Toxicity and Sublethal Effects of Autumn Crocus (Colchicum autumnale) Bulb Powder on Red Imported Fire Ants (Solenopsis invicta)
Abstract
1. Introduction
2. Results
2.1. Colchicine Content in Autumn Crocus Bulb
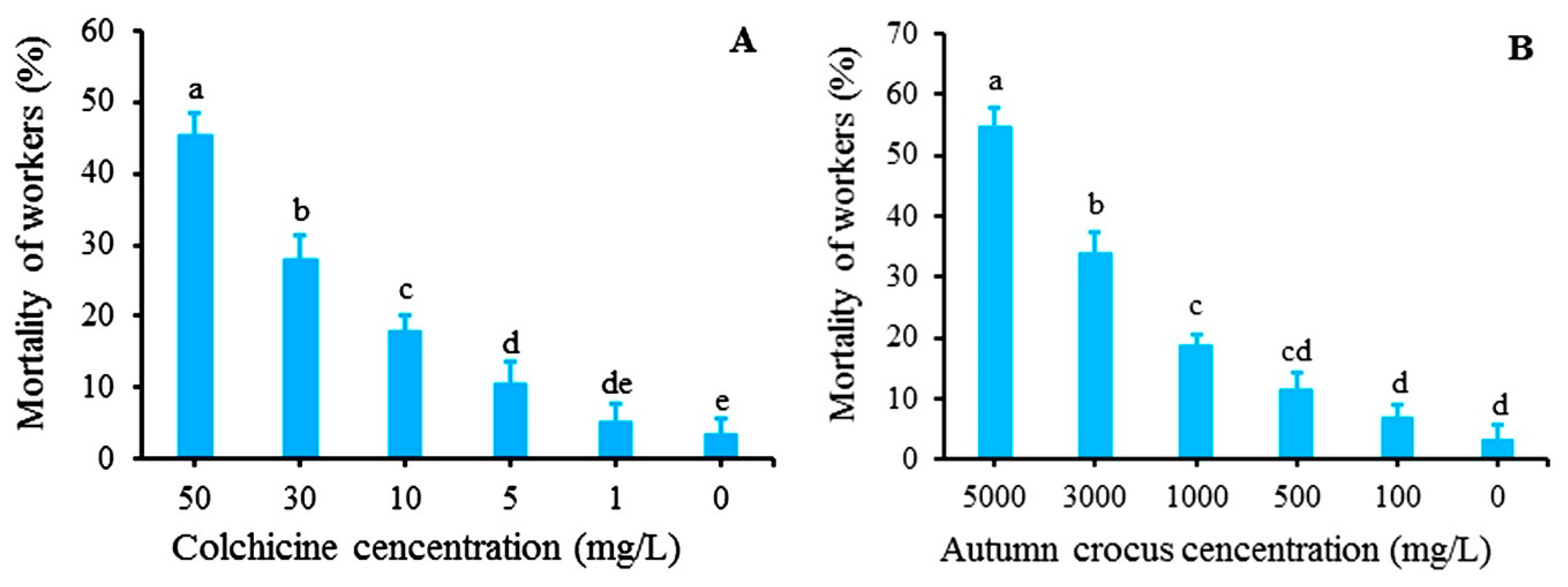
2.2. Toxicity of Colchicine and Autumn Crocus Bulb Powder to S. invicta
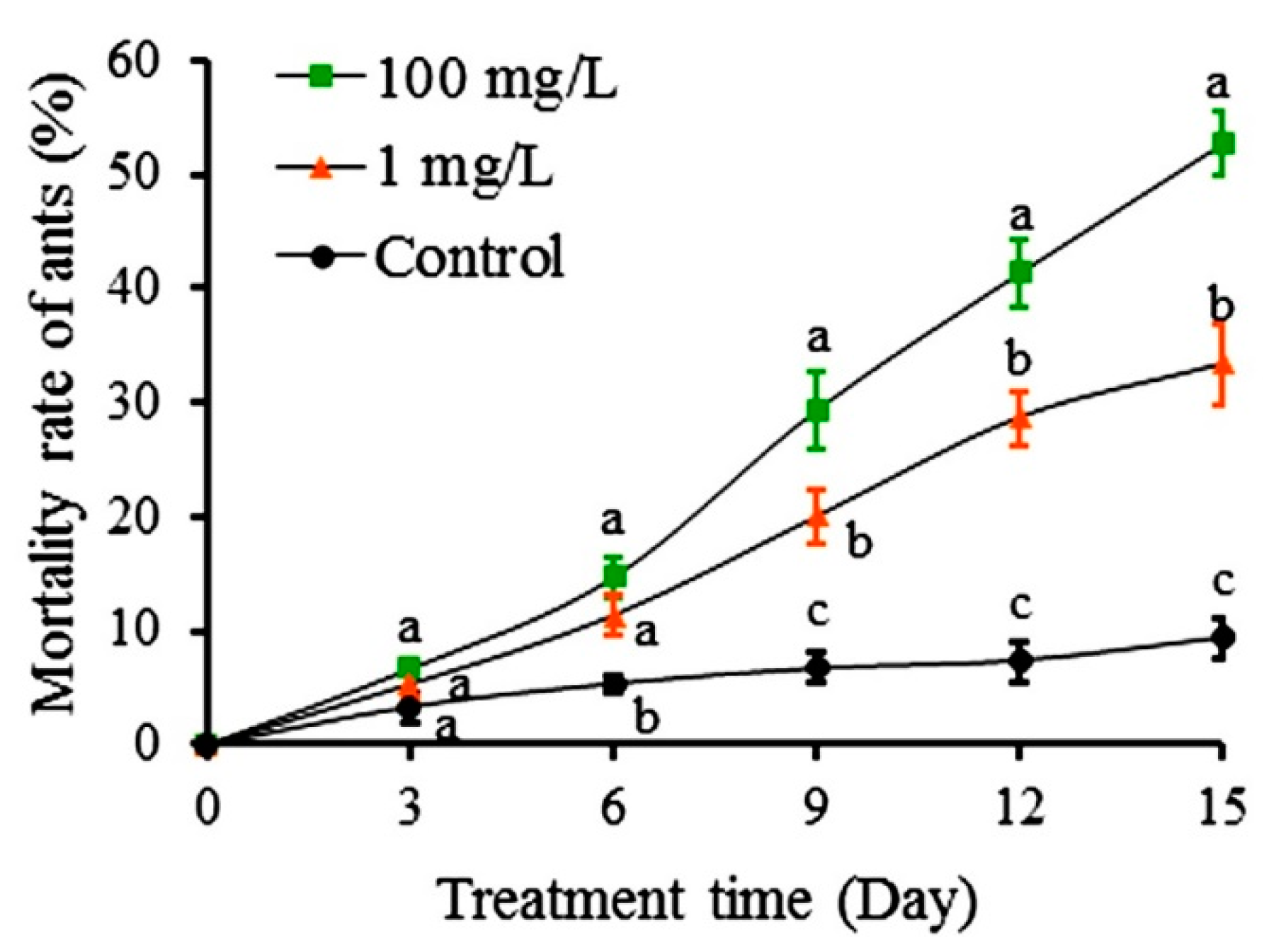
2.3. Effect of Colchicine and Autumn Crocus on Colony Growth of S. invicta
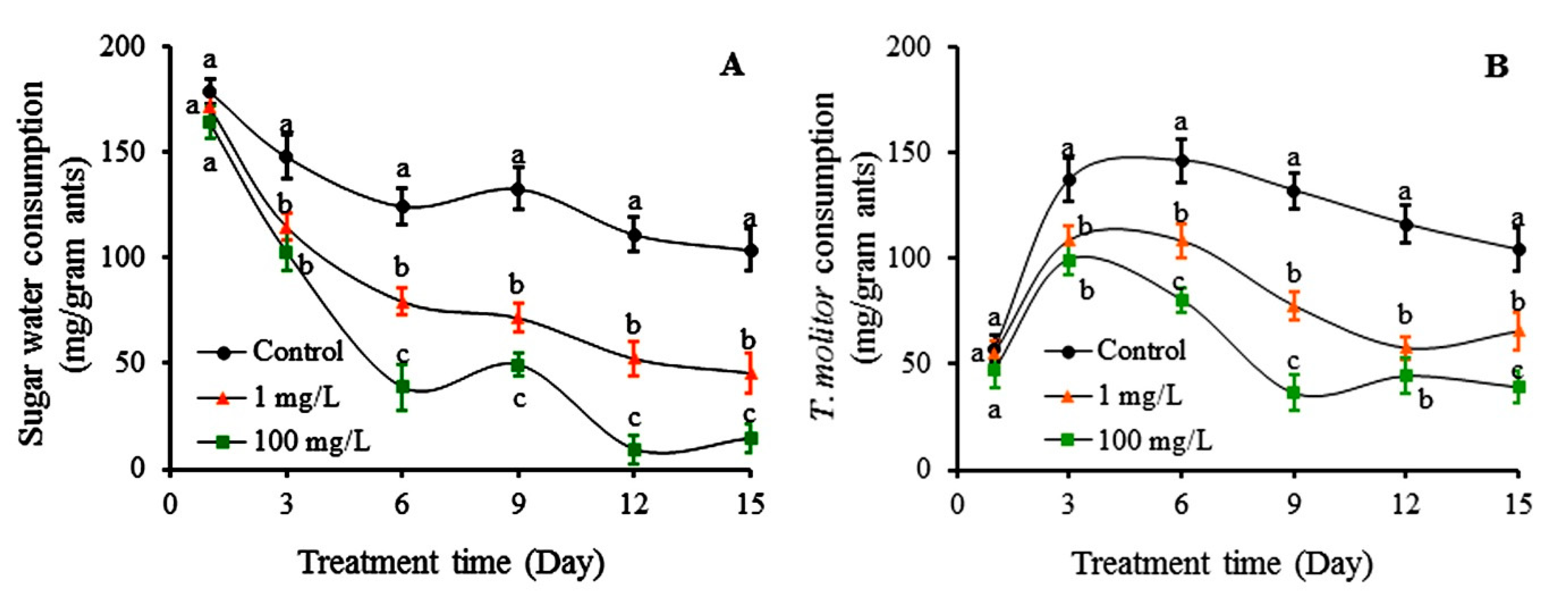
2.4. Effects of Colchicine and Autumn Crocus Bulb Powder on Food Consumption of S. invicta
2.5. Effects of Colchicine and Autumn Crocus on the Aggressiveness of S. invicta
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Insects, Chemicals, and Plant
5.2. HPLC Analysis of Colchicine in Autumn Crocus Powder
5.3. Toxicity of Colchicine and Autumn Crocus Bulb
5.4. Sublethal Effects of Colchicine and Autumn Crocus Bulb Powder on S. invicta Colony Growth
5.5. Effects of Colchicine and Autumn Crocus Bulb Powder on Food Consumption of S. invicta
5.6. Effects of Colchicine and Autumn Crocus Bulb Powder on the Aggressiveness of S. invicta
5.7. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holway, D.A.; Lach, L.; Suarez, A.; Tsutsui, N.; Case, T.J. The causes and consequences of ant invasions. Ann. Rev. Ecol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Tsutsui, N.D.; Suarez, A.V. The colony structure and population biology of invasive ants. Conserv. Biol. 2003, 17, 48–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, J.; Huang, C.; Cheng, D.; Huang, R.; Zhang, Z. Insecticidal Activity of the Soil in the Rhizosphere of Viburnum odoratissimum against Solenopsis invicta (Hymenoptera: Formicidae). Sociobiology 2017, 64, 1–6. [Google Scholar] [CrossRef]
- Tschinkel, W.R. The Fire Ants; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 2006; p. 723. [Google Scholar]
- Buren, W.F.; Allen, G.E.; Whitcomb, W.H.; Lennartz, F.E.; Williams, R.N. Zoogeography of the imported fire ants. J. N. Y. Entomol. Soc. 1974, 82, 113–124. [Google Scholar]
- McCubbin, K.I.; Weiner, J.M. Fire ants in Australia: A new medical and ecological hazard. Med. J. Aust. 2002, 176, 518–519. [Google Scholar] [CrossRef]
- Moloney, S.; Vanderwoude, C. Red imported fire ants: A threat to eastern Australia’s wildlife. Ecol. Manag. Restor. 2002, 3, 167–175. [Google Scholar] [CrossRef]
- Christian, S. Red imported fire ants eradicated from Napier. Bios 2009, 92, 28–29. [Google Scholar]
- Na, J.P.; Lee, C.Y. Identification key to common urban pest ants in Malaysia. Trop. Biomed. 2001, 18, 1–17. [Google Scholar]
- Chen, J.S.C.; Shen, C.H.; Lee, H.J. Monnogynous and polygynous red imported fire ants, Solenopsis invicta Buren (Hymennoptera: Formicidae), in Taiwan. Environ. Entomol. 2006, 35, 167–172. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Liu, N.; Porter, S.D. An overview of the red imported fire ant (Hymenoptera: Formicidae) in mainland China. Fla. Entomol. 2007, 90, 723–731. [Google Scholar] [CrossRef]
- Lai, L.C.; Hua, K.H.; Wu, W.J. Intraspecific and interspecific aggressive interactions between two species of fire ants, Solenopsis geminata and S. invicta (Hymenoptera: Formicidae) in Taiwan. J. Asia-Pacific Entomol. 2015, 18, 93–98. [Google Scholar] [CrossRef]
- Vinson, S.B. Invasion of the red imported fire ant: Spread, biology, and impact. Am. Ent. 1997, 43, 23–39. [Google Scholar] [CrossRef]
- Wang, K.; Tang, L.; Zhang, N.; Zhou, Y.; Li, W.S.; Li, H.; Cheng, D.M.; Zhang, Z.X. Repellent and fumigant activity of Eucalyptus globulus and Artemisia carvifolia essential oils against Solenopsis invicta. Bull. Insectol. 2014, 67, 207–211. [Google Scholar]
- Dar, M.A.; Kaushik, G.; Villarreal-Chiu, J.F. Pollution status and bioremediation of chlorpyrifos in environmental matrices by the application of bacterial communities: A review. J. Environ. Manag. 2019, 239, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Appel, A.G.; Gehret, M.J.; Tanley, M.J. Repellency and toxicity of mint oil mint oil granules to red imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2004, 97, 575–580. [Google Scholar] [CrossRef]
- Vogt, J.T.; Shelton, T.G.; Merchant, M.E.; Russell, S.A.; Tanley, M.J. Efficacy of three citrus oil formulations against Solenopsis invicta Buren (Hymenopter: Formicidae), the red imported fire ant. J. Agric. Urban. Entomol. 2002, 19, 159–171. [Google Scholar]
- Prabha, S. Biopesticides—An alternative and eco-friendly source for the control of pests in agricultural crops. Plant. Arch. 2016, 16, 902–906. [Google Scholar]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Kohl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trend. Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Qin, D.Q.; Huang, R.L.; Li, Z.H.; Wang, S.Y.; Cheng, D.M.; Zhang, Z.X. Volatile component analysis of Michelia alba leaves and their effect on fumigation activity and worker behavior of Solenopsis invicta. Sociobiology 2018, 65, 170–176. [Google Scholar] [CrossRef]
- Abbey, L.; Abbey, J.; Leke-Aladekoba, A.; Iheshiulo, E.M.; Ijenyo, M. Biopesticides and Biofertilizers: Types, Production, Benefits, and Utilization. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; Simpson, B.K., Aryee, A.N.A., Toldrá, F., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2020; pp. 479–500. [Google Scholar]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest. Manag. Sci. 2019, 75, 2325–2340. [Google Scholar]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as promising alternatives to chemical pesticides: A review of the current and future status. Online J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Benschop, M.; Kamenetsky, R.; Le Nard, M.; Okubo, H.; De Hertogh, A. The global flower bulb industry: Production, utilization, and research. Hortic. Rev. 2010, 36, 1–115. [Google Scholar]
- Kupper, J.; Rentsch, K.; Mittelholzer, A.; Artho, R.; Meyer, S.; Kupferschmidt, H.; Naegeli, H. A fatal case of autumn crocus (Colchicum autumnale) poisoning in a heifer: Confirmation by mass-spectrometric colchicine detection. J. Vet. Diagn. Invest. 2010, 22, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D. Saffron (Crocus sativus L.): Phytochemistry, therapeutic significance and omics-based biology. In Medicinal and Aromatic Plants: Expanding Their Horizon through Omics; Aftab, T., Hakeem, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–167. [Google Scholar]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum 2015, 45, 341–350. [Google Scholar] [CrossRef]
- Shekelle, P.G.; Newberry, S.J.; FitzGerald, J.D.; Motala, A.; O’Hanlon, C.E.; Tariq, A.; Okunogbe, A.; Han, D.; Shanman, R. Management of Gout: A Systematic Review in Support of an American College of Physicians Clinical Practice Guideline. Ann. Intern. Med. 2017, 166, 37–51. [Google Scholar] [CrossRef]
- Henny, R.J.; Holm, J.R.; Chen, J.; Scheiber, M. In vitro induction of tetraploids in Dieffenbachia x ‘Star Bright M-1′ by colchicine. HortScience 2009, 44, 646–650. [Google Scholar] [CrossRef]
- Nebel, R. Mechanism of polyploidy through colchicine. Nature 1937, 140, 1101. [Google Scholar] [CrossRef]
- Ascunce, M.S.; Yang, C.C.; Oakey, J.; Calcaterra, L.; Wu, W.J.; Shih, C.J.; Goudet, J.; Ross, K.G.; Shoemaker, D. Global invasion history of the fire ant Solenopsis invicta. Science 2011, 331, 1066–1068. [Google Scholar] [CrossRef]
- Qin, D.; Zhang, P.W.; Zhou, Y.; Zheng, Q.; Hou, R.Q.; Liu, B.J.; Chen, J.; Zhang, Z.X. Different lethal treatments induce changes in piperidine (1,1′-(1,2-ethanediyl)bis-) in the epidermal compounds of red imported fire ants and affect corpse-removal behavior. Ecotoxicol. Environ. Saf. 2020, 194. [Google Scholar] [CrossRef]
- Lard, C.F. An Economic Impact of Imported Fire Ants in the United States of America; Texas A&M University: College Station, TX, USA, 2006; Available online: http://fireantecon.tamu.edu (accessed on 10 August 2020).
- Zeng, L.; Lu, Y.; He, X.; Zhang, W.; Liang, G. Identification of red imported fire ant Solenopsis invicta to invade mainland China and infestation in Wuchuan. Guangdong Chin. Bull. Entomol. 2005, 42, 144–148. [Google Scholar]
- Lei, W.; Xu, Y.; Ling, Z.; Lu, Y. Impact of the red imported fire ant Solenopsis invicta Buren on biodiversity in South China: A review. J. Integr. Agric. 2019, 18, 788–796. [Google Scholar]
- Lin, F.R.; Cheng, D.F.; Qiao, H.B.; Chen, L. Potential threat caused by red imported fire ants to some species in China. Chin. Bul. Entomol. 2006, 43, 608–611. [Google Scholar]
- Wang, L.; Lu, Y.; Xu, Y.; Zeng, L. The current status of research on Solenopsis invicta Buren (Hymenoptera: Formicidae) in mainland China. Asian Myrmecol. 2012, 5, 125–138. [Google Scholar]
- Kafle, L.; Shih, C.J. Insecticidal activities of compounds from sweet flag (Acorus calamus) against red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae). Sociobiology 2017, 64, 398–403. [Google Scholar] [CrossRef]
- Cheng, S.S.; Liua, J.Y.; Lina, C.Y.; Hsui, Y.R.; Lu, M.C.; Wu, W.J.; Chang, S.T. Terminating red imported fire ants using Cinnamomum osmophloeum leaf essential oil. Biores. Technol. 2008, 99, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Frankova, L.; Cibirova, K.; Boka, K.; Gasparikova, O.; Psenak, M. The role of the roots in the life strategy of Colchicum autumnale. Biologia Bratisl. 2004, 59, 87–93. [Google Scholar]
- Jung, L.S.; Winter, S.; Kriechbaum, M.; Eckstein, R.L.; Donath, T.W.; Otte, A. Colchicum autumnale L. Perspect. Plant. Ecol. Evol. Syst. 2011, 13, 227–244. [Google Scholar] [CrossRef]
- Porter, S.D.; Savignano, D.A. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 1990, 71, 2095–2106. [Google Scholar] [CrossRef]
- Wu, B.; Wang, L.; Liang, G.; Lu, Y.; Zeng, L. Food competition mechanism between Solenopsis invicta Buren and Tapinoma Melanocephalum Fabricys. Sociobiology 2014, 61, 265–273. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.X.; Zhou, Y.; Song, X.N.; Xu, H.H.; Cheng, D.M. Insecticidal activity of the whole grass extract of Typha angustifolia and its active component against Solenopsis invicta. Sociobiology 2013, 60, 362–366. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant. Protect. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, D.; Chen, J.; Zhang, Z. Plants in the genus Tephrosia: Valuable resources for botanical insecticides. Insects 2020, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Issakul, K.; Araya, J.; Pawelzik, E.; Jatisatienr, C. Potential of Mammea siamensis as a botanical insecticide: Its efficiency on diamondback moth and side effects on non-target organisms. J. Med. Plant. Res. 2011, 5, 2149–2156. [Google Scholar]
- Asogwa, E.U.; Ndubuaku, T.C.N.; Ugwu, J.A.; Awe, O.O. Prospects of botanical pesticides from neem, Azadirachta indica for routine protection of cocoa farms against the brown cocoa mirid—Sahlbergella singularis in Nigeria. J. Med. Plants Res. 2010, 4, 1–6. [Google Scholar]
- George, D.R.; Sparagano, O.A.E.; Port, G.; Okello, E.; Shiel, R.S.; Guy, J.H. Toxicity of plant essential oils to different life stages of the poultry red mite, Dermanyssus gallinae, and non-target invertebrates. Med. Vet. Entomol. 2010, 24, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Abidin, L.; Khurana, D.; Mujeeb, M. Effect of process parameters on the extraction of colchicine from Colchicum autumnale L. seeds. BMR Biotechnol. 2014, 1, 1–5. [Google Scholar]
- Zhang, Z.X.; Zhou, Y.; Cheng, D.M. Effects of Hematoporphyrin Monomethyl Ether on worker behavior of red imported fire ant Solenopsis invicta. Sociobiology 2013, 60, 169–173. [Google Scholar] [CrossRef][Green Version]
- Pan, F.X.; Lu, Y.; Wang, L. Toxicity and sublethal effects of sulfoxaflor on the red imported fire ant, Solenopsis invicta. Ecotoxicol. Environ. Saf. 2017, 139, 377–383. [Google Scholar] [CrossRef]
- Tang, L.; Sun, Y.Y.; Zhang, Q.P.; Zhou, Y.; Zhang, N.; Zhang, Z.X. Fumigant activity of eight plant essential oils against workers of red imported fire ant, Solenopsis invicta. Sociobiology 2013, 60, 35–40. [Google Scholar] [CrossRef][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Qin, D.; Zhang, Y.; Zheng, Q.; Yang, L.; Cheng, D.; Huang, S.; Chen, J.; Zhang, Z. Toxicity and Sublethal Effects of Autumn Crocus (Colchicum autumnale) Bulb Powder on Red Imported Fire Ants (Solenopsis invicta). Toxins 2020, 12, 731. https://doi.org/10.3390/toxins12110731
Lin S, Qin D, Zhang Y, Zheng Q, Yang L, Cheng D, Huang S, Chen J, Zhang Z. Toxicity and Sublethal Effects of Autumn Crocus (Colchicum autumnale) Bulb Powder on Red Imported Fire Ants (Solenopsis invicta). Toxins. 2020; 12(11):731. https://doi.org/10.3390/toxins12110731
Chicago/Turabian StyleLin, Sukun, Deqiang Qin, Yue Zhang, Qun Zheng, Liupeng Yang, Dongmei Cheng, Suqing Huang, Jianjun Chen, and Zhixiang Zhang. 2020. "Toxicity and Sublethal Effects of Autumn Crocus (Colchicum autumnale) Bulb Powder on Red Imported Fire Ants (Solenopsis invicta)" Toxins 12, no. 11: 731. https://doi.org/10.3390/toxins12110731
APA StyleLin, S., Qin, D., Zhang, Y., Zheng, Q., Yang, L., Cheng, D., Huang, S., Chen, J., & Zhang, Z. (2020). Toxicity and Sublethal Effects of Autumn Crocus (Colchicum autumnale) Bulb Powder on Red Imported Fire Ants (Solenopsis invicta). Toxins, 12(11), 731. https://doi.org/10.3390/toxins12110731






