Presence of Mycotoxins in Milk Thistle (Silybum marianum) Food Supplements: A Review
Abstract
:1. Introduction
2. Botanical Description
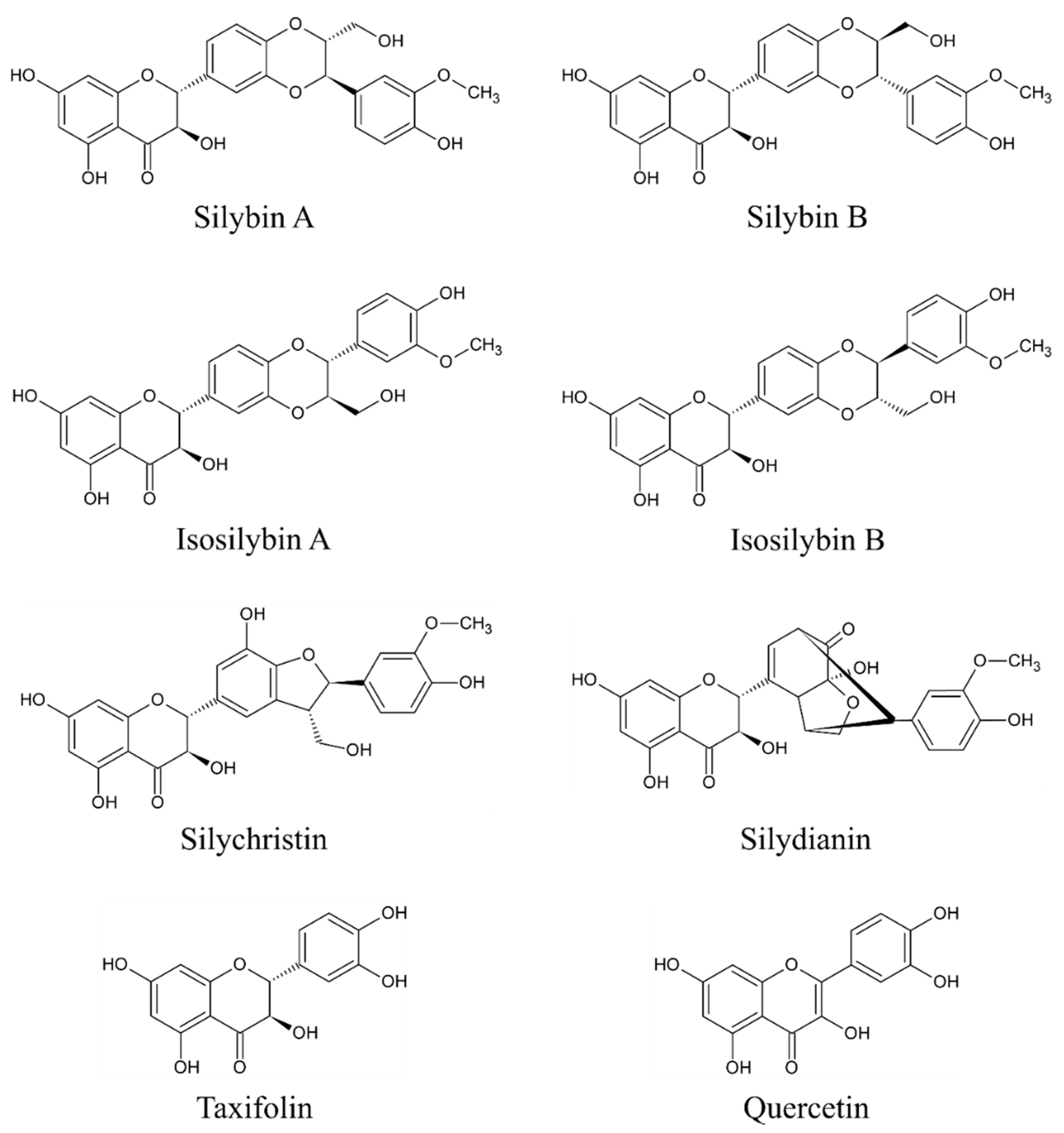
3. Bioactive Compounds of Milk Thistle
4. Beneficial Effects of Milk Thistle-Based Supplements
5. Methods Used in the Determination of Mycotoxins in Milk Thistle-Based Dietary Supplements
6. Micro-fungi in Milk Thistle-Based Dietary Supplements—An Overview
7. Mycotoxin Contamination of Dietary Supplements Based on Milk Thistle—An Overview
7.1. Seeds
7.2. Capsules
7.3. Tablets
7.4. Granules
7.5. Extracts
7.6. Herbs
8. The Most Significant Mycotoxins in Milk Thistle-Based Dietary Supplements
8.1. Alternaria Mycotoxins (AME, AOH, TEN)
8.2. Fusarium Mycotoxins
8.3. Trichothecenes (DON, T-2, HT-2)
8.4. Zearalenone (ZEA)
8.5. Emergent Mycotoxins (BEA, ENNs)
9. Mycotoxin Regulations
10. Mycotoxin Exposure Assessment and Risk Characterization
11. Summary
Author Contributions
Funding
Conflicts of Interest
References
- European Parliament and the Council of the European Union. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the member states relating to food supplements. Off. J. Eur. Communities 2002, L183, 51–57. [Google Scholar]
- Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Multiclass mycotoxin analysis in Silybum marianum by ultra high performance liquid chromatography–tandem mass spectrometry using a procedure based on QuEChERS and dispersive liquid–liquid microextraction. J. Chromatogr. A 2013, 1282, 11–19. [Google Scholar] [CrossRef]
- Fenclova, M.; Novakova, A.; Viktorova, J.; Jonatova, P.; Dzuman, Z.; Ruml, T.; Kren, V.; Hajslova, J.; Vitek, L.; Stranska-Zachariasova, M. Poor chemical and microbiological quality of the commercial milk thistle-based dietary supplements may account for their reported unsatisfactory and non-reproducible clinical outcomes. Sci. Rep. 2019, 9, 11118. [Google Scholar] [CrossRef]
- Fibigr, J.; Šatínský, D.; Solich, P. Current trends in the analysis and quality control of food supplements based on plant extracts. Anal. Chim. Acta 2018, 1036, 1–15. [Google Scholar] [CrossRef]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G. Herbal products and the liver: A review of adverse effects and mechanisms. Gastroenterology 2015, 148, 517–532.e3. [Google Scholar] [CrossRef] [Green Version]
- Ashiq, S.; Hussain, M.; Ahmad, B. Natural occurrence of mycotoxins in medicinal plants: A review. Fungal Genet. Biol. 2014, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mavungu, J.D.D.; Monbaliu, S.; Scippo, M.-L.; Maghuin-Rogister, G.; Schneider, Y.-J.; Larondelle, Y.; Callebaut, A.; Robbens, J.; Peteghem, C.V.; Saeger, S.D. LC-MS/MS multi-analyte method for mycotoxin determination in food supplements. Food Addit. Contam. Part A 2009, 26, 885–895. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Gillespie, M.; Eckl, V.; Knepper, J.; Reynolds, C.M. Herbal supplement sales in US increase by 9.4% in 2018. HerbalGram 2019, 123, 62–73. [Google Scholar]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Cwalina-Ambroziak, B.; Wierzbowska, J.; Damszel, M.; Bowszys, T. The effect of mineral fertilization on achenes yield and fungal communities isolated from the stems of milk thistle Silybum marianum (L.) Gaertner. Acta Sci. Pol. Hortorum Cultus 2012, 11, 157–168. [Google Scholar]
- Rosińska, A.; Dorna, H.; Szopińska, D.; Seidler-Łożykowska, K. Experimental paper. The effect of colour grading of milk thistle (Silybum marianum (L.) Gaertn.) seeds on their quality for sowing. Herba Pol. 2017, 63, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Rosińska, A.; Dorna, H.; Szopińska, D.; Irzykowska, L.; Seidler-Łożykowska, K. Evaluation of milk thistle (Silybum marianum (L.) Gaertn.) seed germination in relation to seed health and seedling emergence. Herba Pol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tournas, V.H.; Calo, J.R.; Sapp, C. Fungal profiles in various milk thistle botanicals from US retail. Int. J. Food Microbiol. 2013, 164, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Veprikova, Z.; Zachariasova, M.; Dzuman, Z.; Zachariasova, A.; Fenclova, M.; Slavikova, P.; Vaclavikova, M.; Mastovska, K.; Hengst, D.; Hajslova, J. Mycotoxins in plant-based dietary supplements: Hidden health risk for consumers. J. Agric. Food Chem. 2015, 63, 6633–6643. [Google Scholar] [CrossRef]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, A.J. Screening of mycotoxin multicontamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009, 89, 1802–1807. [Google Scholar] [CrossRef]
- Tournas, V.H.; Sapp, C.; Trucksess, M.W. Occurrence of aflatoxins in milk thistle herbal supplements. Food Addit. Contam. Part A 2012, 29, 994–999. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Foglia, P.; Samperi, R.; Laganà, A. Multiclass mycotoxin analysis in food, environmental and biological matrices with chromatography/mass spectrometry. Mass Spectrom. Rev. 2012, 31, 466–503. [Google Scholar] [CrossRef]
- Steyn, P.S. Mycotoxins, general view, chemistry and structure. Toxicol. Lett. 1995, 82–83, 843–851. [Google Scholar] [CrossRef]
- Wianowska, D.; Wiśniewski, M. Simplified procedure of silymarin extraction from Silybum marianum L. Gaertner. J. Chromatogr. Sci. 2015, 53, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Andrzejewska, J.; Martinelli, T.; Sadowska, K. Silybum marianum: Non-medical exploitation of the species. Ann. Appl. Biol. 2015, 167, 285–297. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Cultivation of milk thistle (Silybum marianum L. Gaertn.), a medicinal weed. Ind. Crops Prod. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresta, F.; Avola, G.; Guarnaccia, P. Agronomic characterization of some spontaneous genotypes of milk thistle (Silybum marianum L. Gaertn.) in Mediterranean environment. J. Herbs Spices Med. Plants 2006, 12, 51–60. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Fibigr, J.; Šatínský, D.; Solich, P. A new approach to the rapid separation of isomeric compounds in a Silybum marianum extract using UHPLC core-shell column with F5 stationary phase. J. Pharm. Biomed. Anal. 2017, 134, 203–213. [Google Scholar] [CrossRef]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. J. Clin. Ther. 2011, 16, 239–249. [Google Scholar]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 23 July 2020).
- Mitchell, S.T. Chapter 231. Silymarin or Milk Thistle (Silybum Marianum). In Poisoning & Drug Overdose; Olson, K.R., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2012; pp. 551–552. ISBN 0-07-166833-0. [Google Scholar]
- Fanoudi, S.; Alavi, M.S.; Karimi, G.; Hosseinzadeh, H. Milk thistle (Silybum Marianum) as an antidote or a protective agent against natural or chemical toxicities: A review. Drug Chem. Toxicol. 2020, 43, 240–254. [Google Scholar] [CrossRef]
- Aboelwafa, H.R.; El-kott, A.F.; Abd-Ella, E.M.; Yousef, H.N. The possible neuroprotective effect of silymarin against aluminum chloride-prompted Alzheimer’s-like disease in Rats. Brain Sci. 2020, 10, 628. [Google Scholar] [CrossRef]
- Guo, H.; Cao, H.; Cui, X.; Zheng, W.; Wang, S.; Yu, J.; Chen, Z. Silymarin’s inhibition and treatment effects for Alzheimer’s disease. Molecules 2019, 24, 1748. [Google Scholar] [CrossRef] [Green Version]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Helmy, N.N. Modulatory effect of silymarin on apoptosis in testosterone -induced benign prostatic hyperplasia in rats. Pathol. Oncol. Res. 2020, 26, 1947–1956. [Google Scholar] [CrossRef]
- Saberi, Z.; Gorji, N.; Memariani, Z.; Moeini, R.; Shirafkan, H.; Amiri, M. Evaluation of the effect of Silybum Marianum extract on menopausal symptoms: A randomized, double-blind placebo-controlled trial. Phytother. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Othman, S.; Ali, S.M.; Deeb, N.M.E. Protective effect of Silybum marianum extract against doxorubicin induced toxicity in male rats. PSM Biol. Res. 2020, 5, 14–21. [Google Scholar]
- Rašković, A.; Stilinović, N.; Kolarović, J.; Vasović, V.; Vukmirović, S.; Mikov, M. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules 2011, 16, 8601–8613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Rajnochová Svobodová, A. Skin protective activity of silymarin and its flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef] [Green Version]
- Fidrus, E.; Ujhelyi, Z.; Fehér, P.; Hegedűs, C.; Janka, E.A.; Paragh, G.; Vasas, G.; Bácskay, I.; Remenyik, É. Silymarin: Friend or foe of UV exposed keratinocytes? Molecules 2019, 24, 1652. [Google Scholar] [CrossRef] [Green Version]
- Alhidary, I.A.; Rehman, Z.; Khan, R.U.; Tahir, M. Anti-aflatoxin activities of milk thistle (Silybum marianum) in broiler. Worlds Poult. Sci. J. 2017, 73, 559–566. [Google Scholar] [CrossRef]
- Stoev, S.D.; Njobeh, P.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Denev, S.; Dimitrova, B. Selected herbal feed additives showing protective effects against ochratoxin A toxicosis in broiler chicks. World Mycotoxin J. 2019, 12, 257–268. [Google Scholar] [CrossRef]
- Ledur, P.C.; Santurio, J.M. Cytoprotective effects of curcumin and silymarin on PK-15 cells exposed to ochratoxin A, fumonisin B1 and deoxynivalenol. Toxicon 2020, 185, 97–103. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Z.-H.; Liu, M.; Zhang, N.-Y.; Khalil, M.M.; Gu, C.-Q.; Qi, D.-S.; Sun, L.-H. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J. Nutr. 2018, 148, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Gillessen, A.; Schmidt, H.H.-J. Silymarin as supportive treatment in liver diseases: A narrative review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grela, E.R.; Świątkiewicz, M.; Florek, M.; Wojtaszewska, I. Impact of milk thistle (Silybum marianum L.) seeds in fattener diets on pig performance and carcass traits and fatty acid profile and cholesterol of meat, backfat and liver. Livest. Sci. 2020, 239, 104180. [Google Scholar] [CrossRef]
- Kosina, P.; Dokoupilová, A.; Janda, K.; Sládková, K.; Silberová, P.; Pivodová, V.; Ulrichová, J. Effect of Silybum marianum fruit constituents on the health status of rabbits in repeated 42-day fattening experiment. Anim. Feed Sci. Technol. 2017, 223, 128–140. [Google Scholar] [CrossRef]
- Tedesco, D.; Tava, A.; Galletti, S.; Tameni, M.; Varisco, G.; Costa, A.; Steidler, S. Effects of silymarin, a natural hepatoprotector, in periparturient Dairy Cows. J. Dairy Sci. 2004, 87, 2239–2247. [Google Scholar] [CrossRef] [Green Version]
- Khamisabadi, H. Effects of Silymarin on milk production, liver enzymes, oxidative status and HSP70 gene expression in postparturient Sanjabi ewes. Cell. Mol. Biol. 2020, 66, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Šťastník, O.; Mrkvicová, E.; Pavlata, L.; Roztočilová, A.; Umlášková, B.; Anzenbacherová, E. Performance, biochemical profile and antioxidant activity of hens supplemented with addition of milk thistle (Silybum marianum) seed cakes in diet. Acta Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; World Health Organization: Geneva, Switzerland, 2007.
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A.; Mulé, G.; Moretti, A.; Perrone, G. Epidemiology of toxigenic fungi and their associated mycotoxins for some mediterranean Crops. Eur. J. Plant Pathol. 2003, 109, 645–667. [Google Scholar] [CrossRef]
- Romero, S.M.; Comerio, R.M.; Larumbe, G.; Ritieni, A.; Vaamonde, G.; Fernández Pinto, V. Toxigenic fungi isolated from dried vine fruits in Argentina. Int. J. Food Microbiol. 2005, 104, 43–49. [Google Scholar] [CrossRef]
- Andersen, B.; Krøger, E.; Roberts, R.G. Chemical and morphological segregation of Alternaria arborescens, A. infectoria and A. tenuissima species-groups. Mycol. Res. 2002, 106, 170–182. [Google Scholar] [CrossRef]
- Andersen, B.; Hansen, M.E.; Smedsgaard, J. Automated and unbiased image analyses as tools in phenotypic classification of small-spored Alternaria spp. Phytopathology 2005, 95, 1021–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, e04654. [Google Scholar] [CrossRef]
- Aichinger, G.; Krüger, F.; Puntscher, H.; Preindl, K.; Warth, B.; Marko, D. Naturally occurring mixtures of Alternaria toxins: Anti-estrogenic and genotoxic effects in vitro. Arch. Toxicol. 2019, 93, 3021–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessel-Pras, S.; Kieshauer, J.; Roenn, G.; Luckert, C.; Braeuning, A.; Lampen, A. In vitro characterization of hepatic toxicity of Alternaria toxins. Mycotoxin Res. 2019, 35, 157–168. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Eschbach, S.; Metzler, M. Alternaria toxins: DNA strand-breaking activity in mammalian cells in vitro. Mycotoxin Res. 2007, 23, 152. [Google Scholar] [CrossRef]
- Brugger, E.-M.; Wagner, J.; Schumacher, D.M.; Koch, K.; Podlech, J.; Metzler, M.; Lehmann, L. Mutagenicity of the mycotoxin alternariol in cultured mammalian cells. Toxicol. Lett. 2006, 164, 221–230. [Google Scholar] [CrossRef]
- Lehmann, L.; Wagner, J.; Metzler, M. Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem. Toxicol. 2006, 44, 398–408. [Google Scholar] [CrossRef]
- Schmutz, C.; Cenk, E.; Marko, D. The Alternaria mycotoxin alternariol triggers the immune response of IL-1β-stimulated, differentiated Caco-2 cells. Mol. Nutr. Food Res. 2019, 63, 1900341. [Google Scholar] [CrossRef] [Green Version]
- Kollarova, J.; Cenk, E.; Schmutz, C.; Marko, D. The mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation in THP-1 derived macrophages targeting the NF-κB signalling pathway. Arch. Toxicol. 2018, 92, 3347–3358. [Google Scholar] [CrossRef] [Green Version]
- Bansal, M.; Singh, N.; Alam, S.; Pal, S.; Satyanarayana, G.N.V.; Singh, D.; Ansari, K.M. Alternariol induced proliferation in primary mouse keratinocytes and inflammation in mouse skin is regulated via PGE2/EP2/cAMP/p-CREB signaling pathway. Toxicology 2019, 412, 79–88. [Google Scholar] [CrossRef]
- Tiemann, U.; Tomek, W.; Schneider, F.; Müller, M.; Pöhland, R.; Vanselow, J. The mycotoxins alternariol and alternariol methyl ether negatively affect progesterone synthesis in porcine granulosa cells in vitro. Toxicol. Lett. 2009, 186, 139–145. [Google Scholar] [CrossRef]
- Dellafiora, L.; Warth, B.; Schmidt, V.; Del Favero, G.; Mikula, H.; Fröhlich, J.; Marko, D. An integrated in silico/in vitro approach to assess the xenoestrogenic potential of Alternaria mycotoxins and metabolites. Food Chem. 2018, 248, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.T.; Qian, Y.Z.; Zhang, P.E.; Dong, W.H.; Qi, Y.M.; Guo, H. Etiological role of Alternaria alternata in human esophageal cancer. Chin. Med. J. 1992, 105, 394–400. [Google Scholar] [PubMed]
- Frisvad, J.C.; Thrane, U.; Samson, R.A. Mycotoxin producers. In Food Mycology: A Multifaceted Approach to Fungi and Food; Dijksterhuis, J., Samson, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 135–159. [Google Scholar]
- Sun, L.-H.; Lei, M.; Zhang, N.-Y.; Zhao, L.; Krumm, C.S.; Qi, D.-S. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem. Toxicol. 2014, 74, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Han, S.; Chen, Y.; Wang, Y.; Li, D.; Zhu, Q. T-2 Toxin induces oxidative stress, apoptosis and cytoprotective autophagy in chicken hepatocytes. Toxins 2020, 12, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Tang, J.; Geng, F.; Zhu, L.; Chu, X.; Zhang, Y.; Rahman, S.U.; Chen, X.; Jiang, Y.; Zhu, D.; et al. Effects of deoxynivalenol exposure on cerebral lipid peroxidation, neurotransmitter and calcium homeostasis of chicks in vivo. Toxicon 2018, 150, 60–65. [Google Scholar] [CrossRef]
- Guo, P.; Liu, A.; Huang, D.; Wu, Q.; Fatima, Z.; Tao, Y.; Cheng, G.; Wang, X.; Yuan, Z. Brain damage and neurological symptoms induced by T-2 toxin in rat brain. Toxicol. Lett. 2018, 286, 96–107. [Google Scholar] [CrossRef]
- Modra, H.; Palikova, M.; Hyrsl, P.; Bartonkova, J.; Papezikova, I.; Svobodova, Z.; Blahova, J.; Mares, J. Effects of trichothecene mycotoxin T-2 toxin on haematological and immunological parameters of rainbow trout (Oncorhynchus mykiss). Mycotoxin Res. 2020, 36, 319–326. [Google Scholar] [CrossRef]
- Hymery, N.; Léon, K.; Carpentier, F.-G.; Jung, J.-L.; Parent-Massin, D. T-2 toxin inhibits the differentiation of human monocytes into dendritic cells and macrophages. Toxicol. In Vitro 2009, 23, 509–519. [Google Scholar] [CrossRef]
- Vlata, Z.; Porichis, F.; Tzanakakis, G.; Tsatsakis, A.; Krambovitis, E. In vitro cytopathic effects of mycotoxin T-2 on human peripheral blood T lymphocytes. Toxicol. Lett. 2005, 160, 60–68. [Google Scholar] [CrossRef]
- Minervini, F.; Fornelli, F.; Lucivero, G.; Romano, C.; Visconti, A. T-2 toxin immunotoxicity on human B and T lymphoid cell lines. Toxicology 2005, 210, 81–91. [Google Scholar] [CrossRef]
- Hymery, N.; Sibiril, Y.; Parent-Massin, D. In vitro effects of trichothecenes on human dendritic cells. Toxicol. In Vitro 2006, 20, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Yao, Q.; Song, M.; Han, Y.; Shao, B.; Li, Y. T-2 toxin impairs male fertility by disrupting hypothalamic-pituitary-testis axis and declining testicular function in mice. Chemosphere 2019, 234, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Tassis, P.D.; Tsakmakidis, I.A.; Nagl, V.; Reisinger, N.; Tzika, E.; Gruber-Dorninger, C.; Michos, I.; Mittas, N.; Basioura, A.; Schatzmayr, D. Individual and combined in vitro effects of deoxynivalenol and zearalenone on boar semen. Toxins 2020, 12, 495. [Google Scholar] [CrossRef]
- Agrawal, M.; Yadav, P.; Lomash, V.; Bhaskar, A.S.B.; Lakshmana Rao, P.V. T-2 toxin induced skin inflammation and cutaneous injury in mice. Toxicology 2012, 302, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, A.A.; Kalantari, H.; Jalali, A.; Rezai, S.; Zadeh, H.H. Healing effect of quince seed mucilage on T-2 toxin-induced dermal toxicity in rabbit. Exp. Toxicol. Pathol. 2012, 64, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Cho, U.M.; Choi, J.H.; Hwang, H.S. Deoxynivalenol impair skin barrier function through the down regulation of filaggrin and claudin 1/8 in HaCaT keratinocyte. Biotechnol. Bioprocess Eng. 2017, 22, 693–699. [Google Scholar] [CrossRef]
- Zhou, H.; George, S.; Hay, C.; Lee, J.; Qian, H.; Sun, X. Individual and combined effects of aflatoxin B1, deoxynivalenol and zearalenone on HepG2 and RAW 264.7 cell lines. Food Chem. Toxicol. 2017, 103, 18–27. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Elmo, L.; Waldner, T.; Ruiz, M.-J. Cytotoxic effects induced by patulin, deoxynivalenol and toxin T2 individually and in combination in hepatic cells (HepG2). Food Chem. Toxicol. 2018, 120, 12–23. [Google Scholar] [CrossRef]
- Yu, F.-F.; Lin, X.-L.; Wang, X.; Ping, Z.-G.; Guo, X. Comparison of apoptosis and autophagy in human chondrocytes Induced by the T-2 and HT-2 Toxins. Toxins 2019, 11, 260. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Tu, D.; Zhao, Z.; Cui, J. Cytotoxicity and apoptosis induced by mixed mycotoxins (T-2 and HT-2 toxin) on primary hepatocytes of broilers in vitro. Toxicon 2017, 129, 1–10. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occuring Substances: Food Items and Costituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC Press: Lyon, France, 1993; Volume 56, ISBN 92-832-1256-8. [Google Scholar]
- European Food Safety Authority. Scientific opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Sali, N.; Kőszegi, T.; Szente, L.; Peles-Lemli, B. Interactions of zearalenone with native and chemically modified cyclodextrins and their potential utilization. J. Photochem. Photobiol. B 2015, 151, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Kotowicz, N.K.; Frac, M.; Lipiec, J. The importance of Fusarium fungi in wheat cultivation-pathogenicity and mycotoxins production: A review. J. Anim. Plant Sci. 2014, 21, 3326–3343. [Google Scholar]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Krejcárková, A.; Šimoník, O.; Šašková, M.; Krejčířová, R.; Drábek, O.; Rajmon, R. Effects of zearalenone, α-zearalenol, and genistein on boar sperm motility in vitro. Czech J. Anim. Sci. 2017, 62, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Harčárová, M.; Čonková, E.; Proškovcová, M.; Falis, M. In vivo assessment of zearalenone toxicity. Folia Vet. 2020, 64, 60–65. [Google Scholar] [CrossRef]
- Aichinger, G.; Pantazi, F.; Marko, D. Combinatory estrogenic effects of bisphenol A in mixtures with alternariol and zearalenone in human endometrial cells. Toxicol. Lett. 2020, 319, 242–249. [Google Scholar] [CrossRef]
- Althali, N.J.; Hassan, A.M.; Abdel-Wahhab, M.A. Effect of grape seed extract on maternal toxicity and in utero development in mice treated with zearalenone. Environ. Sci. Pollut. Res. 2019, 26, 5990–5999. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, H.; Gao, Q.; Li, Y.-H.; Xu, Y.N.; Kim, N.-H. Melatonin alleviates defects induced by zearalenone during porcine embryo development. Theriogenology 2020, 151, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhi, Y.; Xu, H.; Fang, H.; Jia, X. Zearalenone causes embryotoxicity and induces oxidative stress and apoptosis in differentiated human embryonic stem cells. Toxicol. In Vitro 2019, 54, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.B.; Boussabbeh, M.; Neffati, F.; Najjar, M.; Abid-Essefi, S.; Bacha, H. Zearalenone-induced changes in biochemical parameters, oxidative stress and apoptosis in cardiac tissue: Protective role of crocin. Hum. Exp. Toxicol. 2016, 35, 623–634. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, M.; Qu, Z.; Zhang, Y.; Yin, S.; Shan, A. Toxic effects of zearalenone on oxidative stress, inflammatory cytokines, biochemical and pathological changes induced by this toxin in the kidney of pregnant rats. Environ. Toxicol. Pharmacol. 2014, 37, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Bázár, G.; Kocsó, D.; Ali, O.; Kovács, M. Individual and combined effects of fumonisin B1, deoxynivalenol and zearalenone on the hepatic and renal membrane lipid integrity of rats. Toxins 2018, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.R.; Kim, J.W.; Roh, Y.-S.; Kim, J.-H.; Han, K.M.; Kwon, H.-J.; Lim, C.W.; Kim, B. Evaluation of immunomodulatory effects of zearalenone in mice. J. Immunotoxicol. 2017, 14, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [Green Version]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef] [Green Version]
- Bouaziz, C.; Sharaf el dein, O.; El Golli, E.; Abid-Essefi, S.; Brenner, C.; Lemaire, C.; Bacha, H. Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 2008, 254, 19–28. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Bulgaru, C.V.; Taranu, I. Cytotoxic and inflammatory effects of individual and combined exposure of HepG2 cells to zearalenone and its metabolites. Naunyn. Schmiedebergs Arch. Pharmacol. 2019, 392, 937–947. [Google Scholar] [CrossRef]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sørlie, M.; Ropstad, E.; Muller, M.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Fang, H.; Zhao, Y.; Jin, Y.; Shen, J.; Zhou, C.; Zhou, Y.; Fu, Y.; Wang, J.; et al. Transcriptional profiling of zearalenone-induced inhibition of IPEC-J2 cell proliferation. Toxicon 2019, 172, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H.; Deng, H.D.; Deng, Y.T.; Deng, J.L.; Zuo, Z.C.; Yu, S.M.; Shen, L.H.; Cui, H.M.; Xu, Z.W.; Hu, Y.C. Effect of the Fusarium toxins, zearalenone and deoxynivalenol, on the mouse brain. Environ. Toxicol. Pharmacol. 2016, 46, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Liu, W.; Zhao, L.; Cao, L.; Shen, Z. Low doses of individual and combined deoxynivalenol and zearalenone in naturally moldy diets impair intestinal functions via inducing inflammation and disrupting epithelial barrier in the intestine of piglets. Toxicol. Lett. 2020, 333, 159–169. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Shan, A.; Xu, L. Changes in intestinal barrier functions and gut microbiota in rats exposed to zearalenone. Ecotoxicol. Environ. Saf. 2020, 204, 111072. [Google Scholar] [CrossRef]
- Lahjouji, T.; Bertaccini, A.; Neves, M.; Puel, S.; Oswald, I.P.; Soler, L. Acute exposure to zearalenone disturbs intestinal homeostasis by modulating the Wnt/β-Catenin signaling pathway. Toxins 2020, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin in Serbian maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef] [Green Version]
- Tonshin, A.A.; Teplova, V.V.; Andersson, M.A.; Salkinoja-Salonen, M.S. The Fusarium mycotoxins enniatins and beauvericin cause mitochondrial dysfunction by affecting the mitochondrial volume regulation, oxidative phosphorylation and ion homeostasis. Toxicology 2010, 276, 49–57. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium -mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Devreese, M.; Broekaert, N.; De Mil, T.; Fraeyman, S.; De Backer, P.; Croubels, S. Pilot toxicokinetic study and absolute oral bioavailability of the Fusarium mycotoxin enniatin B1 in pigs. Food Chem. Toxicol. 2014, 63, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosperini, A.; Juan-García, A.; Font, G.; Ruiz, M.J. Beauvericin-induced cytotoxicity via ROS production and mitochondrial damage in Caco-2 cells. Toxicol. Lett. 2013, 222, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Font, G.; Ruiz, M.J. Interaction effects of Fusarium enniatins (A, A1, B and B1) combinations on in vitro cytotoxicity of Caco-2 cells. Toxicol. In Vitro 2014, 28, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Comparative structure–activity analysis of the antimicrobial activity, cytotoxicity, and mechanism of action of the fungal cyclohexadepsipeptides enniatins and eeauvericin. Toxins 2019, 11, 514. [Google Scholar] [CrossRef] [Green Version]
- Agahi, F.; Font, G.; Juan, C.; Juan-García, A. Individual and combined effect of zearalenone derivates and beauvericin mycotoxins on SH-SY5Y Cells. Toxins 2020, 12, 212. [Google Scholar] [CrossRef] [Green Version]
- Mamur, S.; Yuzbasioglu, D.; Yılmaz, S.; Erikel, E.; Unal, F. Assessment of cytotoxic and genotoxic effects of enniatin—A in vitro. Food Addit. Contam. Part A 2018, 35, 1633–1644. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, F.-T.; Chan, W.-H. Enniatin B1 exerts embryotoxic effects on mouse blastocysts and induces oxidative stress and immunotoxicity during embryo development. Environ. Toxicol. 2019, 34, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Büchter, C.; Koch, K.; Freyer, M.; Baier, S.; Saier, C.; Honnen, S.; Wätjen, W. The mycotoxin beauvericin impairs development, fertility and life span in the nematode Caenorhabditis elegans accompanied by increased germ cell apoptosis and lipofuscin accumulation. Toxicol. Lett. 2020, 334, 102–109. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- European Commission. European Union Commission Regulation (EU) No. 105/2010 of 5 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A. Off. J. Eur. Union 2010, L35, 7–8. [Google Scholar]
- JECFA FAO/WHO. Evaluation of Certain Contaminants in Food Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 959; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4-120959-5.
- European Food Safety Authority. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef]
- JECFA FAO/WHO. Evaluation of Certain Mycotoxins in Food. Fifty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 906; World Health Organization: Geneva, Switzerland, 2002; ISBN 92-4-120906-2.
- European Food Safety Authority. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, e04972. [Google Scholar] [CrossRef]
- JECFA FAO/WHO. Evaluation of Certain food Additives and Contaminants. Fifty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 896; World Health Organization: Geneva, Switzerland, 2000; ISBN 92-4-120896-1.
- European Food Safety Authority. Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 1–46. [Google Scholar] [CrossRef]
- Krogh, P.; Axelsen, N.H.; Elling, F.; Gyrd-Hansen, N.; Hald, B.; Hyldgaard-Jensen, J.; Larsen, A.E.; Madsen, A.; Mortensen, H.P.; Moller, T.; et al. Experimental porcine nephropathy. Changes of renal function and structure induced by ochratoxin A- contaminated feed. Acta Pathol. Microbiol. Scand. Suppl. 1974, 84, 1–21. [Google Scholar]
- European Food Safety Authority. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 1–150. [Google Scholar] [CrossRef]
- European Food Safety Authority. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 1–112. [Google Scholar] [CrossRef]
- EU. SCF Opinion of the Scientific Committee on Food on Fusarium-Toxins Part 1: Deoxynivalenol (DON); (Expressed on 2 December 1999); EU: Brussel, Belgium, 1999.
- European Food Safety Authority. Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Ostry, V.; Skarkova, J.; Ruprich, J. Alternaria Mycotoxins in Foodstuffs–Current Information for Health Risk Assessment; Mycotoxin: Bratislava, Slovakia, 2009; pp. 1–9. [Google Scholar]


| Supplement Form | Mycotoxins | Clean-up Method | Analysis | References |
|---|---|---|---|---|
| Seeds | 7 mycotoxins | multifunctional columns (for AFs, ZEA, DON, FBs, T-2); polyamide column (for CIT); no clean-up (for OTA) | ELISA | [16] |
| Seeds, herbs, tea, alcohol-based liquid seed extract, oil-based liquid seed extract | AFs, AFB1 | immunoaffinity column clean-up | RPLC-FLD | [17] |
| Seeds, extract | 15 mycotoxins | QuEChERS + DLLME (for AFB1, AFB2, AFG1, AFG2, CIT, HT-2, OTA, STEG, T-2, ZEA) | UHPLC-MS/MS | [2] |
| Capsules with dried powder/oil-based matrix, seeds, tablets, granules, tea | 57 mycotoxins | QuEChERS | UHPLC-MS/MS | [15] |
| Encapsulated oily paste, capsules with dried powder | 55 mycotoxins | QuEChERS | UHPLC-HRMS | [3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Presence of Mycotoxins in Milk Thistle (Silybum marianum) Food Supplements: A Review. Toxins 2020, 12, 782. https://doi.org/10.3390/toxins12120782
Pickova D, Ostry V, Toman J, Malir F. Presence of Mycotoxins in Milk Thistle (Silybum marianum) Food Supplements: A Review. Toxins. 2020; 12(12):782. https://doi.org/10.3390/toxins12120782
Chicago/Turabian StylePickova, Darina, Vladimir Ostry, Jakub Toman, and Frantisek Malir. 2020. "Presence of Mycotoxins in Milk Thistle (Silybum marianum) Food Supplements: A Review" Toxins 12, no. 12: 782. https://doi.org/10.3390/toxins12120782
APA StylePickova, D., Ostry, V., Toman, J., & Malir, F. (2020). Presence of Mycotoxins in Milk Thistle (Silybum marianum) Food Supplements: A Review. Toxins, 12(12), 782. https://doi.org/10.3390/toxins12120782






