]
- Canadian Adverse Reaction Newsletter, 2003. Case presentation: RespirActin. Available online: http://www.hc-sc.gc.ca/dhp-mps/medeff/bulletin/carn-bcei_v13n1-eng.php (accessed on 6 January 2003).
- Complementary Medicines Evaluation Committee (CMEC) A. Polygonum Multiflorum May Harm the Liver in Some People (Updated 2008). Available online: http://www.tga.gov.au/archive/labelling-rasml-notices-080414.htm (accessed on 23 April 2008).
- Medicines and Healthcare products Regulatory Agency (MHRA). MHRA Raises Concerns about the Safety of Polygonum Multiflorum (Updated 2006). Available online: https://www.who.int/medicines/publications/newsletter/pn2006_3.pdf?ua=1 (accessed on 28 April 2006).
- China Food and Drug Administration (CFDA). CFDA reminds about the risk of liver injury induced by oral assumption of PMR (Updated 2014). Available online: https://www.nmpa.gov.cn/yaopin/ypjgdt/20140716145801865.html (accessed on 16 July 2014).
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of EMODIN (CAS NO. 518-82-1) Feed Studies in F344/N Rats and B6C3F1 Mice. Natl. Toxicol. Program Tech. Rep. Ser. 2001, 493, 1–278. [Google Scholar] [PubMed]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef]
- Feng, Y.U.; Bounda, G.-A. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacogn. Res. 2015, 7, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, Q.; Yang, J.; Guo, X.; Liu, W.; Ma, S.; Li, S. Polygonum multiflorum Thunb.: A Review on Chemical Analysis, Processing Mechanism, Quality Evaluation, and Hepatotoxicity. Front. Pharmacol. 2018, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sang, M.; Liu, E.; Banahene, P.O.; Zhang, Y.; Wang, T.; Han, L.; Gao, X. Rapid profiling and pharmacokinetic studies of major compounds in crude extract from Polygonum multiflorum by UHPLC-Q-TOF-MS and UPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 140, 45–61. [Google Scholar] [CrossRef]
- Yao, S.; Li, Y.; Kong, L. Preparative isolation and purification of chemical constituents from the root of Polygonum multiflorum by high-speed counter-current chromatography. J. Chromatogr. A 2006, 1115, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, W.-F.; Li, J.; He, J.; Zhang, P.; Zheng, F.; Zhang, B.-L.; Gao, X.; Chang, Y.-X. Influence of processing on pharmacokinetic of typical constituents in radix polygoni multiflori after oral administration by LC–ESI–MS/MS. J. Ethnopharmacol. 2013, 148, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, H.-B.; Yu, Z.-L.; Zhao, Z. Comparison of raw and processed Radix Polygoni Multiflori (Heshouwu) by high performance liquid chromatography and mass spectrometry. Chin. Med. 2010, 5, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong Kong Chinese Materia Medica Standards V. Available online: http://www.cmd.gov.hk/hkcmms/vol2/main.html (accessed on 1 July 2008).
- Cheng, W.; Li, Y.; Yang, W.; Wu, S.; Wei, M.; Gao, Y.; Kang, C.; Zhang, S.-F.; Li, Y. Simultaneous Determination of 13 Constituents of Radix Polygoni Multiflori in Rat Plasma and Its Application in a Pharmacokinetic Study. Int. J. Anal. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Lin, L.; Lin, H.; Qu, C.; Yan, L.; Ni, J. Interpretation the Hepatotoxicity Based on Pharmacokinetics Investigated Through Oral Administrated Different Extraction Parts of Polygonum multiflorum on Rats. Front. Pharmacol. 2018, 9, 505. [Google Scholar] [CrossRef]
- Lin, L.; Ni, B.; Lin, H.; Cao, S.; Yang, C.; Zhao, Y.; Xue, D.; Ni, J. Simultaneous determination and pharmacokinetic study of P-hydroxybenzaldehyde, 2,3,5,4′-tetrahydroxystilbene-2-O-β-glucoside, emodin-8-O-β-d-glucopyranoside, and emodin in rat plasma by liquid chromatography tandem mass spectrometry after oral administration of Polygonum multiflorum. Anal. Methods 2015, 7, 244–252. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, L.; He, Y.-S.; Li, H.-J. Hepatotoxic assessment of Polygoni Multiflori Radix extract and toxicokinetic study of stilbene glucoside and anthraquinones in rats. J. Ethnopharmacol. 2015, 162, 61–68. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, L.; Deng, T.; Li, C.-L.; He, Y.-S.; Li, H.-J.; Li, P. Stilbene glucoside inhibits the glucuronidation of emodin in rats through the down-regulation of UDP-glucuronosyltransferases 1A8: Application to a drug–drug interaction study in Radix Polygoni Multiflori. J. Ethnopharmacol. 2013, 147, 335–340. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Feng, Y.; Chen, D.Q.; Xi, Z.H.; Du, X.; Bai, X.; Lin, R. Pharmacokinetics of 2,3,5,4’-tetrahydroxystilbene-2-O-beta-D-glucoside in rat using ultra-performance LC-quadrupole TOF-MS. J. Sep. Sci. 2013, 36, 863–871. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Z.; Liu, X.; Gao, S.; Ye, L.; Yang, Z.; Hu, M.; Liu, Z. Sensitive and robust UPLC–MS/MS method to determine the gender-dependent pharmacokinetics in rats of emodin and its glucuronide. J. Pharm. Biomed. Anal. 2011, 54, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. Pharmacokinetics of oral administration of 2,3,5,4’-tetrahydroxystilbene-2-O-β-d-glucoside from Polygonum multiflorum in beagle dogs. Afr. J. Pharm. Pharmacol. 2012, 6. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Ren, Y.; Wang, H.; Cong, Z.; Wu, G.; Du, L.; Li, H.; Zhang, X. Pharmacokinetics and tissue distribution of emodin loaded nanoemulsion in rats. J. Drug Deliv. Sci. Technol. 2015, 30, 242–249. [Google Scholar] [CrossRef]
- Cai, J.Z.; Chen, S.H.; Zhang, Q.W.; Zhuang, R. Determination of Emodin in Rat Plasma by Gradient Elution LC-ESI-MS and its Application to Pharmacokinetics. Lat. Am. J. Pharm. 2013, 32, 269–274. [Google Scholar]
- Feng, S.X.; Zhang, L.; Hao, R.; Zhou, T.Q.; LI, X.H. Determination of physcion in plasma by SPE-HPLC and its pharmacokinetic study in rats. J. Jinan Univ. (Nat. Sci. Med. Ed.) 2017, 38, 109–113. [Google Scholar]
- Wang, C.; Zhou, Y.; Gong, X.; Zheng, L.; Li, Y. In vitro and in situ study on characterization and mechanism of the intestinal absorption of 2,3,5,4’-tetrahydroxy-stilbene-2-O-β-D-glucoside. BMC Pharmacol. Toxicol. 2020, 21, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Feng, Q.; Li, Y.; Ye, L.; Hu, M.; Liu, Z. Coupling of UDP-glucuronosyltransferases and multidrug resistance-associated proteins is responsible for the intestinal disposition and poor bioavailability of emodin. Toxicol. Appl. Pharmacol. 2012, 265, 316–324. [Google Scholar] [CrossRef]
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal Absorption and First-Pass Metabolism of Polyphenol Compounds in Rat and Their Transport Dynamics in Caco-2 Cells. PLoS ONE 2012, 7, e29647. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Wang, J.R.; Yu, Y.; Meng, X. Study of intestinal absorption of emodin in one-way intestinal perfusion rat model. Tradit. Chin. Drug Res. Clin. Pharmacol. 2012, 23, 286–290. [Google Scholar]
- Teng, Z.-H.; Zhou, S.-Y.; Ran, Y.-H.; Liu, X.-Y.; Yang, R.-T.; Yang, X.; Yuan, C.-J.; Mei, Q.-B. Cellular Absorption of Anthraquinones Emodin and Chrysophanol in Human Intestinal Caco-2 Cells. Biosci. Biotechnol. Biochem. 2007, 71, 1636–1643. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Meng, X.L.; Wang, J.R.; Liu, H.; Yang, Y.; Liu, R. Intestinal absorption kinetics of rhubarb mixture free anthraquinones in rats. Lishizhen Med. Mater. Med. Res. 2011, 22, 790–792. [Google Scholar]
- Bachmann, M.; Schlatter, C. Metabolism of [14C] emodin in the rat. Xenobiotica 1981, 11, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.X.; Chen, Q.H. Absorption, distribution and excretion of physcion in rat. J. China Pharm. Univ. 1988, 19, 179–181. [Google Scholar]
- Wang, C.Y.; Guo, D.; Yuan, Z.F.; Feng, X.; Zhang, L. Metabolism of stilbene glycoside in rats and in vitro. Chin. J. Pharm. 2009, 40, 120–123. [Google Scholar]
- Liu, W.; Tang, L.; Ye, L.; Cai, Z.; Xia, B.; Zhang, J.; Hu, M.; Liu, Z. Species and Gender Differences Affect the Metabolism of Emodin via Glucuronidation. AAPS J. 2010, 12, 424–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shia, C.-S.; Hou, Y.-C.; Tsai, S.-Y.; Huieh, P.-H.; Leu, Y.-L.; Chao, P.-D.L. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats**Chi-Sheng Shia and Yu-Chi Hou contributed equally to this work. J. Pharm. Sci. 2010, 99, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.-H.; Zhou, S.-Y.; Yang, R.-T.; Liu, X.-Y.; Liu, R.-W.; Yang, X.; Zhang, B.-L.; Yang, J.-Y.; Cao, D.-Y.; Mei, Q.-B. Quantitation assay for absorption and first-pass metabolism of emodin in isolated rat small intestine using liquid chromatography-tandem mass spectrometry. Biol. Pharm. Bull. 2007, 30, 1628–1633. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Xu, F.; Zhang, Z.; Liu, Y.; Dong, H.; Tian, Y. Structural elucidation ofin vitrometabolites of emodin by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2008, 22, 1230–1236. [Google Scholar] [CrossRef]
- Mueller, S.O.; Stopper, H.; Dekant, W. Biotansfermatioin of the anthraquinones emodin and chrysophanole by cytochrome P450 enzymes. Drug Metab. Dispos. 1998, 26, 540–546. [Google Scholar]
- Qin, X.; Peng, Y.; Zheng, J. In Vitro and in Vivo Studies of the Electrophilicity of Physcion and its Oxidative Metabolites. Chem. Res. Toxicol. 2018, 31, 340–349. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, L.; Wang, C.; Zhang, Y.; Zhang, Y.; Hu, L.; Gao, X.; Han, L.; Yang, W. Pharmacokinetic studies unveiled the drug-drug interaction between trans-2,3,5,4’-tetrahydroxystilbene-2-O-beta-d-glucopyranoside and emodin that may contribute to the idiosyncratic hepatotoxicity of Polygoni Multiflori Radix. J. Pharm. Biomed. Anal. 2019, 164, 672–680. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, L.-L.; Luo, N.; Fan, Y.-X.; Ma, J.; Li, P.; Li, H.-J. Enhanced absorption and inhibited metabolism of emodin by 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucopyranoside: Possible mechanisms for Polygoni Multiflori Radix-induced liver injury. Chin. J. Nat. Med. 2017, 15, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-R.; Liu, X.; Feng, S.; Shu, S.-N.; Wang, P.-Y.; Zhang, N.; Li, J.-S.; Qu, L. Pharmacodynamics of Five Anthraquinones (Aloe-emodin, Emodin, Rhein, Chysophanol, and Physcion) and Reciprocal Pharmacokinetic Interaction in Rats with Cerebral Ischemia. Molecules 2019, 24, 1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.; Chen, J.; Ren, J.; Li, Y.; Zhai, J.; Mu, W.; Zhang, L.; Zheng, W.; Tian, G.; Shang, H. Liver Damage Associated with Polygonum multiflorum Thunb.: A Systematic Review of Case Reports and Case Series. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Liu, S.; Wang, J.; Song, H.; Li, Y.; He, T.; Ma, X.; Wang, Z.X.; Wang, L.; Zhou, K.; et al. Clinical analysis of drug-induced liver injury caused by Polygonum multiflorum and its preparations. Chin. J. Integr. Tradit. West. Med. 2015, 35, 1442–1447. [Google Scholar]
- Liu, Y.; Wang, W.; Sun, M.; Ma, B.; Pang, L.; Du, Y.; Dong, X.; Yin, X.; Ni, J. Polygonum multiflorum-Induced Liver Injury: Clinical Characteristics, Risk Factors, Material Basis, Action Mechanism and Current Challenges. Front. Pharmacol. 2019, 10, 1467. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Teschke, R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int. J. Mol. Sci. 2015, 17, 14. [Google Scholar] [CrossRef]
- Li, D.-K.; Chen, J.; Ge, Z.-Z.; Sun, Z.-X. Hepatotoxicity in Rats Induced by Aqueous Extract of Polygoni Multiflori Radix, Root of Polygonum multiflorum Related to the Activity Inhibition of CYP1A2 or CYP2E1. Evid. Based Complement. Altern. Med. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Shimada, T.; Yamazaki, H.; Mimura, M.; Inui, Y.; Guengerich, F.P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994, 270, 414–423. [Google Scholar]
- Ma, K.; Zhang, X.; Jia, H. CYP1A2 polymorphism in Chinese patients with acute liver injury induced by Polygonum multiflorum. Genet. Mol. Res. 2014, 13, 5637–5643. [Google Scholar] [CrossRef]
- Li, C.; Rao, T.; Chen, X.; Zou, Z.; Wei, A.; Tang, J.; Xiong, P.; Li, P.; Jing, J.; He, T.; et al. HLA-B*35:01Allele Is a Potential Biomarker for PredictingPolygonum multiflorum–Induced Liver Injury in Humans. Hepatology 2019, 70, 346–357. [Google Scholar] [CrossRef]
- Malhi, H.; Gores, G.J. Cellular and Molecular Mechanisms of Liver Injury. Gastroenterology 2008, 134, 1641–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014, 5, e996. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Liu, Y.; Chen, C.; Xu, W.; Xiao, H. Emodin induces liver injury by inhibiting the key enzymes of FADH/NADPH transport in rat liver. Toxicol. Res. 2018, 7, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Liu, Y.; Fu, S.; Qu, C.; Li, H.; Ni, J. Inhibition of Mitochondrial Complex Function—The Hepatotoxicity Mechanism of Emodin Based on Quantitative Proteomic Analyses. Cells 2019, 8, 263. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Ni, B.; Fu, J.; Yin, X.; You, L.; Leng, X.; Liang, X.; Ni, J. Emodin induces apoptosis in human hepatocellular carcinoma HepaRG cells via the mitochondrial caspase-dependent pathway. Oncol. Rep. 2018, 40, 1985–1993. [Google Scholar] [CrossRef]
- Schadt, H.S.; Wolf, A.; Pognan, F.; Chibout, S.-D.; Merz, M.; Kullak-Ublick, G.A. Bile acids in drug induced liver injury: Key players and surrogate markers. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.-L.; Zhao, N.-S.; Fan, Y.-X.; Yu, Q.; Lai, Y.-S.; Li, P.; Li, H.-J. Transcriptome analysis to assess the cholestatic hepatotoxicity induced by Polygoni Multiflori Radix: Up-regulation of key enzymes of cholesterol and bile acid biosynthesis. J. Proteom. 2018, 177, 40–47. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.-Y.; Zhou, Z.-X.; Jiang, Z.-Z.; Li, Y.-Y.; Zhang, L.; Zhang, L.-Y. Study on hepatotoxicity of aqueous extracts of Polygonum multiflorum in rats after 28-day oral administration: Cholestasis-related mechanism. China J. Chin. Mater. Medica 2015, 40, 2163–2167. [Google Scholar]
- Zhao, D.S.; Jiang, L.; Fan, Y.; Dong, L.; Ma, J.; Dong, X.; Xu, X.; Li, P.; Li, H. Identification of urine tauro-beta-muricholic acid as a promising biomarker in Polygoni Multiflori Radix-induced hepatotoxicity by targeted metabolomics of bile acids. Food Chem. Toxicol. 2017, 108, 532–542. [Google Scholar] [CrossRef]
- Dong, Q.; Li, N.; Li, Q.; Zhang, C.-E.; Feng, W.-W.; Li, G.-Q.; Li, R.-Y.; Tu, C.; Han, X.; Bai, Z.-F.; et al. Screening for biomarkers of liver injury induced by Polygonum multiflorum: A targeted metabolomic study. Front. Pharmacol. 2015, 6, 217. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Chen, J.; Fu, L.; Han, L.; Gao, X.; Sarhene, M.; Hu, L.; Zhang, Y.; Fan, G. Polygonum multiflorum Thunb suppress bile acid synthesis by activating Fxr-Fgf15 signaling in the intestine. J. Ethnopharmacol. 2019, 235, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Si, L.; Rao, J.; Li, D.; Wu, Y.; Wu, S.; Wu, M.; He, S.; Zhu, W.; Wu, Y.; et al. Polygoni Multiflori Radix derived anthraquinones alter bile acid disposition in sandwich-cultured rat hepatocytes. Toxicol. In Vitro 2017, 40, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Etu, C.; Egao, D.; Eli, X.-F.; Eli, C.-Y.; Eli, R.-S.; Ezhao, Y.-L.; Eli, N.; Ejia, G.-L.-C.; Epang, J.-Y.; Ecui, H.-R.; et al. Inflammatory stress potentiates emodin-induced liver injury in rats. Front. Pharmacol. 2015, 6, 233. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-H.; Yang, X.-W.; Dai, Y.-H.; Xiao, H.-B. Effects of emodin on lipid accumulation and inflammation in hepatocytes. China J. Chin. Mater. Med. 2019, 44, 2820–2826. [Google Scholar]
- Li, H.; Wang, X.; Liu, Y.; Pan, D.; Wang, Y.; Yang, N.; Xiang, L.; Cai, X.; Feng, Y. Hepatoprotection and hepatotoxicity of Heshouwu, a Chinese medicinal herb: Context of the paradoxical effect. Food Chem. Toxicol. 2017, 108, 407–418. [Google Scholar] [CrossRef]
- Dang, S.; Zhang, X.; Jia, X.-L.; Cheng, Y.-A.; Song, P.; Liu, E.-Q.; He, Q.; Li, Z.-F. Protective effects of emodin and astragalus polysaccharides on chronic hepatic injury in rats. Chin. Med. J. 2008, 121, 1010–1014. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Liu, H.; Zhan, Z.; Liang, Z.; Zhang, T.; Cai, Z.; Ye, L.; Liu, M.; Zhao, J.; et al. Emodin-induced hepatotoxicity was exacerbated by probenecid through inhibiting UGTs and MRP2. Toxicol. Appl. Pharmacol. 2018, 359, 91–101. [Google Scholar] [CrossRef]
- Di, X.; Wang, X.; Liu, Y. Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J. Pharm. Biomed. Anal. 2015, 115, 144–149. [Google Scholar] [CrossRef]
- Gao, D.; Pang, J.-Y.; Zhang, C.-E.; Li, C.-Y.; Tu, C.; Zhang, H.-Z.; Niu, M.; Xiong, Y.; Xiao, X.-H.; Zhao, K.-J.; et al. Poria Attenuates Idiosyncratic Liver Injury Induced by Polygoni Multiflori Radix Praeparata. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.E. Toxicokinetic modeling and its applications in chemical risk assessment. Toxicol. Lett. 2003, 138, 9–27. [Google Scholar] [CrossRef]

| Dose of RPM Extract (Equivalent Dose of Raw/Processed RPM) and Its Major Components | Pharmacokinetics Parameters | TSG | EMG | Emodin | Aloe emodin | Physcion | Oxyresveratrol | Rhein | Ref. |
|---|---|---|---|---|---|---|---|---|---|
1.7 g/kg (10 g/kg of raw RPM)
| Tmax (h) | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.2 | 0.2 ± 0.0 | 0.2 ± 0.1 | ND | ND | [25] |
| Cmax (ng/mL) | 74.4 ± 18.5 | 65.1 ± 58.3 | 152.4 ± 36.6 | 29.6 ± 7.4 | 11.6 ± 5.5 | ND | ND | ||
| AUC0→∞ (ng h/mL) | 195.8 ± 151.2 | 95.3 ± 94.9 | 233.5 ± 120.5 | 137.9 ± 105.3 | 35.2 ± 21.9 | ND | ND | ||
| T1/2 (h) | NA | NA | NA | NA | NA | NA | NA | ||
3.4 g/kg (20 g/kg of raw RPM)
| Tmax (h) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.2 | ND | ND | |
| Cmax (ng/mL) | 189.2 ± 46.7 | 115.1 ± 38.9 | 224.5 ± 131.1 | 40.7 ± 23.0 | 30.8 ± 11.0 | ND | ND | ||
| AUC0→∞ (ng h/mL) | 350.4 ± 321.6 | 371.1 ± 340.6 | 683.0 ± 268.9 | 281.7 ± 203.5 | 203.3 ± 130.4 | ND | ND | ||
| T1/2 (h) | NA | NA | NA | NA | NA | NA | NA | ||
6.8 g/kg (40 g/kg of raw RPM)
| Tmax (h) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.4 | 0.2 ± 0.0 | 0.5 ± 0.2 | |
| Cmax (ng/mL) | 784.5 ± 543.9 | 160.3 ± 44.3 | 348.1 ± 131.5 | 106.2 ± 33.4 | 95.8 ± 51.6 | 0.6 ± 0.8 | 0.4 ± 0.2 | ||
| AUC0→∞ (ng h/mL) | 2019.0 ± 431.9 | 492.1 ± 143.4 | 1042.2 ± 589.0 | 485.7 ± 151.0 | 438.1 ± 163.0 | 7.7 ± 13.1 | 3.1 ± 4.1 | ||
| T1/2 (h) | NA | NA | NA | NA | ND | NA | NA | ||
NA (36 g/kg of raw RPM)
| Tmax (h) | 0.3 ± 0.1 | 0.3± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | ND | NA | 0.5 ± 0.1 | [30] |
| Cmax (ng/mL) | 1743.0 ± 401.0 | 101.0 ± 47.4 | 175.0 ± 33.8 | 11.3 ± 3.1 | ND | NA | 1.1 ± 0.2 | ||
| AUC0→∞ (ng h/mL) | 1871.0 ± 581.0 | 83.7 ± 32.3 | 801.0 ± 233.0 | 8.5 ± 3.4 | ND | NA | 2.3 ± 0.5 | ||
| T1/2 (h) | 6.0 ± 2.62 | 3.9 ± 2.5 | 8.4 ± 4.2 | 3.4 ± 1.4 | ND | NA | 1.2 ± 0.4 | ||
3.3 g/kg (19.19 g/kg of raw RPM)
| Tmax (h) | 0.5 ± 0.2 | NA | 0.2 ± 0.0 | NA | NA | NA | NA | [27] |
| Cmax (ng/mL) | 884.0 ± 146.0 | NA | 89.9 ± 13.6 | ||||||
| AUC0→∞ (ng h/mL) | 3292.0 ± 707.0 | NA | 1842.0 ± 425.0 | ||||||
| T1/2 (h) | 1.1 ± 0.5 | NA | 2.8 ± 1.6 | ||||||
1.7 g/kg (18.00 g/kg of processed RPM)
| Tmax (h) | 0.4 ± 0.1 | NA | 0.2 ± 0.0 | |||||
| Cmax (ng/mL) | 491.6 ± 179.7 | NA | 61.3 ± 9.2 | ||||||
| AUC0→∞ (ng h/mL) | 1137.0 ± 401.6 | NA | 879.9 ± 195.0 | ||||||
| T1/2 (h) | 0.3 ± 0.0 | NA | 1.7 ± 0.6 | ||||||
NA (6 g/kg of raw RPM)
| Tmax (h) | 0.2 ± 0.1 | 0.3 ± 0.1 | 1.6 ± 3.1 | [31] | ||||
| Cmax (ng/mL) | 69.6 ± 51.8 | 21.5 ± 25.6 | 86.7 ± 19.7 | ||||||
| AUC0→∞ (ng h/mL) | 90.2 ± 35.8 | 14.3 ± 15.9 | 506.3 ± 61.6 | ||||||
| T1/2 (h) | 2.2 ± 1.6 | 0.2 ± 0.1 | 5.0 ± 1.7 | ||||||
NA (10 g/kg of raw RPM)
| Tmax (h) | 0.7 ± 0.1 | 1.0 ± 0.6 | 0.5 ± 0.3 | [32] | ||||
| Cmax (ng/mL) | 240.2 ± 114.0 | 204.4 ± 85.9 | 76.7 ± 13.2 | ||||||
| AUC0→∞ (ng h/mL) | 373.6 ± 142.7 | 489.7 ± 129.7 | 395.2 ± 208.3 | ||||||
| T1/2 (h) | 1.6 ± 0.6 | 1.7 ± 0.5 | 6.5 ± 1.4 |
| Compounds | Species | Dose, Route of Administrations | Cmax (μg/mL) | Tmax (h) | AUC0→t (μg h/mL) | AUC0→∞ (μg h/mL) | T1/2α (h) | T1/2β (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| TSG | Beagle dogs | 0.52 g/kg, p.o. | 0.83 ± 0.04 | 1.00 ± 0.00 | 1.53 ± 0.07 | 2.04 ± 0.02 | 0.20 ± 0.02 | 0.56 ± 0.05 | [37] |
| 0.78 g/kg, p.o. | 1.16 ± 0.06 | 1.00 ± 0.00 | 2.30 ± 0.06 | 3.00 ± 0.19 | 0.10 ± 0.02 | 0.60 ± 0.03 | |||
| 1.04 g/kg, p.o. | 2.17 ± 0.23 | 1.00 ± 0.00 | 3.60 ± 0.02 | 4.59 ± 0.35 | 0.14 ± 0.02 | 0.64 ± 0.15 | |||
| SD rats | 10 mg/kg, i.v. | 22.80 ± 2.60 | - | 5.10 ± 0.33 | 5.84 ± 0.19 | NA | NA | [35] | |
| 20 mg/kg, i.v. | 64.20 ± 3.60 | - | 11.01 ± 0.58 | 12.23 ± 0.98 | NA | NA | |||
| 50 mg/kg, p.o. | 5.70 ± 1.60 | 0.25 ± 0.02 | 5.99 ± 0.59 | 7.09 ± 1.87 | NA | NA | |||
| 100 mg/kg, p.o. | 21.90 ± 2.50 | 0.24 ± 0.02 | 20.70 ± 0.64 | 21.29 ± 0.63 | NA | NA | |||
| Emodin | SD rats | 4 mg/kg, i.v. | 5.83 ± 2.34 | - | 7.18 ± 1.84 | NA | NA | 1.38 ± 0.59 | [36] |
| 8 mg/kg, p.o. | 0.21 ± 0.09 | 0.30 ± 0.11 | 1.33 ± 0.53 | NA | NA | 6.42 ± 1.72 | |||
| 10 mg/kg, p.o. | 0.08 ± 0.02 | 0.75 ± 0.00 | 0.39 ± 0.04 | 0.42 ± 0.05 | NA | 2.98 ± 0.71 | [38] | ||
| 20 mg/kg, p.o. | 6.04 ± 1.14 | NA | 13.18 ± 2.99 | 13.28 ± 3.00 | NA | 1.22 ± 0.29 | [39] | ||
| 82.4 mg/kg, p.o. | 0.10 ± 0.01 | NA | 1.26 ± 0.08 | 1.30 ± 0.02 | 4.56 ± 0.76 | NA | [34] | ||
| Physcion | SD rats | 26.4 mg/kg, p.o. | 0.29 ± 0.12 | 1.00 ± 0.76 | 45.84 ± 36.00 | NA | NA | 13.25 ± 5.60 | [40] |
| 52.8 mg/kg, p.o | 0.41 ± 0.15 | 1.00 ± 0.42 | 47.52 ± 33.60 | NA | NA | 14.23 ± 11.00 | |||
| 105.6 mg/kg, p.o | 0.49 ± 0.17 | 0.75 ± 0.56 | 78.70 ± 31.20 | NA | NA | 10.97 ± 6.60 |
| Mechanisms | Model | Substance | Dose/Duration | Findings |
|---|---|---|---|---|
| Metabolic enzymes alteration and genetic polymorphism | SD rats [31] | RPM extract | 6 g raw RPM/kg/bolus | Protein expression: CYP3A4, CYP2C19, CYP2E1, UGT1A1 and UGT1A8 ↓; ALT and AST ↑. |
| SD rats [63] | RPM aqueous extract | 40 g raw RPM/kg/3 weeks | CYP1A2 or CYP2E1 inhibitors + RPM: ALT and AST↑; moderate liver injury. | |
| Human (43 cases) [65] | RPM | NR | CYP1A2*1C frequency: 46.5%: RPM induced liver injury patients; 27.9%: healthy controls. | |
| Human (87 cases) [66] | RPM | 4 weeks | HLA-B*35:01 allele: 45.4%: RPM induced liver injury patients; 2.7%: Han Chinese population. | |
| Hepatocytes apoptosis | SD rats [69] | Emodin | 1500 mg/kg/7 days | Emodin: ↑caspase-9, caspase-3, and Cyt c → mitochondrial apoptosis and liver injury |
| L02 cells [70] | Emodin | 50 μM | Emodin: ↑caspase-3 and ROS, ↓mitochondrial membrane potential, disrupting ATP synthesis → mitochondrial damage and hepatocyte apoptosis. | |
| HepaRG cells [71] | Emodin | 20–80 μM | Emodin: cell cycle arrest and ROS generation → mitochondrial apoptosis → cell apoptosis. | |
| Bile acids homeostasis disruption | SD rats [73] | Extracts of raw RPM (75% EtOH) | 1 and 20g extract/kg/90 days | Protein expression of 3-hydroxy-3-methylglutaryl CoA reductase and CYP7A1 ↑ in a dose-dependent manner. |
| SD rats [74] | RPM concentrated powder (1:10) | 30 and 60g extract/kg/28 days | mRNA and protein expression of MRP2/Mrp2, MRP3/Mrp3, BSEP/Bsep, FXR/Fxr, CYP7A1/Cyp7a1 ↑. | |
| SD rats [75] | Extracts of raw RPM (75% EtOH) | 1 and 20 g extract/kg/ 3, 6, 7 weeks |
| |
| SD rats [76] | Extracts of raw and processed RPM (75% EtOH) | 50 g extract/kg/42 days | GDCA in bile, as well as HDCA in serum, could be selected as potential biomarkers for RPM induced liver injury. | |
| C57BL/6J mice [77] | Extracts of processed RPM (60% EtOH) | 1.275 and 3.825g extract/kg/7 days |
| |
| Sandwich cultured rat hepatocytes [78] | Emodin, Physcion, Chrysophanol | 1–50 μM | All compounds could alter bile acids disposition through direct ↓BA transporters as well as regulated expression of bile acids transporters and metabolic enzymes. | |
| Inflammatory damage | SD rats [79] | Emodin | 20, 40, 80 mg/kg | Emodin + lipopolysaccharide: ↑ proinflammatory cytokines (TNF-α, IL-1β and IL-6) → ALT and AST ↑. |
| L02 cells [80] | Emodin | 10.93, 54.09, 267.7 μM | Emodin: ↑ p-NF-κB and IL-6 → inflammatory damage. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yang, M.; Zuo, Z. Overview of Pharmacokinetics and Liver Toxicities of Radix Polygoni Multiflori. Toxins 2020, 12, 729. https://doi.org/10.3390/toxins12110729
Li D, Yang M, Zuo Z. Overview of Pharmacokinetics and Liver Toxicities of Radix Polygoni Multiflori. Toxins. 2020; 12(11):729. https://doi.org/10.3390/toxins12110729
Chicago/Turabian StyleLi, Dan, Mengbi Yang, and Zhong Zuo. 2020. "Overview of Pharmacokinetics and Liver Toxicities of Radix Polygoni Multiflori" Toxins 12, no. 11: 729. https://doi.org/10.3390/toxins12110729
APA StyleLi, D., Yang, M., & Zuo, Z. (2020). Overview of Pharmacokinetics and Liver Toxicities of Radix Polygoni Multiflori. Toxins, 12(11), 729. https://doi.org/10.3390/toxins12110729





