Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review
Abstract
:1. Introduction
1.1. Historical Perspective
1.2. Purpose of the Review
2. Epidemiology and Microbiology
2.1. The Infectious Agent
2.1.1. Nomenclature: Shiga Toxin, Vero Toxin-Producing, or Enterohemorrhagic E. coli
2.1.2. Evolution of E. coli and Phage Acquisition of Stx Gene
2.1.3. EHEC: Microbiological Characteristics of Classic O157:H7 and Emergent Non-O157 Serotypes
2.2. Shiga Toxins: Structure and Nomenclature
3. STEC-HUS as a Zoonosis: Reservoirs, Sources, and Modes of Transmission
3.1. Global Burden, Spatial and Temporal Distribution of STEC-HUS Cases
3.2. Propensity to Develop STEC-HUS
4. Pathogenesis
4.1. Colonization of the Bowel: The Attaching and Effacing Phenotype
4.2. Shiga Toxin Production and Effect: Gb3 Fixation and Trafficking
4.3. Mechanisms of Shiga Toxin Cytotoxicity
4.4. Activation of Complement Pathways: Culprit or Innocent Bystander?
4.5. Endothelial Damage: From Stx Cytotoxicity to Thrombotic Microangiopathy
5. Diagnosis
5.1. Clinical Presentation
5.2. From Colitis to HUS
5.3. Clinical Predictors of Evolution Towards HUS
5.4. Renal Involvement
5.5. Extra-Renal Involvement
5.5.1. Neurologic Involvement
5.5.2. Cardiac Involvement
5.5.3. STEC-HUS and Diabetes Mellitus
5.6. Recurrence
5.7. Unusual Invasive Infections
5.8. Paraclinical Signs
5.8.1. Thrombotic Microangiopathy
5.8.2. Inflammatory Features and Coagulation Activation
5.8.3. Biological Predictors of Evolution Towards HUS
5.8.4. Histopathology
5.9. Microbiology
5.9.1. Identification of EHEC: Culture and Characterization
5.9.2. Identification of Shiga Toxin: Non-Culture Assays
Molecular Biology
Immunological Tests
Serodiagnosis
5.10. Differential Diagnosis
6. Treatment
6.1. Prevention
6.1.1. Primary Prevention
Individual Level
Farm and Industry Level
Slaughterhouse Hygiene and Meat Processing
6.1.2. Secondary Prevention
Community Level
Individual Level
6.2. Supportive Therapy
6.2.1. Volume, Electrolytic Balance, and Nutrition
6.2.2. Blood Pressure Control
6.2.3. Renal Replacement Therapy (RRT)
6.2.4. Transfusion
6.2.5. Detrimental Effect of Antimotility Agents
6.3. Specific Therapies
6.3.1. Plasma Exchange and Immunoadsorption
6.3.2. Complement Blockade Therapy
6.3.3. Gb3 Receptor Analogues, Shiga Toxin-Binding Agents, and Monoclonal Antibodies
6.3.4. Manganese
6.3.5. Other Abandoned Therapies
7. Prognosis
7.1. Renal Sequelae
7.2. Extra-Renal Sequelae
7.3. Predicting the Risk of Long-Term Sequelae
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- George, J.N.; Nester, C.M. Syndromes of thrombotic microangiopathy. N. Engl. J. Med. 2014, 371, 654–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loirat, C.; Fakhouri, F.; Ariceta, G.; Besbas, N.; Bitzan, M.; Bjerre, A.; Coppo, R.; Emma, F.; Johnson, S.; Karpman, D.; et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr. Nephrol. Berl. Ger. 2016, 31, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.; Coppo, P.; Rubia, J.; de la Friedman, K.D.; Hovinga, J.K.; Lämmle, B.; Matsumoto, M.; Pavenski, K.; Sadler, E.; et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J. Thromb. Haemost. 2016, 15, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Shigatoxin/Verocytotoxin-Producing Escherichia coli (STEC/VTEC) Infection—AER. 2016. Available online: https://ecdc.europa.eu/en/STEC/AER2016 (accessed on 23 March 2018).
- Gasser, C.; Gautier, E.; Steck, A.; Siebenmann, R.E.; Oechslin, R. [Hemolytic-uremic syndrome: Bilateral necrosis of the renal cortex in acute acquired hemolytic anemia]. Schweiz Med. Wochenschr. 1955, 85, 905–909. [Google Scholar] [PubMed]
- Karmali, M.A.; Steele, B.T.; Petric, M.; Lim, C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet Lond. Engl. 1983, 1, 619–620. [Google Scholar] [CrossRef]
- O’Brien, A.D.; Lively, T.A.; Chang, T.W.; Gorbach, S.L. Purification of Shigella dysenteriae 1 (Shiga)-like toxin from Escherichia coli O157:H7 strain associated with haemorrhagic colitis. Lancet Lond. Engl. 1983, 2, 573. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; an der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef] [Green Version]
- Butler, T. Haemolytic uraemic syndrome during shigellosis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.D.; Lampel, K.A.; Strockbine, N.A.; Fernandez, R.E.; Melton-Celsa, A.R.; Maurelli, A.T. Clinical Isolates of Shiga Toxin 1a–Producing Shigella flexneri with an Epidemiological Link to Recent Travel to Hispañiola. Emerg. Infect. Dis. CDC 2014, 20, 1669–1677. [Google Scholar] [CrossRef]
- Gray, M.D.; Lacher, D.W.; Leonard, S.R.; Abbott, J.; Zhao, S.; Lampel, K.A.; Prothery, E.; Gouali, M.; Weill, F.-X.; Maurelli, A.T.; et al. Prevalence of Shiga toxin-producing Shigella species isolated from French travellers returning from the Caribbean: An emerging pathogen with international implications. Clin. Microbiol. Infect. 2015, 21, 765.e9–765.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamba, K.; Nelson, J.A.; Kimura, A.C.; Poe, A.; Collins, J.; Kao, A.S.; Cruz, L.; Inami, G.; Vaishampayan, J.; Garza, A.; et al. Shiga Toxin 1–Producing Shigella sonnei Infections, California, United States, 2014–2015. Emerg. Infect. Dis. CDC 2016, 22, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, H.; Montag, M.; Bockemühl, J.; Heesemann, J.; Karch, H. Shiga-like toxin II-related cytotoxins in Citrobacter freundii strains from humans and beef samples. Infect. Immun. 1993, 61, 534–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinescu, A.R.; Bitzan, M.; Weiss, L.S.; Christen, E.; Kaplan, B.S.; Cnaan, A.; Trachtman, H. Non-enteropathic hemolytic uremic syndrome: Causes and short-term course. Am. J. Kidney Dis. 2004, 43, 976–982. [Google Scholar] [CrossRef]
- Spinale, J.M.; Ruebner, R.L.; Kaplan, B.S.; Copelovitch, L. Update on Streptococcus pneumoniae associated hemolytic uremic syndrome. Curr. Opin. Pediatr. 2013, 25, 203–208. [Google Scholar] [CrossRef]
- Riley, L.W.; Remis, R.S.; Helgerson, S.D.; McGee, H.B.; Wells, J.G.; Davis, B.R.; Hebert, R.J.; Olcott, E.S.; Johnson, L.M.; Hargrett, N.T.; et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983, 308, 681–685. [Google Scholar] [CrossRef]
- Johnson, W.M.; Lior, H.; Bezanson, G.S. Cytotoxic Escherichia coli O157:H7 associated with haemorrhagic colitis in Canada. Lancet Lond. Engl. 1983, 1, 76. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P.; Ng, L.-K.; Lukinmaa, S.; Kam, K.-M.; Rolando, S.; Gutiérrez, E.P.; Binsztein, N. Building PulseNet International: An interconnected system of laboratory networks to facilitate timely public health recognition and response to foodborne disease outbreaks and emerging foodborne diseases. Foodborne Pathog. Dis. 2006, 3, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Köck, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 2011, 11, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Boerlin, P.; McEwen, S.A.; Boerlin-Petzold, F.; Wilson, J.B.; Johnson, R.P.; Gyles, C.L. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 1999, 37, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, L.; Vanstone, G.L.; Perry, N.T.; Launders, N.; Adak, G.K.; Godbole, G.; Grant, K.A.; Smith, R.; Jenkins, C. Epidemiology and microbiology of Shiga toxin-producing Escherichia coli other than serogroup O157 in England, 2009–2013. J. Med. Microbiol. 2014, 63, 1181–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmali, M.A.; Mascarenhas, M.; Shen, S.; Ziebell, K.; Johnson, S.; Reid-Smith, R.; Isaac-Renton, J.; Clark, C.; Rahn, K.; Kaper, J.B.; et al. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 2003, 41, 4930–4940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, N.; Lee, K.-I.; Yamazaki, A.; Saito, S.; Furukawa, I.; Kono, T.; Maeda, E.; Isobe, J.; Sugita-Konishi, Y.; Hara-Kudo, Y.; et al. Virulence gene profiles and population genetic analysis for exploration of pathogenic serogroups of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 2013, 51, 4022–4028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogura, Y.; Ooka, T.; Iguchi, A.; Toh, H.; Asadulghani, M.; Oshima, K.; Kodama, T.; Abe, H.; Nakayama, K.; Kurokawa, K.; et al. Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc. Natl. Acad. Sci. USA 2009, 106, 17939–17944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, L.M.; Qi, W.; Lacher, D.W.; Whittam, T.S. Evolution of genomic content in the stepwise emergence of Escherichia coli O157:H7. J. Bacteriol. 2005, 187, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Lampel, K.A.; Karch, H.; Whittam, T.S. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J. Infect. Dis. 1998, 177, 1750–1753. [Google Scholar] [CrossRef]
- Newland, J.W.; Strockbine, N.A.; Miller, S.F.; O’Brien, A.D.; Holmes, R.K. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science 1985, 230, 179–181. [Google Scholar] [CrossRef]
- Krüger, A.; Lucchesi, P.M.A. Shiga toxins and stx phages: Highly diverse entities. Microbiology Read. Engl. 2015, 161 Pt 3, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Mauro, S.A.; Koudelka, G.B. Shiga toxin: Expression, distribution, and its role in the environment. Toxins 2011, 3, 608–625. [Google Scholar] [CrossRef]
- Krüger, A.; Lucchesi, P.M.A.; Parma, A.E. Verotoxins in Bovine and Meat Verotoxin-Producing Escherichia coli Isolates: Type, Number of Variants, and Relationship to Cytotoxicity. Appl. Environ. Microbiol. 2011, 77, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimmitt, P.T.; Harwood, C.R.; Barer, M.R. Toxin gene expression by shiga toxin-producing Escherichia coli: The role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 2000, 6, 458–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, P.L.; Waldor, M.K. Bacteriophage control of bacterial virulence. Infect. Immun. 2002, 70, 3985–3993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, P.L.; Livny, J.; Neely, M.N.; Acheson, D.W.K.; Friedman, D.I.; Waldor, M.K. Bacteriophage control of Shiga toxin 1 production and release by Escherichia coli. Mol. Microbiol. 2002, 44, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.C.H.; Monday, S.R.; Lacher, D.W.; Allison, L.; Siitonen, A.; Keys, C.; Eklund, M.; Nagano, H.; Karch, H.; Keen, J.; et al. Genetic diversity among clonal lineages within Escherichia coli O157:H7 stepwise evolutionary model. Emerg. Infect. Dis. 2007, 13, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Werber, D.; Bielaszewska, M.; Frank, C.; Stark, K.; Karch, H. Watch out for the even eviler cousin-sorbitol-fermenting E coli O157. Lancet 2011, 377, 298–299. [Google Scholar] [CrossRef]
- Alpers, K.; Werber, D.; Frank, C.; Koch, J.; Friedrich, A.W.; Karch, H.; HEIDEN, M.A.; PRAGER, R.; FRUTH, A.; BIELASZEWSKA, M.; et al. Sorbitol-fermenting enterohaemorrhagic Escherichia coli O157:H- causes another outbreak of haemolytic uraemic syndrome in children. Epidemiol. Infect. 2009, 137, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Ammon, A.; Petersen, L.R.; Karch, H. A large outbreak of hemolytic uremic syndrome caused by an unusual sorbitol-fermenting strain of Escherichia coli O157:H-. J. Infect. Dis. 1999, 179, 1274–1277. [Google Scholar] [CrossRef] [Green Version]
- Orth, D.; Grif, K.; Zimmerhackl, L.B.; Würzner, R. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157 in Austria. Wien. Klin. Wochenschr. 2009, 121, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.H.; Mody, R.K.; Ong, K.L.; Clogher, P.; Cronquist, A.B.; Garman, K.N.; Lathrop, S.; Medus, C.; Spina, N.L.; Webb, T.H.; et al. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000-2010: Epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog. Dis. 2013, 10, 453–460. [Google Scholar] [CrossRef]
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; Strockbine, N.A. Non-O157 Shiga Toxin–Producing Escherichia coli Infections in the United States, 1983–2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedican, E.B.; Medus, C.; Besser, J.M.; Juni, B.A.; Koziol, B.; Taylor, C.; Smith, K.E. Characteristics of O157 versus Non-O157 Shiga Toxin-Producing Escherichia coli Infections in Minnesota, 2000–2006. Clin. Infect. Dis. 2009, 49, 358–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cointe, A.; Birgy, A.; Mariani-Kurkdjian, P.; Liguori, S.; Courroux, C.; Blanco, J.; Delannoy, S.; Fach, P.; Loukiadis, E.; Bidet, P.; et al. Emerging Multidrug-Resistant Hybrid Pathotype Shiga Toxin-Producing Escherichia coli O80 and Related Strains of Clonal Complex 165, Europe. Emerg. Infect. Dis. 2018, 24, 2262–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soysal, N.; Mariani-Kurkdjian, P.; Smail, Y.; Liguori, S.; Gouali, M.; Loukiadis, E.; Fach, P.; Bruyand, M.; Blanco, J.; Bidet, P.; et al. Enterohemorrhagic Escherichia coli Hybrid Pathotype O80:H2 as a New Therapeutic Challenge. Emerg. Infect. Dis. 2016, 22, 1604–1612. [Google Scholar] [CrossRef] [Green Version]
- Mariani-Kurkdjian, P.; Lemaître, C.; Bidet, P.; Perez, D.; Boggini, L.; Kwon, T.; Bonacorsi, S. Haemolytic-uraemic syndrome with bacteraemia caused by a new hybrid Escherichia coli pathotype. New Microbes New Infect. 2014, 2, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Fierz, L.; Cernela, N.; Hauser, E.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of Shigatoxin-Producing Escherichia coli Strains Isolated during 2010-2014 from Human Infections in Switzerland. Front. Microbiol. 2017, 8, 1471. [Google Scholar] [CrossRef]
- Wijnsma, K.L.; Schijvens, A.M.; Rossen, J.W.A.; Kooistra-Smid, A.M.D.M.; Schreuder, M.F.; van de Kar, N.C.A.J. Unusual severe case of hemolytic uremic syndrome due to Shiga toxin 2d-producing E. coli O80:H2. Pediatr. Nephrol. 2017, 32, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Nüesch-Inderbinen, M.; Cernela, N.; Wüthrich, D.; Egli, A.; Stephan, R. Genetic characterization of Shiga toxin producing Escherichia coli belonging to the emerging hybrid pathotype O80:H2 isolated from humans 2010–2017 in Switzerland. Int. J. Med. Microbiol. IJMM 2018, 308, 534–538. [Google Scholar]
- Hadler, J.L.; Clogher, P.; Hurd, S.; Phan, Q.; Mandour, M.; Bemis, K.; Marcus, R. Ten-Year Trends and Risk Factors for Non-O157 Shiga Toxin–Producing Escherichia coli Found Through Shiga Toxin Testing, Connecticut, 2000–2009. Clin. Infect. Dis. 2011, 53, 269–276. [Google Scholar] [CrossRef]
- Braune, S.A.; Wichmann, D.; von Heinz, M.C.; Nierhaus, A.; Becker, H.; Meyer, T.N.; Meyer, G.P.; Müller-Schulz, M.; Fricke, J.; De Weerth, A.; et al. Clinical features of critically ill patients with Shiga toxin-induced hemolytic uremic syndrome. Crit. Care Med. 2013, 41, 1702–1710. [Google Scholar] [CrossRef]
- Rasko, D.A.; Webster, D.R.; Sahl, J.W.; Bashir, A.; Boisen, N.; Scheutz, F.; Paxinos, E.E.; Sebra, R.; Chin, C.-S.; Iliopoulos, D.; et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 2011, 365, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Zimmerhackl, L.-B.; Rosales, A.; Hofer, J.; Riedl, M.; Jungraithmayr, T.; Mellmann, A.; Bielaszewska, M.; Karch, H. Enterohemorrhagic Escherichia coli O26:H11-Associated Hemolytic Uremic Syndrome: Bacteriology and Clinical Presentation. Semin. Thromb. Hemost. 2010, 36, 586–593. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Bletz, S.; Zhang, W.; Köck, R.; Kossow, A.; Prager, R.; Fruth, A.; Orth-Höller, D.; Marejková, M.; et al. Enterohemorrhagic Escherichia coli O26:H11/H-: A new virulent clone emerges in Europe. Clin. Infect. Dis. 2013, 56, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, O.K. Shiga, Bacteriologist. Science 1957, 126, 113. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K. Ueber den Disenteriebacillus (Bacillus dysenteriae). Zentralblat Fuer Bakteriol Parasitenkd Infekt Erste Abt. 1898, 24, 913–918. [Google Scholar]
- Johannes, L.; Römer, W. Shiga toxins—From cell biology to biomedical applications. Nat. Rev. Microbiol. 2010, 8, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.J.; Dodd, J.E.; Hautbergue, G.M. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef] [Green Version]
- Ling, H.; Boodhoo, A.; Hazes, B.; Cummings, M.D.; Armstrong, G.D.; Brunton, J.L.; Read, R.J. Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry 1998, 37, 1777–1788. [Google Scholar] [CrossRef]
- Stein, P.E.; Boodhoo, A.; Tyrrell, G.J.; Brunton, J.L.; Read, R.J. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature 1992, 355, 748–750. [Google Scholar] [CrossRef]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [Green Version]
- Ostroff, S.M.; Tarr, P.I.; Neill, M.A.; Lewis, J.H.; Hargrett-Bean, N.; Kobayashi, J.M. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 1989, 160, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Matussek, A.; Einemo, I.-M.; Jogenfors, A.; Löfdahl, S.; Löfgren, S. Shiga Toxin-Producing Escherichia coli in Diarrheal Stool of Swedish Children: Evaluation of Polymerase Chain Reaction Screening and Duration of Shiga Toxin Shedding. J. Pediatr. Infect. Dis. Soc. 2016, 5, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Bielaszewska, M.; Friedrich, A.W.; Aldick, T.; Schürk-Bulgrin, R.; Karch, H. Shiga toxin activatable by intestinal mucus in Escherichia coli isolated from humans: Predictor for a severe clinical outcome. Clin. Infect. Dis. 2006, 43, 1160–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orth, D.; Grif, K.; Khan, A.B.; Naim, A.; Dierich, M.P.; Würzner, R. The Shiga toxin genotype rather than the amount of Shiga toxin or the cytotoxicity of Shiga toxin in vitro correlates with the appearance of the hemolytic uremic syndrome. Diagn. Microbiol. Infect. Dis. 2007, 59, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.R.M.; Peiris, J.S.M.; Cryz, S.J.; O’Brien, A.D. Escherichia coli strains isolated from pigs with edema disease produce a variant of Shiga-like toxin II. FEMS Microbiol. Lett. 1987, 44, 33–38. [Google Scholar] [CrossRef]
- Schmidt, H.; Scheef, J.; Morabito, S.; Caprioli, A.; Wieler, L.H.; Karch, H. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 2000, 66, 1205–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seto, K.; Taguchi, M.; Kobayashi, K.; Kozaki, S. Biochemical and molecular characterization of minor serogroups of Shiga toxin-producing Escherichia coli isolated from humans in Osaka prefecture. J. Vet. Med. Sci. 2007, 69, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Etoh, Y.; Murakami, K.; Ichihara, S.; Sera, N.; Hamasaki, M.; Takenaka, S.; Horikawa, K.; Kawano, K.; Takeishi, T.; Kuwana, Y.; et al. Isolation of Shiga toxin 2f-producing Escherichia coli (O115:HNM) from an adult symptomatic patient in Fukuoka Prefecture, Japan. Jpn. J. Infect. Dis. 2009, 62, 315–317. [Google Scholar]
- Sonntag, A.-K.; Zenner, E.; Karch, H.; Bielaszewska, M. Pigeons as a possible reservoir of Shiga toxin 2f-producing Escherichia coli pathogenic to humans. Berl. Munch. Tierarztl. Wochenschr. 2005, 118, 464–470. [Google Scholar]
- Jenkins, C.; Willshaw, G.A.; Evans, J.; Cheasty, T.; Chart, H.; Shaw, D.J.; Dougan, G.; Frankel, G.; Smith, H.R. Subtyping of virulence genes in verocytotoxin-producing Escherichia coli (VTEC) other than serogroup O157 associated with disease in the United Kingdom. J. Med. Microbiol. 2003, 52 Pt 11, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Pierard, D.; Huyghens, L.; Lauwers, S.; Lior, H. Diarrhoea associated with Escherichia coli producing porcine oedema disease verotoxin. Lancet Lond. Engl. 1991, 338, 762. [Google Scholar] [CrossRef]
- Friesema, I.; Zwaluw, K.; van der Schuurman, T.; Kooistra-Smid, M.; Franz, E.; van Duynhoven, Y.; van Pelt, W. Emergence of Escherichia coli encoding Shiga toxin 2f in human Shiga toxin-producing E. coli (STEC) infections in the Netherlands, January 2008 to December 2011. Eurosurveillance 2014, 19. [Google Scholar] [CrossRef] [Green Version]
- De Rauw, K.; Jacobs, S.; Piérard, D. Twenty-seven years of screening for Shiga toxin-producing Escherichia coli in a university hospital. Brussels, Belgium, 1987–2014. PLoS ONE 2018, 13, e0199968. [Google Scholar] [CrossRef] [PubMed]
- Prager, R.; Fruth, A.; Siewert, U.; Strutz, U.; Tschäpe, H. Escherichia coli encoding Shiga toxin 2f as an emerging human pathogen. Int. J. Med. Microbiol. IJMM 2009, 299, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Ogden, I.D.; Hepburn, N.F.; MacRae, M.; Strachan, N.J.C.; Fenlon, D.R.; Rusbridge, S.M.; Pennington, T.H. Long-term survival of Escherichia coli O157 on pasture following an outbreak associated with sheep at a scout camp. Lett. Appl. Microbiol. 2002, 34, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Bielaszewska, M.; Janda, J.; Bláhová, K.; Minaríková, H.; Jíková, E.; Karmali, M.A.; Laubova, J.; Scikulova, J.; Preston, M.A.; Khakhria, R.; et al. Human Escherichia coli O157:H7 infection associated with the consumption of unpasteurized goat’s milk. Epidemiol. Infect. 1997, 119, 299–305. [Google Scholar] [CrossRef]
- Hoey, D.E.E.; Sharp, L.; Currie, C.; Lingwood, C.A.; Gally, D.L.; Smith, D.G.E. Verotoxin 1 binding to intestinal crypt epithelial cells results in localization to lysosomes and abrogation of toxicity. Cell Microbiol. 2003, 5, 85–97. [Google Scholar] [CrossRef]
- Ekong, P.S.; Sanderson, M.W.; Cernicchiaro, N. Prevalence and concentration of Escherichia coli O157 in different seasons and cattle types processed in North America: A systematic review and meta-analysis of published research. Prev. Vet. Med. 2015, 121, 74–85. [Google Scholar] [CrossRef]
- Bruyand, M.; Mariani-Kurkdjian, P.; Hello, S.L.; King, L.-A.; Cauteren, D.V.; Lefevre, S.; Gouali, M.; Silva, N.J.-D.; Mailles, A.; Donguy, M.-P.; et al. Paediatric haemolytic uraemic syndrome related to Shiga toxin-producing Escherichia coli, an overview of 10 years of surveillance in France, 2007 to 2016. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Elder, R.O.; Keen, J.E.; Siragusa, G.R.; Barkocy-Gallagher, G.A.; Koohmaraie, M.; Laegreid, W.W. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 2000, 97, 2999–3003. [Google Scholar] [CrossRef]
- Harris, S.M.; Yue, W.-F.; Olsen, S.A.; Hu, J.; Means, W.J.; McCormick, R.J.; Du, M.; Zhu, M.-J. Salt at concentrations relevant to meat processing enhances Shiga toxin 2 production in Escherichia coli O157:H7. Int. J. Food Microbiol. 2012, 159, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Munns, K.D.; Selinger, L.B.; Stanford, K.; Guan, L.; Callaway, T.R.; McAllister, T.A. Perspectives on super-shedding of Escherichia coli O157:H7 by cattle. Foodborne Pathog. Dis. 2015, 12, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Smith-Spangler, C.; Brandeau, M.L.; Hunter, G.E.; Bavinger, J.C.; Pearson, M.; Eschbach, P.J.; Sundaram, V.; Liu, H.; Schirmer, P.; Stave, C.; et al. Are organic foods safer or healthier than conventional alternatives: A systematic review. Ann. Intern. Med. 2012, 157, 348–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-C.; Chui, L.; Wang, Y.; Shen, J.; Jeon, B. Expansion of Shiga Toxin–Producing Escherichia coli by Use of Bovine Antibiotic Growth Promoters. Emerg. Infect. Dis. 2016, 22, 802–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, S.W.; Gally, D.L.; Low, J.C. Enterohaemorrhagic E. coli in veterinary medicine. Int. J. Med. Microbiol. IJMM 2005, 295, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Louie, M.; Read, S.; Louie, L.; Ziebell, K.; Rahn, K.; Borczyk, A.; Lior, H. Molecular typing methods to investigate transmission of Escherichia coli O157:H7 from cattle to humans. Epidemiol. Amp. Infect. 1999, 123, 17–24. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Importance of culture confirmation of shiga toxin-producing Escherichia coli infection as illustrated by outbreaks of gastroenteritis—New York and North Carolina, 2005. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 1042–1045. [Google Scholar]
- Salvadori, M.I.; Sontrop, J.M.; Garg, A.X.; Moist, L.M.; Suri, R.S.; Clark, W.F. Factors that led to the Walkerton tragedy. Kidney Int. Suppl. 2009, S33–S34. [Google Scholar] [CrossRef] [Green Version]
- Buchholz, U.; Bernard, H.; Werber, D.; Böhmer, M.M.; Remschmidt, C.; Wilking, H.; Deleré, Y.; an der Heiden, M.; Adlhoch, C.; Dreesman, J.; et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 2011, 365, 1763–1770. [Google Scholar] [CrossRef]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157:H7 Outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar] [CrossRef]
- Park, S.; Szonyi, B.; Gautam, R.; Nightingale, K.; Anciso, J.; Ivanek, R. Risk Factors for Microbial Contamination in Fruits and Vegetables at the Preharvest Level: A Systematic Review. J. Food Prot. 2012, 75, 2055–2081. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Chinen, I.; Miliwebsky, E.; Masana, M. Risk Factors for Shiga Toxin-Producing Escherichia coli-Associated Human Diseases. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Frenzen, P.D.; Drake, A.; Angulo, F.J. Emerging Infections Program FoodNet Working Group. Economic cost of illness due to Escherichia coli O157 infections in the United States. J. Food Prot. 2005, 68, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Buzby, J.C.; Roberts, T. The economics of enteric infections: Human foodborne disease costs. Gastroenterology 2009, 136, 1851–1862. [Google Scholar] [CrossRef]
- Williams, D.M.; Sreedhar, S.S.; Mickell, J.J.; Chan, J.C.M. Acute kidney failure: A pediatric experience over 20 years. Arch. Pediatr. Adolesc. Med. 2002, 156, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, C.M. Shiga toxin-producing Escherichia coli infection. Clin. Infect. Dis. 2004, 38, 1298–1303. [Google Scholar] [CrossRef] [Green Version]
- Rivas, M.; Miliwebsky, E.; Chinen, I.; Roldán, C.D.; Balbi, L.; García, B.; Fiorilli, G.; Sosa-Estani, S.; Kincaid, J.; Rangel, J.; et al. Characterization and epidemiologic subtyping of Shiga toxin-producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodborne Pathog. Dis. 2006, 3, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Elliott, E.J.; Robins-Browne, R.M.; O’Loughlin, E.V.; Bennett-Wood, V.; Bourke, J.; Henning, P.; Hogg, G.G.; Knight, J.; Powell, H.; Redmond, D.; et al. Nationwide study of haemolytic uraemic syndrome: Clinical, microbiological, and epidemiological features. Arch. Dis. Child. 2001, 85, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Vally, H.; Hall, G.; Dyda, A.; Raupach, J.; Knope, K.; Combs, B.; Desmarchelier, P. Epidemiology of Shiga toxin producing Escherichia coli in Australia, 2000–2010. BMC Public Health 2012, 12, 63. [Google Scholar] [CrossRef] [Green Version]
- Karmali, M.A.; Mascarenhas, M.; Petric, M.; Dutil, L.; Rahn, K.; Ludwig, K.; Arbus, G.S.; Michel, P.; Sherman, P.M.; Wilson, J.; et al. Age-Specific Frequencies of Antibodies to Escherichia coli Verocytotoxins (Shiga Toxins) 1 and 2 among Urban and Rural Populations in Southern Ontario. J. Infect. Dis. 2003, 188, 1724–1729. [Google Scholar] [CrossRef] [Green Version]
- Kistemann, T.; Zimmer, S.; Vågsholm, I.; Andersson, Y. GIS-supported investigation of human EHEC and cattle VTEC O157 infections in Sweden: Geographical distribution, spatial variation and possible risk factors. Epidemiol. Infect. 2004, 132, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.M.; Tauxe, R.V. The Epidemiology of Infections Caused by Escherichia coli O157: H7, Other Enterohemorrhagic, E. coli, and the Associated Hemolytic Uremic Syndrome. Epidemiol. Rev. 1991, 13, 60–98. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.M.; Scheutz, F.; Torpdahl, M. Continuous Surveillance of Shiga Toxin–Producing Escherichia coli Infections by Pulsed-Field Gel Electrophoresis Shows That Most Infections Are Sporadic. Foodborne Pathog. Dis. 2006, 3, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Grisaru, S.; Midgley, J.P.; Hamiwka, L.A.; Wade, A.W.; Samuel, S.M. Diarrhea-associated hemolytic uremic syndrome in southern Alberta: A long-term single-centre experience. Paediatr. Child Health 2011, 16, 337–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, S.D.; Motiwala, A.S.; Springman, A.C.; Qi, W.; Lacher, D.W.; Ouellette, L.M.; Mladonicky, J.M.; Somsel, P.; Rudrik, J.T.; Dietrich, S.E.; et al. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc. Natl. Acad. Sci. USA 2008, 105, 4868–4873. [Google Scholar] [CrossRef] [Green Version]
- Dundas, S.; Todd, W.T.; Stewart, A.I.; Murdoch, P.S.; Chaudhuri, A.K.; Hutchinson, S.J. The central Scotland Escherichia coli O157:H7 outbreak: Risk factors for the hemolytic uremic syndrome and death among hospitalized patients. Clin. Infect. Dis. 2001, 33, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Gould, L.H.; Demma, L.; Jones, T.F.; Hurd, S.; Vugia, D.J.; Smith, K.; Shiferaw, B.; Segler, S.; Palmer, A.; Zansky, S.; et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000–2006. Clin. Infect. Dis. 2009, 49, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Whitney, B.M.; Mainero, C.; Humes, E.; Hurd, S.; Niccolai, L.; Hadler, J.L. Socioeconomic Status and Foodborne Pathogens in Connecticut, USA, 2000–2011(1). Emerg. Infect. Dis. 2015, 21, 1617–1624. [Google Scholar] [CrossRef] [Green Version]
- Newburg, D.S.; Chaturvedi, P.; Lopez, E.L.; Devoto, S.; Fayad, A.; Cleary, T.G. Susceptibility to hemolytic-uremic syndrome relates to erythrocyte glycosphingolipid patterns. J. Infect. Dis. 1993, 168, 476–479. [Google Scholar] [CrossRef]
- Watarai, S.; Yokota, K.; Kishimoto, T.; Kanadani, T.; Taketa, K.; Oguma, K. Relationship between susceptibility to hemolytic-uremic syndrome and levels of globotriaosylceramide in human sera. J. Clin. Microbiol. 2001, 39, 798–800. [Google Scholar] [CrossRef] [Green Version]
- Taranta, A.; Gianviti, A.; Palma, A.; De Luca, V.; Mannucci, L.; Procaccino, M.A.; Ghiggeri, G.M.; Caridi, G.; Fruci, D.; Ferracuti, S.; et al. Genetic risk factors in typical haemolytic uraemic syndrome. Nephrol. Dial. Transplant. 2009, 24, 1851–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, Y.; Numata, S.; Nakamura, Y.; Honda, T.; Furukawa, K.; Urano, T.; Wiels, J.; Uchikawa, M.; Ozaki, N.; Matsuo, S.; et al. Murine glycosyltransferases responsible for the expression of globo-series glycolipids: cDNA structures, mRNA expression, and distribution of their products. Glycobiology 2005, 15, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Argyle, J.C.; Hogg, R.J.; Pysher, T.J.; Silva, F.G.; Siegler, R.L. A clinicopathological study of 24 children with hemolytic uremic syndrome. Pediatr. Nephrol. 1990, 4, 52–58. [Google Scholar] [CrossRef]
- Inward, C.D.; Howie, A.J.; Fitzpatrick, M.M.; Rafaat, F.; Milford, D.V.; Taylor, C.M. Renal histopathology in fatal cases of diarrhoea-associated haemolytic uraemic syndrome. Pediatr. Nephrol. 1997, 11, 556–559. [Google Scholar] [CrossRef]
- Keepers, T.R.; Psotka, M.A.; Gross, L.K.; Obrig, T.G. A Murine Model of HUS: Shiga Toxin with Lipopolysaccharide Mimics the Renal Damage and Physiologic Response of Human Disease. J. Am. Soc. Nephrol. 2006, 17, 3404–3414. [Google Scholar] [CrossRef] [Green Version]
- Keepers, T.R.; Gross, L.K.; Obrig, T.G. Monocyte Chemoattractant Protein 1, Macrophage Inflammatory Protein 1α, and RANTES Recruit Macrophages to the Kidney in a Mouse Model of Hemolytic-Uremic Syndrome. Infect. Immun. 2007, 75, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Roche, J.K.; Keepers, T.R.; Gross, L.K.; Seaner, R.M.; Obrig, T.G. CXCL1/KC and CXCL2/MIP-2 Are Critical Effectors and Potential Targets for Therapy of Escherichia coli O157:H7-Associated Renal Inflammation. Am. J. Pathol. 2007, 170, 526–537. [Google Scholar] [CrossRef] [Green Version]
- Mayer, C.L.; Leibowitz, C.S.; Kurosawa, S.; Stearns-Kurosawa, D.J. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins 2012, 4, 1261–1287. [Google Scholar] [CrossRef] [Green Version]
- Mohawk, K.L.; O’Brien, A.D. Mouse models of Escherichia coli O157:H7 infection and shiga toxin injection. J. Biomed. Biotechnol. 2011, 2011, 258185. [Google Scholar] [CrossRef] [Green Version]
- Proulx, F.; Seidman, E.; Mariscalco, M.M.; Lee, K.; Caroll, S. Increased Circulating Levels of Lipopolysaccharide Binding Protein in Children with Escherichia coli O157:H7 Hemorrhagic Colitis and Hemolytic Uremic Syndrome. Clin. Diagn. Lab. Immunol. 1999, 6, 773. [Google Scholar] [CrossRef] [Green Version]
- Koster, F.; Levin, J.; Walker, L.; Tung, K.S.; Gilman, R.H.; Rahaman, M.M.; Majid, M.A.; Islam, S.; Williams, J.R. Hemolytic-uremic syndrome after shigellosis. Relation to endotoxemia and circulating immune complexes. N. Engl. J. Med. 1978, 298, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Dennhardt, S.; Pirschel, W.; Wissuwa, B.; Daniel, C.; Gunzer, F.; Lindig, S.; Medyukhina, A.; Kiehntopf, M.; Rudolph, W.W.; Zipfel, P.F.; et al. Modeling Hemolytic-Uremic Syndrome: In-Depth Characterization of Distinct Murine Models Reflecting Different Features of Human Disease. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hews, C.L.; Tran, S.-L.; Wegmann, U.; Brett, B.; Walsham, A.D.S.; Kavanaugh, D.; Ward, N.J.; Juge, N.; Schüller, S. The StcE metalloprotease of enterohaemorrhagic Escherichia coli reduces the inner mucus layer and promotes adherence to human colonic epithelium ex vivo. Cell. Microbiol. 2017, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDaniel, T.K.; Jarvis, K.G.; Donnenberg, M.S.; Kaper, J.B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 1995, 92, 1664–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdem, A.L.; Avelino, F.; Xicohtencatl-Cortes, J.; Girón, J.A. Host Protein Binding and Adhesive Properties of H6 and H7 Flagella of Attaching and Effacing Escherichia coli. J. Bacteriol. 2007, 189, 7426–7435. [Google Scholar] [CrossRef] [Green Version]
- Xicohtencatl-Cortes, J.; Monteiro-Neto, V.; Ledesma, M.A.; Jordan, D.M.; Francetic, O.; Kaper, J.B.; Puente, J.L.; Girón, J.A. Intestinal adherence associated with type IV pili of enterohemorrhagic Escherichia coli O157:H7. J. Clin. Investig. 2007, 117, 3519–3529. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.M.; Sinclair, J.F.; Smith, M.J.; O’Brien, A.D. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. USA 2006, 103, 9667–9672. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.M.; Ladwein, M.; Schmidt, D.; Ehinger, J.; Lommel, S.; Städing, K.; Beutling, U.; Disanza, A.; Frank, R.; Jänsch, L.; et al. IRSp53 links the enterohemorrhagic E. coli effectors Tir and EspFU for actin pedestal formation. Cell Host Microbe 2009, 5, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Garmendia, J.; Phillips, A.D.; Carlier, M.-F.; Chong, Y.; Schüller, S.; Marches, O.; Dahan, S.; Oswald, E.; Shaw, R.K.; Knutton, S.; et al. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell Microbiol. 2004, 6, 1167–1183. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Skehan, B.M.; Campellone, K.G.; Leong, J.M.; Rosen, M.K. Structural mechanism of WASP activation by the enterohaemorrhagic E. coli effector EspF(U). Nature 2008, 454, 1009–1013. [Google Scholar] [CrossRef] [Green Version]
- Kovbasnjuk, O.; Mourtazina, R.; Baibakov, B.; Wang, T.; Elowsky, C.; Choti, M.A.; Kane, A.; Donowitz, M. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 19087–19092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schüller, S.; Heuschkel, R.; Torrente, F.; Kaper, J.B.; Phillips, A.D. Shiga toxin binding in normal and inflamed human intestinal mucosa. Microbes Infect. 2007, 9, 35–39. [Google Scholar] [CrossRef]
- Malyukova, I.; Murray, K.F.; Zhu, C.; Boedeker, E.; Kane, A.; Patterson, K.; Peterson, J.R.; Donowitz, M.; Kovbasnjuk, O. Macropinocytosis in Shiga toxin 1 uptake by human intestinal epithelial cells and transcellular transcytosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G78–G92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schüller, S. Shiga Toxin Interaction with Human Intestinal Epithelium. Toxins 2011, 3, 626–639. [Google Scholar] [CrossRef] [Green Version]
- Brigotti, M. The Interactions of Human Neutrophils with Shiga Toxins and Related Plant Toxins: Danger or Safety? Toxins 2012, 4, 157–190. [Google Scholar] [CrossRef] [Green Version]
- Te Loo, D.M.; van Hinsbergh, V.W.; van den Heuvel, L.P.; Monnens, L.A. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J. Am. Soc. Nephrol. JASN 2001, 12, 800–806. [Google Scholar]
- Brigotti, M.; Caprioli, A.; Tozzi, A.E.; Tazzari, P.L.; Ricci, F.; Conte, R.; Carnicelli, D.; Procaccino, M.A.; Minelli, F.; Ferretti, A.V.S.; et al. Shiga toxins present in the gut and in the polymorphonuclear leukocytes circulating in the blood of children with hemolytic-uremic syndrome. J. Clin. Microbiol. 2006, 44, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Geelen, J.M.; van der Velden, T.J.A.M.; Te Loo, D.M.W.M.; Boerman, O.C.; van den Heuvel, L.P.W.J.; Monnens, L.A.H. Lack of specific binding of Shiga-like toxin (verocytotoxin) and non-specific interaction of Shiga-like toxin 2 antibody with human polymorphonuclear leucocytes. Nephrol. Dial. Transplant. 2007, 22, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Flagler, M.J.; Strasser, J.E.; Chalk, C.L.; Weiss, A.A. Comparative analysis of the abilities of Shiga toxins 1 and 2 to bind to and influence neutrophil apoptosis. Infect. Immun. 2007, 75, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Brigotti, M.; Carnicelli, D.; Ravanelli, E.; Barbieri, S.; Ricci, F.; Bontadini, A.; Tozzi, A.E.; Scavia, G.; Caprioli, A.; Tazzari, P.L.; et al. Interactions between Shiga toxins and human polymorphonuclear leukocytes. J. Leukoc. Biol. 2008, 84, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.E.; Rotman, T.A.; Jay, V.; Smith, C.R.; Becker, L.E.; Petric, M.; Olivieri, N.F.; Karmali, M.A. Experimental verocytotoxemia in rabbits. Infect. Immun. 1992, 60, 4154–4167. [Google Scholar] [CrossRef] [Green Version]
- Brigotti, M.; Tazzari, P.L.; Ravanelli, E.; Carnicelli, D.; Rocchi, L.; Arfilli, V.; Scavia, G.; Minelli, F.; Ricci, F.; Pagliaro, P.; et al. Clinical relevance of shiga toxin concentrations in the blood of patients with hemolytic uremic syndrome. Pediatr. Infect. Dis. J. 2011, 30, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Obrig, T.G.; Louise, C.B.; Lingwood, C.A.; Boyd, B.; Barley-Maloney, L.; Daniel, T.O. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 1993, 268, 15484–15488. [Google Scholar] [PubMed]
- Khan, F.; Proulx, F.; Lingwood, C.A. Detergent-resistant globotriaosyl ceramide may define verotoxin/glomeruli-restricted hemolytic uremic syndrome pathology. Kidney Int. 2009, 75, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Cooling, L.L.W.; Walker, K.E.; Gille, T.; Koerner, T.A.W. Shiga Toxin Binds Human Platelets via Globotriaosylceramide (Pk Antigen) and a Novel Platelet Glycosphingolipid. Infect. Immun. 1998, 66, 4355–4366. [Google Scholar] [PubMed]
- Mangeney, M.; Richard, Y.; Coulaud, D.; Tursz, T.; Wiels, J. CD77: An antigen of germinal center B cells entering apoptosis. Eur. J. Immunol. 1991, 21, 1131–1140. [Google Scholar] [CrossRef]
- Obata, F.; Tohyama, K.; Bonev, A.D.; Kolling, G.L.; Keepers, T.R.; Gross, L.K.; Nelson, M.T.; Sato, S.; Obrig, T.G. Shiga Toxin 2 Affects the Central Nervous System through Receptor Globotriaosylceramide Localized to Neurons. J. Infect. Dis. 2008, 198, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Tokuda, N.; Numata, S.; Ito, M.; Ohta, M.; Kawamura, K.; Wiels, J.; Urano, T.; Tajima, O.; Furukawa, K.; et al. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J. Biol. Chem. 2006, 281, 10230–10235. [Google Scholar] [CrossRef] [Green Version]
- Römer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windschiegl, B.; Tenza, D.; Aly, M.R.E.; Fraisier, V.; Florent, J.-C.; Perrais, D.; et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef]
- Müthing, J.; Schweppe, C.H.; Karch, H.; Friedrich, A.W. Shiga toxins, glycosphingolipid diversity, and endothelial cell injury. Thromb. Haemost. 2009, 101, 252–264. [Google Scholar]
- Torgersen, M.L.; Lauvrak, S.U.; Sandvig, K. The A-subunit of surface-bound Shiga toxin stimulates clathrin-dependent uptake of the toxin. FEBS J. 2005, 272, 4103–4113. [Google Scholar] [CrossRef] [PubMed]
- Villysson, A.; Tontanahal, A.; Karpman, D. Microvesicle Involvement in Shiga Toxin-Associated Infection. Toxins 2017, 9, 376. [Google Scholar] [CrossRef] [Green Version]
- Johannes, L.; Parton, R.G.; Bassereau, P.; Mayor, S. Building endocytic pits without clathrin. Nat. Rev. Mol. Cell Biol. 2015, 16, 311–321. [Google Scholar] [CrossRef]
- Sandvig, K.; Garred, O.; Prydz, K.; Kozlov, J.V.; Hansen, S.H.; van Deurs, B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 1992, 358, 510–512. [Google Scholar] [CrossRef]
- Garred, O.; Dubinina, E.; Polesskaya, A.; Olsnes, S.; Kozlov, J.; Sandvig, K. Role of the disulfide bond in Shiga toxin A-chain for toxin entry into cells. J. Biol. Chem. 1997, 272, 11414–11419. [Google Scholar] [CrossRef] [Green Version]
- Endo, Y.; Tsurugi, K.; Yutsudo, T.; Takeda, Y.; Ogasawara, T.; Igarashi, K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 1988, 171, 45–50. [Google Scholar] [CrossRef]
- Kavaliauskiene, S.; Dyve Lingelem, A.B.; Skotland, T.; Sandvig, K. Protection against Shiga Toxins. Toxins 2017, 9, 44. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Linstedt, A.D. Manganese Blocks Intracellular Trafficking of Shiga Toxin and Protects Against Shiga Toxicosis. Science 2012, 335, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Cherla, R.P.; Lee, S.Y.; Mees, P.L.; Tesh, V.L. Shiga toxin 1-induced cytokine production is mediated by MAP kinase pathways and translation initiation factor eIF4E in the macrophage-like THP-1 cell line. J. Leukoc. Biol. 2006, 79, 397–407. [Google Scholar] [CrossRef]
- Jandhyala, D.M.; Ahluwalia, A.; Schimmel, J.J.; Rogers, A.B.; Leong, J.M.; Thorpe, C.M. Activation of the Classical Mitogen-Activated Protein Kinases Is Part of the Shiga Toxin-Induced Ribotoxic Stress Response and Contribute to Shiga Toxin-Induced Inflammation. Infect. Immun. 2016, 84, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Tesh, V.L. Activation of cell stress response pathways by Shiga toxins. Cell Microbiol. 2011, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Van Setten, P.A.; Monnens, L.A.; Verstraten, R.G.; van den Heuvel, L.P.; van Hinsbergh, V.W. Effects of verocytotoxin-1 on nonadherent human monocytes: Binding characteristics, protein synthesis, and induction of cytokine release. Blood 1996, 88, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Ramegowda, B.; Tesh, V.L. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 1996, 64, 1173–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falguières, T.; Mallard, F.; Baron, C.; Hanau, D.; Lingwood, C.; Goud, B.; Salamero, J.; Johannes, L. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 2001, 12, 2453–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, P.B.; Chaturvedi, P.; Fine, R.E.; Ritchie, A.J.; Pober, J.S.; Cleary, T.G.; Newburg, D.S. Tumor necrosis factor alpha increases human cerebral endothelial cell Gb3 and sensitivity to Shiga toxin. Infect. Immun. 2001, 69, 1889–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stricklett, P.K.; Hughes, A.K.; Kohan, D.E. Inhibition of p38 mitogen-activated protein kinase ameliorates cytokine up-regulated shigatoxin-1 toxicity in human brain microvascular endothelial cells. J. Infect. Dis. 2005, 191, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.K.; Kolling, G.L.; Lindner, M.H.; Obrig, T.G. p38 mitogen-activated protein kinase mediates lipopolysaccharide and tumor necrosis factor alpha induction of shiga toxin 2 sensitivity in human umbilical vein endothelial cells. Infect. Immun. 2008, 76, 1115–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matussek, A.; Lauber, J.; Bergau, A.; Hansen, W.; Rohde, M.; Dittmar, K.E.J.; Gunzer, M.; Mengel, M.; Gatzlaff, P.; Hartmann, M.; et al. Molecular and functional analysis of Shiga toxin–induced response patterns in human vascular endothelial cells. Blood 2003, 102, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Koo, S.; Jeong, D.G.; Tesh, V.L. Shiga Toxins as Multi-Functional Proteins: Induction of Host Cellular Stress Responses, Role in Pathogenesis and Therapeutic Applications. Toxins 2016, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Gobert, A.P.; Vareille, M.; Glasser, A.-L.; Hindré, T.; de Sablet, T.; Martin, C. Shiga toxin produced by enterohemorrhagic Escherichia coli inhibits PI3K/NF-kappaB signaling pathway in globotriaosylceramide-3-negative human intestinal epithelial cells. J. Immunol. 2007, 178, 8168–8174. [Google Scholar]
- Sellier-Leclerc, A.-L.; Fremeaux-Bacchi, V.; Dragon-Durey, M.-A.; Macher, M.-A.; Niaudet, P.; Guest, G.; Boudailliez, B.; Bouissou, F.; Deschenes, G.; Gie, S.; et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. JASN 2007, 18, 2392–2400. [Google Scholar] [CrossRef]
- Noris, M.; Caprioli, J.; Bresin, E.; Mossali, C.; Pianetti, G.; Gamba, S.; Daina, E.; Fenili, C.; Castelletti, F.; Sorosina, A.; et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 1844–1859. [Google Scholar] [CrossRef]
- Frémeaux-Bacchi, V.; Miller, E.C.; Liszewski, M.K.; Strain, L.; Blouin, J.; Brown, A.L.; Moghal, N.; Kaplan, B.S.; Weiss, R.A.; Lhotta, K.; et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 2008, 112, 4948–4952. [Google Scholar] [CrossRef] [Green Version]
- Fremeaux-Bacchi, V.; Dragon-Durey, M.-A.; Blouin, J.; Vigneau, C.; Kuypers, D.; Boudailliez, B.; Loirat, C.; Rondeau, E.; Fridman, W.H. Complement factor I: A susceptibility gene for atypical haemolytic uraemic syndrome. J. Med. Genet. 2004, 41, e84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jorge, E.G.; Harris, C.L.; Esparza-Gordillo, J.; Carreras, L.; Arranz, E.A.; Garrido, C.A.; Lopez-Trascasa, M.; Sanchez-Corral, P.; Morgan, B.P.; de Cordoba, S.R.; et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragon-Durey, M.-A.; Loirat, C.; Cloarec, S.; Macher, M.-A.; Blouin, J.; Nivet, H.; Weiss, L.; Fridman, W.H.; Frémeaux-Bacchi, V. Anti–Factor H Autoantibodies Associated with Atypical Hemolytic Uremic Syndrome. J. Am. Soc. Nephrol. 2005, 16, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Noris, M.; Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009, 361, 1676–1687. [Google Scholar] [CrossRef]
- Fakhouri, F.; Zuber, J.; Frémeaux-Bacchi, V.; Loirat, C. Haemolytic uraemic syndrome. Lancet Lond. Engl. 2017, 390, 681–696. [Google Scholar] [CrossRef]
- Legendre, C.M.; Licht, C.; Muus, P.; Greenbaum, L.A.; Babu, S.; Bedrosian, C.; Bingham, C.; Cohen, D.J.; Delmas, Y.; Douglas, K.; et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2013, 368, 2169–2181. [Google Scholar] [CrossRef] [Green Version]
- Licht, C.; Greenbaum, L.A.; Muus, P.; Babu, S.; Bedrosian, C.L.; Cohen, D.J.; Delmas, Y.; Douglas, K.; Furman, R.R.; Gaber, O.A.; et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015, 87, 1061–1073. [Google Scholar] [CrossRef] [Green Version]
- Fakhouri, F.; Hourmant, M.; Campistol, J.M.; Cataland, S.R.; Espinosa, M.; Gaber, A.O.; Menne, J.; Minetti, E.E.; Provôt, F.; Rondeau, E.; et al. Terminal Complement Inhibitor Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome: A Single-Arm, Open-Label Trial. Am. J. Kidney Dis. 2016, 68, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Noris, M.; Mescia, F.; Remuzzi, G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 2012, 8, 622–633. [Google Scholar] [CrossRef]
- Thurman, J.M.; Marians, R.; Emlen, W.; Wood, S.; Smith, C.; Akana, H.; Holers, V.M.; Lesser, M.; Kline, M.; Hoffman, C.; et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 1920–1924. [Google Scholar] [CrossRef] [Green Version]
- Ståhl, A.; Sartz, L.; Karpman, D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood 2011, 117, 5503–5513. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Hertel, B.; Emden, S.H.; Beneke, J.; Menne, J.; Haller, H.; von Vietinghoff, S. Microparticle generation and leucocyte death in Shiga toxin-mediated HUS. Nephrol. Dial. Transplant. 2012, 27, 2768–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orth, D.; Khan, A.B.; Naim, A.; Grif, K.; Brockmeyer, J.; Karch, H.; Joannidis, M.; Clark, S.J.; Day, A.J.; Fidanzi, S.; et al. Shiga toxin activates complement and binds factor H: Evidence for an active role of complement in hemolytic uremic syndrome. J. Immunol. 2009, 182, 6394–6400. [Google Scholar] [CrossRef] [Green Version]
- Poolpol, K.; Orth-Höller, D.; Speth, C.; Zipfel, P.F.; Skerka, C.; de Córdoba, S.R.; Brockmeyer, J.; Bielaszewska, M.; Würzner, R. Interaction of Shiga toxin 2 with complement regulators of the factor H protein family. Mol. Immunol. 2014, 58, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Galbusera, M.; Gastoldi, S.; Locatelli, M.; Buelli, S.; Pezzotta, A.; Pagani, C.; Noris, M.; Gobbi, M.; Stravalaci, M.; et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J. Immunol. 2011, 187, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Westra, D.; Volokhina, E.B.; van der Molen, R.G.; van der Velden, T.J.A.M.; Jeronimus-Klaasen, A.; Goertz, J.; Gracchi, V.; Dorresteijn, E.M.; Bouts, A.H.M.; Keijzer-Veen, M.G.; et al. Serological and genetic complement alterations in infection-induced and complement-mediated hemolytic uremic syndrome. Pediatr. Nephrol. 2017, 32, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.C.; Mayer, C.L.; Leibowitz, C.S.; Stearns-Kurosawa, D.J.; Kurosawa, S. Quiescent complement in nonhuman primates during E coli Shiga toxin-induced hemolytic uremic syndrome and thrombotic microangiopathy. Blood 2013, 122, 803–806. [Google Scholar] [CrossRef] [Green Version]
- Porubsky, S.; Federico, G.; Müthing, J.; Jennemann, R.; Gretz, N.; Büttner, S.; Obermüller, N.; Jung, O.; Hauser, I.A.; Gröne, E.; et al. Direct acute tubular damage contributes to Shigatoxin-mediated kidney failure. J. Pathol. 2014, 234, 120–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoja, C.; Locatelli, M.; Pagani, C.; Corna, D.; Zanchi, C.; Isermann, B.; Remuzzi, G.; Conway, E.M.; Noris, M. Lack of the lectin-like domain of thrombomodulin worsens Shiga toxin-associated hemolytic uremic syndrome in mice. J. Immunol. 2012, 189, 3661–3668. [Google Scholar] [CrossRef] [Green Version]
- Delvaeye, M.; Noris, M.; De Vriese, A.; Esmon, C.T.; Esmon, N.L.; Ferrell, G.; Del-Favero, J.; Plaisance, S.; Claes, B.; Lambrechts, D.; et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009, 361, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; Francois, B.; Zabolotskikh, I.; Daga, M.K.; Lascarrou, J.B.; Kirov, M.Y.; Pettilä, V.; Wittebole, X.; Meziani, F.; Mercier, E.; et al. Effect of a Recombinant Human Soluble Thrombomodulin on Mortality in Patients With Sepsis-Associated Coagulopathy: The SCARLET Randomized Clinical Trial. JAMA 2019, 321, 1993–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, T.; Ogata, S.; Mineo, E.; Nagamori, Y.; Nakamura, S.; Bando, Y.; Ishii, M. A novel strategy for hemolytic uremic syndrome: Successful treatment with thrombomodulin α. Pediatrics 2013, 131, e928–e933. [Google Scholar] [CrossRef]
- Hughes, A.K.; Ergonul, Z.; Stricklett, P.K.; Kohan, D.E. Molecular Basis for High Renal Cell Sensitivity to the Cytotoxic Effects of Shigatoxin-1: Upregulation of Globotriaosylceramide Expression. J. Am. Soc. Nephrol. 2002, 13, 2239–2245. [Google Scholar] [CrossRef] [Green Version]
- Zoja, C.; Buelli, S.; Morigi, M. Shiga toxin-associated hemolytic uremic syndrome: Pathophysiology of endothelial dysfunction. Pediatr. Nephrol. 2010, 25, 2231–2240. [Google Scholar] [CrossRef]
- Petruzziello-Pellegrini, T.N.; Moslemi-Naeini, M.; Marsden, P.A. New insights into Shiga toxin-mediated endothelial dysfunction in hemolytic uremic syndrome. Virulence 2013, 4, 556–563. [Google Scholar] [CrossRef] [Green Version]
- Chandler, W.L.; Jelacic, S.; Boster, D.R.; Ciol, M.A.; Williams, G.D.; Watkins, S.L.; Igarashi, T.; Tarr, P.I. Prothrombotic coagulation abnormalities preceding the hemolytic-uremic syndrome. N. Engl. J. Med. 2002, 346, 23–32. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Nakagawa, T.; Johnson, R.J.; Thurman, J.M. The role of endothelial cell injury in thrombotic microangiopathy. Am. J. Kidney Dis. 2010, 56, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Petruzziello-Pellegrini, T.N.; Yuen, D.A.; Page, A.V.; Patel, S.; Soltyk, A.M.; Matouk, C.C.; Wong, D.K.; Turgeon, P.J.; Fish, J.E.; Ho, J.J.D.; et al. The CXCR4/CXCR7/SDF-1 pathway contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome in humans and mice. J. Clin. Investig. 2012, 122, 759–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestoridi, E.; Tsukurov, O.; Kushak, R.I.; Ingelfinger, J.R.; Grabowski, E.F. Shiga toxin enhances functional tissue factor on human glomerular endothelial cells: Implications for the pathophysiology of hemolytic uremic syndrome*. J. Thromb. Haemost. 2005, 3, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Haberichter, S.L.; Sadler, J.E. The B subunits of Shiga-like toxins induce regulated VWF secretion in a phospholipase D1-dependent manner. Blood 2012, 120, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Huang, J.; Sadler, J.E. Shiga toxin (Stx)1B and Stx2B induce von Willebrand factor secretion from human umbilical vein endothelial cells through different signaling pathways. Blood 2011, 118, 3392–3398. [Google Scholar] [CrossRef] [Green Version]
- Karpman, D.; Papadopoulou, D.; Nilsson, K.; Sjögren, A.C.; Mikaelsson, C.; Lethagen, S. Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood 2001, 97, 3100–3108. [Google Scholar] [CrossRef] [Green Version]
- Morigi, M.; Micheletti, G.; Figliuzzi, M.; Imberti, B.; Karmali, M.A.; Remuzzi, A.; Remuzzi, G.; Zoja, C. Verotoxin-1 promotes leukocyte adhesion to cultured endothelial cells under physiologic flow conditions. Blood 1995, 86, 4553–4558. [Google Scholar] [CrossRef] [Green Version]
- Zoja, C.; Angioletti, S.; Donadelli, R.; Zanchi, C.; Tomasoni, S.; Binda, E.; Imberti, B.; te Loo, M.; Monnens, L.; Remuzzi, G.; et al. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 2002, 62, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Pijpers, A.H.; van Setten, P.A.; van den Heuvel, L.P.; Assmann, K.J.; Dijkman, H.B.; Pennings, A.H.; Monnens, L.A.; van Hinsbergh, V.W. Verocytotoxin-induced apoptosis of human microvascular endothelial cells. J. Am. Soc. Nephrol. JASN 2001, 12, 767–778. [Google Scholar]
- Karpman, D.; Håkansson, A.; Perez, M.T.; Isaksson, C.; Carlemalm, E.; Caprioli, A.; Svanborg, C. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: In vivo and in vitro studies. Infect. Immun. 1998, 66, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Owens, A.P.; Mackman, N. Tissue factor and thrombosis: The clot starts here. Thromb. Haemost. 2010, 104, 432–439. [Google Scholar] [CrossRef]
- Arvidsson, I.; Ståhl, A.-L.; Hedström, M.M.; Kristoffersson, A.-C.; Rylander, C.; Westman, J.S.; Storry, J.R.; Olsson, M.L.; Karpman, D. Shiga toxin-induced complement-mediated hemolysis and release of complement-coated red blood cell-derived microvesicles in hemolytic uremic syndrome. J. Immunol. 2015, 194, 2309–2318. [Google Scholar] [CrossRef] [Green Version]
- Clogher, P.; Hurd, S.; Hoefer, D.; Hadler, J.L.; Pasutti, L.; Cosgrove, S.; Segler, S.; Tobin-D’Angelo, M.; Nicholson, C.; Booth, H.; et al. Assessment of physician knowledge and practices concerning Shiga toxin-producing Escherichia coli infection and enteric illness, 2009, Foodborne Diseases Active Surveillance Network (FoodNet). Clin. Infect. Dis. 2012, 54 (Suppl. 5), S446–S452. [Google Scholar] [CrossRef] [Green Version]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Reiss, G.; Kunz, P.; Koin, D.; Keeffe, E.B. Escherichia coli O157:H7 infection in nursing homes: Review of literature and report of recent outbreak. J. Am. Geriatr. Soc. 2006, 54, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.O.; Borczyk, A.A.; Carlson, J.A.; Harvey, B.; Hockin, J.C.; Karmali, M.A.; Krishnan, C.; Korn, D.A.; Lior, H. A severe outbreak of Escherichia coli O157:H7--associated hemorrhagic colitis in a nursing home. N. Engl. J. Med. 1987, 317, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- McDonough, M.A.; Butterton, J.R. Spontaneous tandem amplification and deletion of the Shiga toxin operon in Shigella dysenteriae 1. Mol. Microbiol. 1999, 34, 1058–1069. [Google Scholar] [CrossRef]
- Mody, R.K.; Gu, W.; Griffin, P.M.; Jones, T.F.; Rounds, J.; Shiferaw, B.; Tobin-D’Angelo, M.; Smith, G.; Spina, N.; Hurd, S.; et al. Postdiarrheal hemolytic uremic syndrome in United States children: Clinical spectrum and predictors of in-hospital death. J. Pediatr. 2015, 166, 1022–1029. [Google Scholar] [CrossRef]
- Gould, L.H. Update: Recommendations for Diagnosis of Shiga Toxin-Producing Escherichia coli Infections by Clinical Laboratories. Clin. Microbiol. Newsl. 2012, 34, 75–83. [Google Scholar] [CrossRef]
- Mody, R.K.; Luna-Gierke, R.E.; Jones, T.F.; Comstock, N.; Hurd, S.; Scheftel, J.; Lathrop, S.; Smith, G.; Palmer, A.; Strockbine, N.; et al. Infections in pediatric postdiarrheal hemolytic uremic syndrome: Factors associated with identifying shiga toxin-producing Escherichia coli. Arch. Pediatr. Adolesc. Med. 2012, 166, 902–909. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.S.; Mooney, J.C.; Brandt, J.R.; Staples, A.O.; Jelacic, S.; Boster, D.R.; Watkins, S.L.; Tarr, P.I. Risk Factors for the Hemolytic Uremic Syndrome in Children Infected With Escherichia coli O157:H7: A Multivariable Analysis. Clin. Infect. Dis. 2012, 55, 33–41. [Google Scholar] [CrossRef] [Green Version]
- King, L.A.; Nogareda, F.; Weill, F.-X.; Mariani-Kurkdjian, P.; Loukiadis, E.; Gault, G.; Jourdan-DaSilva, N.; Bingen, E.; Macé, M.; Thevenot, D.; et al. Outbreak of Shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin. Infect. Dis. 2012, 54, 1588–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, A.W.; Ratcliff, R.M.; Doyle, R.M.; Seymour-Murray, J.; Davos, D.; Lanser, J.A.; Paton, J.C. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J. Clin. Microbiol. 1996, 34, 1622–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, J.; Gomez, T.; Doyle, M.P.; Wells, J.G.; Zhao, T.; Tauxe, R.V.; Griffin, P.M. Lessons from a large outbreak of Escherichia coli O157:H7 infections: Insights into the infectious dose and method of widespread contamination of hamburger patties. Epidemiol. Infect. 1999, 122, 185–192. [Google Scholar] [CrossRef]
- Keene, W.E.; McAnulty, J.M.; Hoesly, F.C.; Williams, L.P.; Hedberg, K.; Oxman, G.L.; Barrett, T.J.; Pfaller, M.A.; Fleming, D.W. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7 and Shigella sonnei. N. Engl. J. Med. 1994, 331, 579–584. [Google Scholar] [CrossRef]
- Fukushima, H.; Hashizume, T.; Morita, Y.; Tanaka, J.; Azuma, K.; Mizumoto, Y.; Kaneno, M.; Matsuura, M.; Konma, K.; Kitani, T. Clinical experiences in Sakai City Hospital during the massive outbreak of enterohemorrhagic Escherichia coli O157 infections in Sakai City, 1996. Pediatr. Int. 1999, 41, 213–217. [Google Scholar] [CrossRef]
- Tarr, P.I. Shiga toxin-associated hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: Distinct mechanisms of pathogenesis. Kidney Int. 2009, 75, S29–S32. [Google Scholar] [CrossRef] [Green Version]
- Rahman, R.C.; Cobeñas, C.J.; Drut, R.; Amoreo, O.R.; Ruscasso, J.D.; Spizzirri, A.P.; Suarez, A.D.C.; Zalba, J.H.; Ferrari, C.; Gatti, M.C. Hemorrhagic colitis in postdiarrheal hemolytic uremic syndrome: Retrospective analysis of 54 children. Pediatr. Nephrol. 2012, 27, 229–233. [Google Scholar] [CrossRef]
- De Buys Roessingh, A.S.; de Lagausie, P.; Baudoin, V.; Loirat, C.; Aigrain, Y. Gastrointestinal Complications of Post-Diarrheal Hemolytic Uremic Syndrome. Eur. J. Pediatr. Surg. 2007, 17, 328–334. [Google Scholar] [CrossRef]
- Ostroff, S.M.; Kobayashi, J.M.; Lewis, J.H. Infections with Escherichia coli 0157:H7 in Washington State: The First Year of Statewide Disease Surveillance. JAMA 1989, 262, 355–359. [Google Scholar] [CrossRef]
- Ake, J.A.; Jelacic, S.; Ciol, M.A.; Watkins, S.L.; Murray, K.F.; Christie, D.L.; Klein, E.J.; Tarr, P.I. Relative Nephroprotection During Escherichia coli O157:H7 Infections: Association With Intravenous Volume Expansion. Pediatrics 2005, 115, e673–e680. [Google Scholar] [CrossRef] [Green Version]
- Balestracci, A.; Martin, S.M.; Toledo, I.; Alvarado, C.; Wainsztein, R.E. Dehydration at admission increased the need for dialysis in hemolytic uremic syndrome children. Pediatr. Nephrol. 2012, 27, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ida, O.; Kimoto, K.; Takatorige, T.; Nakanishi, N.; Tatara, K. Predictors for the development of haemolytic uraemic syndrome with Escherichia coli O157:H7 infections: With focus on the day of illness. Epidemiol. Infect. 2000, 124, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zoufaly, A.; Cramer, J.P.; Vettorazzi, E.; Sayk, F.; Bremer, J.P.; Koop, I.; de Weerth, A.; Schmiedel, S.; Jordan, S.; Fraedrich, K.; et al. Risk Factors for Development of Hemolytic Uremic Syndrome in a Cohort of Adult Patients with STEC 0104:H4 Infection. PLoS ONE 2013, 8, e59209. [Google Scholar] [CrossRef]
- Tserenpuntsag, B.; Chang, H.-G.; Smith, P.F.; Morse, D.L. Hemolytic Uremic Syndrome Risk and Escherichia coli O157:H7. Emerg. Infect. Dis. 2005, 11, 1955–1957. [Google Scholar] [CrossRef]
- Bell, B.P.; Griffin, P.M.; Lozano, P.; Christie, D.L.; Kobayashi, J.M.; Tarr, P.I. Predictors of Hemolytic Uremic Syndrome in Children During a Large Outbreak of Escherichia coli O157:H7 Infections. Pediatrics 1997, 100, e12. [Google Scholar] [CrossRef] [Green Version]
- Siegler, R.L.; Milligan, M.K.; Burningham, T.H.; Christofferson, R.D.; Chang, S.Y.; Jorde, L.B. Long-term outcome and prognostic indicators in the hemolytic-uremic syndrome. J. Pediatr. 1991, 118, 195–200. [Google Scholar] [CrossRef]
- Gerber, A.; Karch, H.; Allerberger, F.; Verweyen, H.M.; Zimmerhackl, L.B. Clinical Course and the Role of Shiga Toxin–Producing Escherichia coli Infection in the Hemolytic-Uremic Syndrome in Pediatric Patients, 1997–2000, in Germany and Austria: A Prospective Study. J. Infect. Dis. 2002, 186, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Oakes, R.S.; Kirkhamm, J.K.; Nelson, R.D.; Siegler, R.L. Duration of oliguria and anuria as predictors of chronic renal-related sequelae in post-diarrheal hemolytic uremic syndrome. Pediatr. Nephrol. 2008, 23, 1303–1308. [Google Scholar] [CrossRef]
- Rosales, A.; Hofer, J.; Zimmerhackl, L.-B.; Jungraithmayr, T.C.; Riedl, M.; Giner, T.; Strasak, A.; Orth-Höller, D.; Würzner, R.; Karch, H.; et al. Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin. Infect. Dis. 2012, 54, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Lukasz, A.; Beneke, J.; Menne, J.; Vetter, F.; Schmidt, B.M.W.; Schiffer, M.; Haller, H.; Kümpers, P.; Kielstein, J.T. Serum neutrophil gelatinase-associated lipocalin (NGAL) in patients with Shiga toxin mediated haemolytic uraemic syndrome (STEC-HUS). Thromb. Haemost. 2014, 111, 365–372. [Google Scholar]
- Trachtman, H.; Austin, C.; Lewinski, M.; Stahl, R.A.K. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat. Rev. Nephrol. 2012, 8, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Oakes, R.S.; Siegler, R.L.; McReynolds, M.A.; Pysher, T.; Pavia, A.T. Predictors of Fatality in Postdiarrheal Hemolytic Uremic Syndrome. Pediatrics 2006, 117, 1656–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnus, T.; Röther, J.; Simova, O.; Meier-Cillien, M.; Repenthin, J.; Möller, F.; Gbadamosi, J.; Panzer, U.; Wengenroth, M.; Hagel, C.; et al. The neurological syndrome in adults during the 2011 northern German E. coli serotype O104:H4 outbreak. Brain 2012, 135, 1850–1859. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, K.J.; Boyd, S.G.; Tasker, R.C. Acute neurology and neurophysiology of haemolytic-uraemic syndrome. Arch. Dis. Child. 2001, 84, 434–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathanson, S.; Kwon, T.; Elmaleh, M.; Charbit, M.; Launay, E.A.; Harambat, J.; Brun, M.; Ranchin, B.; Bandin, F.; Cloarec, S.; et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 1218–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimolai, N.; Morrison, B.J.; Carter, J.E. Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic-uremic syndrome. Pediatrics 1992, 90, 616–621. [Google Scholar] [PubMed]
- Kleimann, A.; Toto, S.; Eberlein, C.K.; Kielstein, J.T.; Bleich, S.; Frieling, H.; Sieberer, M. Psychiatric symptoms in patients with Shiga toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome. PLoS ONE 2014, 9, e101839. [Google Scholar] [CrossRef] [Green Version]
- Donnerstag, F.; Ding, X.; Pape, L.; Bültmann, E.; Lücke, T.; Zajaczek, J.; Hoy, L.; Das, A.M.; Lanfermann, H.; Ehrich, J.; et al. Patterns in early diffusion-weighted MRI in children with haemolytic uraemic syndrome and CNS involvement. Eur. Radiol. 2012, 22, 506–513. [Google Scholar] [CrossRef]
- Weissenborn, K.; Donnerstag, F.; Kielstein, J.T.; Heeren, M.; Worthmann, H.; Hecker, H.; Schmitt, R.; Schiffer, M.; Pasedag, T.; Schuppner, R.; et al. Neurologic manifestations of E coli infection-induced hemolytic-uremic syndrome in adults. Neurology 2012, 79, 1466–1473. [Google Scholar] [CrossRef]
- Thomas, N.J.; Messina, J.J.; DeBruin, W.J.; Carcillo, J.A. Cardiac failure in hemolytic uremic syndrome and rescue with extracorporeal life support. Pediatr. Cardiol. 2005, 26, 104–106. [Google Scholar] [CrossRef]
- Gallo, G.E.; Gianantonio, C.A. Extrarenal involvement in diarrhoea-associated haemolytic-uraemic syndrome. Pediatr. Nephrol. 1995, 9, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Siegler, R.L.; Christofferson, R.D.; Milligan, M.K.; Pavia, A.T. A 20-Year Population-Based Study of Postdiarrheal Hemolytic Uremic Syndrome in Utah. Pediatrics 1994, 94, 35–40. [Google Scholar] [PubMed]
- Jenssen, G.R.; Vold, L.; Hovland, E.; Bangstad, H.-J.; Nygård, K.; Bjerre, A. Clinical features, therapeutic interventions and long-term aspects of hemolytic-uremic syndrome in Norwegian children: A nationwide retrospective study from 1999–2008. BMC Infect. Dis. 2016, 16, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thayu, M.; Chandler, W.L.; Jelacic, S.; Gordon, C.A.; Rosenthal, G.L.; Tarr, P.I. Cardiac ischemia during hemolytic uremic syndrome. Pediatr. Nephrol. 2003, 18, 286–289. [Google Scholar] [CrossRef]
- Mohammed, J.; Filler, G.; Price, A.; Sharma, A.P. Cardiac tamponade in diarrhoea-positive haemolytic uraemic syndrome. Nephrol. Dial. Transplant. 2009, 24, 679–681. [Google Scholar] [CrossRef]
- Suri, R.S.; Clark, W.F.; Barrowman, N.; Mahon, J.L.; Thiessen-Philbrook, H.R.; Rosas-Arellano, M.P.; Zarnke, K.; Garland, J.S.; Garg, A.X. Diabetes during diarrhea-associated hemolytic uremic syndrome: A systematic review and meta-analysis. Diabetes Care 2005, 28, 2556–2562. [Google Scholar] [CrossRef] [Green Version]
- Suri, R.S.; Mahon, J.L.; Clark, W.F.; Moist, L.M.; Salvadori, M.; Garg, A.X. Relationship between Escherichia coli O157:H7 and diabetes mellitus. Kidney Int. Suppl. 2009, 75, S44–S46. [Google Scholar] [CrossRef] [Green Version]
- Grodinsky, S.; Telmesani, A.; Robson, W.L.; Fick, G.; Scott, R.B. Gastrointestinal manifestations of hemolytic uremic syndrome: Recognition of pancreatitis. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 518–524. [Google Scholar] [CrossRef]
- Caillaud, C.; Zaloszyc, A.; Licht, C.; Pichault, V.; Frémeaux-Bacchi, V.; Fischbach, M. CFH gene mutation in a case of Shiga toxin-associated hemolytic uremic syndrome (STEC-HUS). Pediatr. Nephrol. 2016, 31, 157–161. [Google Scholar] [CrossRef]
- Siegler, R.L.; Griffin, P.M.; Barrett, T.J.; Strockbine, N.A. Recurrent Hemolytic Uremic Syndrome Secondary to Escherichia coli 0157:H7 Infection. Pediatrics 1993, 91, 666–668. [Google Scholar]
- Commereuc, M.; Weill, F.-X.; Loukiadis, E.; Gouali, M.; Gleizal, A.; Kormann, R.; Ridel, C.; Frémeaux-Bacchi, V.; Rondeau, E.; Hertig, A.; et al. Recurrent Hemolytic and Uremic Syndrome Induced by Escherichia Coli. Medicine 2016, 95, e2050. [Google Scholar] [CrossRef] [PubMed]
- Buvens, G.; De Rauw, K.; Roisin, S.; Vanfraechem, G.; Denis, O.; Jacobs, F.; Scheutz, F.; Piérard, D. Verocytotoxin-producing Escherichia coli O128ab:H2 bacteremia in a 27-year-old male with hemolytic-uremic syndrome. J. Clin. Microbiol. 2013, 51, 1633–1635. [Google Scholar] [CrossRef] [Green Version]
- Chiurchiu, C.; Firrincieli, A.; Santostefano, M.; Fusaroli, M.; Remuzzi, G.; Ruggenenti, P. Adult nondiarrhea hemolytic uremic syndrome associated with Shiga toxin Escherichia coli O157:H7 bacteremia and urinary tract infection. Am. J. Kidney Dis. 2003, 41, e4.1–e4.4. [Google Scholar] [CrossRef] [PubMed]
- Lienemann, T.; Salo, E.; Rimhanen-Finne, R.; Rönnholm, K.; Taimisto, M.; Hirvonen, J.J.; Tarkka, E.; Kuusi, M.; Siitonen, A. Shiga toxin-producing Escherichia coli serotype O78:H(-) in family, Finland, 2009. Emerg. Infect. Dis. 2012, 18, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.-V.; Hochstrasser, L.; Chuard, C.; Hächler, H.; Regamey, C.; Descombes, E. Adult hemolytic-uremic syndrome associated with urosepsis due to Shigatoxin-producing Escherichia coli O138:H-. Ren. Fail. 2007, 29, 747–750. [Google Scholar] [CrossRef]
- Starr, M.; Bennett-Wood, V.; Bigham, A.K.; de Koning-Ward, T.F.; Bordun, A.M.; Lightfoot, D.; Bettelheim, K.A.; Jones, C.L.; Robins-Browne, R.M. Hemolytic-uremic syndrome following urinary tract infection with enterohemorrhagic Escherichia coli: Case report and review. Clin. Infect. Dis. 1998, 27, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Bonacorsi, S.; Clermont, O.; Houdouin, V.; Cordevant, C.; Brahimi, N.; Marecat, A.; Tinsley, C.; Nassif, X.; Lange, M.; Bingen, E.; et al. Molecular analysis and experimental virulence of French and North American Escherichia coli neonatal meningitis isolates: Identification of a new virulent clone. J. Infect. Dis. 2003, 187, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Peigne, C.; Bidet, P.; Mahjoub-Messai, F.; Plainvert, C.; Barbe, V.; Médigue, C.; Frapy, E.; Nassif, X.; Denamur, E.; Bingen, E.; et al. The plasmid of Escherichia coli strain S88 (O45:K1:H7) that causes neonatal meningitis is closely related to avian pathogenic E. coli plasmids and is associated with high-level bacteremia in a neonatal rat meningitis model. Infect. Immun. 2009, 77, 2272–2284. [Google Scholar] [CrossRef] [Green Version]
- Cobeñas, C.J.; Bresso, P.S.; Lombardi, L.L.; Amoreo, O.R.; Ruscasso, J.D.; Spizzirri, A.P.; del Suarez, Â.C.; Zalba, J.H.; Rahman, R.C.; Risso, P.; et al. Relationship between red blood cell transfusion requirements and severity of renal disease during the acute stage of hemolytic uremic syndrome. Pediatr. Nephrol. 2015, 30, 2115–2119. [Google Scholar] [CrossRef]
- Pape, L.; Ahlenstiel, T.; Kreuzer, M.; Drube, J.; Froede, K.; Franke, D.; Ehrich, J.H.H.; Haubitz, M. Early erythropoietin reduced the need for red blood cell transfusion in childhood hemolytic uremic syndrome—A randomized prospective pilot trial. Pediatr. Nephrol. 2009, 24, 1061–1064. [Google Scholar] [CrossRef]
- Balestracci, A.; Martin, S.M.; Toledo, I.; Alvarado, C.; Wainsztein, R.E. Early erythropoietin in post-diarrheal hemolytic uremic syndrome: A case–control study. Pediatr. Nephrol. 2015, 30, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Trachtman, H.; Cnaan, A.; Christen, E.; Gibbs, K.; Zhao, S.; Acheson, D.W.K.; Weiss, R.; Kaskel, F.J.; Spitzer, A.; Hirschman, G.H.; et al. Effect of an Oral Shiga Toxin–Binding Agent on Diarrhea-Associated Hemolytic Uremic Syndrome in Children: A Randomized Controlled Trial. JAMA 2003, 290, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Nadon, C.; Van Walle, I.; Gerner-Smidt, P.; Campos, J.; Chinen, I.; Concepcion-Acevedo, J.; Gilpin, B.; Smith, A.M.; Kam, K.M.; Perez, E.; et al. PulseNet International: Vision for the implementation of whole genome sequencing (WGS) for global food-borne disease surveillance. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Voetsch, A.C.; Angulo, F.J.; Rabatsky-Ehr, T.; Shallow, S.; Cassidy, M.; Thomas, S.M.; Swanson, E.; Zansky, S.M.; Hawkins, M.A.; Jones, T.F.; et al. Laboratory Practices for Stool-Specimen Culture for Bacterial Pathogens, Including Escherichia coli O157:H7, in the FoodNet Sites, 1995–2000. Clin. Infect. Dis. 2004, 38 (Suppl. 3), S190–S197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarr, P.I.; Neill, M.A.; Clausen, C.R.; Watkins, S.L.; Christie, D.L.; Hickman, R.O. Escherichia coli O157:H7 and the hemolytic uremic syndrome: Importance of early cultures in establishing the etiology. J. Infect. Dis. 1990, 162, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Cornick, N.A.; Jelacic, S.; Ciol, M.A.; Tarr, P.I. Escherichia coli O157:H7 infections: Discordance between filterable fecal shiga toxin and disease outcome. J. Infect. Dis. 2002, 186, 57–63. [Google Scholar] [CrossRef]
- March, S.B.; Ratnam, S. Sorbitol-MacConkey medium for detection of Escherichia coli O157:H7 associated with hemorrhagic colitis. J. Clin. Microbiol. 1986, 23, 869–872. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.S.; Bollinger, L.M. Influence of Selective Media on Successful Detection of Shiga Toxin–Producing Escherichia coli in Food, Fecal, and Environmental Samples. Foodborne Pathog. Dis. 2008, 5, 227–244. [Google Scholar] [CrossRef]
- Bettelheim, K.A.; Evangelidis, H.; Pearce, J.L.; Sowers, E.; Strockbine, N.A. Isolation of a Citrobacter freundii strain which carries the Escherichia coli O157 antigen. J. Clin. Microbiol. 1993, 31, 760–761. [Google Scholar] [CrossRef] [Green Version]
- Zelyas, N.; Poon, A.; Patterson-Fortin, L.; Johnson, R.P.; Lee, W.; Chui, L. Assessment of commercial chromogenic solid media for the detection of non-O157 Shiga toxin-producing Escherichia coli (STEC). Diagn. Microbiol. Infect. Dis. 2016, 85, 302–308. [Google Scholar] [CrossRef]
- Pollock, K.G.J.; Locking, M.E.; Beattie, T.J.; Maxwell, H.; Ramage, I.; Hughes, D.; Cowieson, J.; Allison, L.; Hanson, M.; Cowden, J.M.; et al. Sorbitol-fermenting Escherichia coli O157, Scotland. Emerg. Infect. Dis. 2010, 16, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Wijnsma, K.L.; van Bommel, S.A.M.; van der Velden, T.; Volokhina, E.; Schreuder, M.F.; van den Heuvel, L.P.; van de Kar, N.C.A.J. Fecal diagnostics in combination with serology: Best test to establish STEC-HUS. Pediatr. Nephrol. 2016, 31, 2163–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallières, E.; Saint-Jean, M.; Rallu, F. Comparison of Three Different Methods for Detection of Shiga Toxin-Producing Escherichia coli in a Tertiary Pediatric Care Center. J. Clin. Microbiol. 2013, 51, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielaszewska, M.; Prager, R.; Köck, R.; Mellmann, A.; Zhang, W.; Tschäpe, H.; Tarr, P.I.; Karch, H. Shiga Toxin Gene Loss and Transfer In Vitro and In Vivo during Enterohemorrhagic Escherichia coli O26 Infection in Humans. Appl. Environ. Microbiol. 2007, 73, 3144–3150. [Google Scholar] [CrossRef] [Green Version]
- Bielaszewska, M.; Köck, R.; Friedrich, A.W.; von Eiff, C.; Zimmerhackl, L.B.; Karch, H.; Mellmann, A. Shiga Toxin-Mediated Hemolytic Uremic Syndrome: Time to Change the Diagnostic Paradigm? PLoS ONE 2007, 2, e1024. [Google Scholar] [CrossRef]
- Scientific Opinion on VTEC-seropathotype and scientific criteria regarding pathogenicity assessment. EFSA J. 2013, 11, 3138. [CrossRef]
- Sharma, V.K.; Dean-Nystrom, E.A. Detection of enterohemorrhagic Escherichia coli O157:H7 by using a multiplex real-time PCR assay for genes encoding intimin and Shiga toxins. Vet. Microbiol. 2003, 93, 247–260. [Google Scholar] [CrossRef]
- Holmes, A.; Allison, L.; Ward, M.; Dallman, T.J.; Clark, R.; Fawkes, A.; Murphy, L.; Hanson, M. Utility of Whole-Genome Sequencing of Escherichia coli O157 for Outbreak Detection and Epidemiological Surveillance. J. Clin. Microbiol. 2015, 53, 3565–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joensen, K.G.; Tetzschner, A.M.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and Easy In Silico Serotyping of Escherichia coli Isolates by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Alikhan, N.-F.; Mohamed, K.; Achtman, M.; Fan, Y. The Agama Study Group. The user’s guide to comparative genomics with EnteroBase. Three case studies: Micro-clades within Salmonella enterica serovar Agama, ancient and modern populations of Yersinia pestis, and core genomic diversity of all Escherichia. bioRxiv 2019, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Karch, H.; Bielaszewska, M.; Bitzan, M.; Schmidt, H. Epidemiology and diagnosis of Shiga toxin-producing Escherichia coli infections. Diagn. Microbiol. Infect. Dis. 1999, 34, 229–243. [Google Scholar] [CrossRef]
- Chui, L.; Patterson-Fortin, L.; Kuo, J.; Li, V.; Boras, V. Evaluation of enzyme immunoassays and real-time PCR for detecting Shiga toxin-producing Escherichia coli in Southern Alberta, Canada. J. Clin. Microbiol. 2015, 53, 1019–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.; Klein, E.J.; Galanakis, E.; Thomas, A.A.; Stapp, J.R.; Rich, S.; Buccat, A.M.; Tarr, P.I. Real-Time PCR Assay for Detection and Differentiation of Shiga Toxin-Producing Escherichia coli from Clinical Samples. J. Clin. Microbiol. 2015, 53, 2148–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chart, H.; Perry, N.T.; Cheasty, T.; Wright, P.A. The kinetics of antibody production to antigens of Escherichia coli O157 in a pregnant woman with haemolytic uraemic syndrome. J. Med. Microbiol. 2002, 51, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Chart, H.; Jenkins, C. The serodiagnosis of infections caused by Verocytotoxin-producing Escherichia coli. J. Appl. Microbiol. 1999, 86, 731–740. [Google Scholar] [CrossRef]
- Holtz, L.R.; Neill, M.A.; Tarr, P.I. Acute bloody diarrhea: A medical emergency for patients of all ages. Gastroenterology 2009, 136, 1887–1898. [Google Scholar] [CrossRef]
- Trotter, J.M.; Hunt, L.; Peter, M.B. Ischaemic colitis. BMJ 2016, 355, i6600. [Google Scholar] [CrossRef]
- Miller, F.H.; Ma, J.J.; Scholz, F.J. Imaging Features of Enterohemorrhagic Escherichia coli Colitis. Am. J. Roentgenol. 2001, 177, 619–623. [Google Scholar] [CrossRef]
- Joseph, A.; Rafat, C.; Zafrani, L.; Mariani-Kurkdjian, P.; Veyradier, A.; Hertig, A.; Rondeau, E.; Mariotte, E.; Azoulay, E. Early Differentiation of Shiga Toxin-Associated Hemolytic Uremic Syndrome in Critically Ill Adults With Thrombotic Microangiopathy Syndromes. Crit. Care Med. 2018, 46, e904–e911. [Google Scholar] [CrossRef]
- Coppo, P.; Schwarzinger, M.; Buffet, M.; Wynckel, A.; Clabault, K.; Presne, C.; Poullin, P.; Malot, S.; Vanhille, P.; Azoulay, E.; et al. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: The French TMA reference center experience. PLoS ONE 2010, 5, e10208. [Google Scholar] [CrossRef]
- Bentley, M.J.; Lehman, C.M.; Blaylock, R.C.; Wilson, A.R.; Rodgers, G.M. The utility of patient characteristics in predicting severe ADAMTS13 deficiency and response to plasma exchange. Transfusion (Paris) 2010, 50, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M.; Cugno, M. The complex differential diagnosis between thrombotic thrombocytopenic purpura and the atypical hemolytic uremic syndrome: Laboratory weapons and their impact on treatment choice and monitoring. Thromb. Res. 2015, 136, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Cataland, S.R.; Wu, H.M. How I treat: The clinical differentiation and initial treatment of adult patients with atypical hemolytic uremic syndrome. Blood 2014, 123, 2478–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendapudi, P.K.; Hurwitz, S.; Fry, A.; Marques, M.B.; Waldo, S.W.; Li, A.; Sun, L.; Upadhyay, V.; Hamdan, A.; Brunner, A.M.; et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: A cohort study. Lancet Haematol. 2017, 4, e157–e164. [Google Scholar] [CrossRef]
- Ejemot, R.I.; Ehiri, J.E.; Meremikwu, M.M.; Critchley, J.A. Hand washing for preventing diarrhoea. Cochrane Database Syst. Rev. 2008, CD004265. [Google Scholar] [CrossRef] [Green Version]
- WHO. The Five Keys to Safer Food Programme. Available online: http://www.who.int/foodsafety/areas_work/food-hygiene/5keys/en/ (accessed on 20 January 2020).
- Ahmed, A.; Li, J.; Shiloach, Y.; Robbins, J.B.; Szu, S.C. Safety and immunogenicity of Escherichia coli O157 O-specific polysaccharide conjugate vaccine in 2-5-year-old children. J. Infect. Dis. 2006, 193, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.J.; Teel, L.D.; Carvalho, H.M.; Melton-Celsa, A.R.; O’Brien, A.D. Development of a hybrid Shiga holotoxoid vaccine to elicit heterologous protection against Shiga toxins types 1 and 2. Vaccine 2006, 24, 4122–4129. [Google Scholar] [CrossRef]
- Wen, S.X.; Teel, L.D.; Judge, N.A.; O’Brien, A.D. A plant-based oral vaccine to protect against systemic intoxication by Shiga toxin type 2. Proc. Natl. Acad. Sci. USA 2006, 103, 7082–7087. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Cai, K.; Shi, J.; Liu, H.; Hou, X.; Tu, W.; Xiao, L.; Wang, Q.; Wang, H. Immunogenicity of a novel Stx2B-Stx1B fusion protein in a mice model of Enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine 2009, 27, 2070–2076. [Google Scholar] [CrossRef]
- Bentancor, L.V.; Bilen, M.; Brando, R.J.F.; Ramos, M.V.; Ferreira, L.C.S.; Ghiringhelli, P.D.; Palermo, M.S. A DNA Vaccine Encoding the Enterohemorragic Escherichia coli Shiga-Like Toxin 2 A2 and B Subunits Confers Protective Immunity to Shiga Toxin Challenge in the Murine Model. Clin. Vaccine Immunol. 2009, 16, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Szu, S.C.; Ahmed, A. Clinical Studies of Escherichia coli O157:H7 Conjugate Vaccines in Adults and Young Children. Microbiol. Spectr. 2014, 2, EHEC-0016-2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.E.; Elliott, E.J. Interventions for preventing diarrhea-associated hemolytic uremic syndrome: Systematic review. BMC Public Health 2013, 13, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, N.P.; Dick, P.; Wilson, J. Assessing the existing information on the efficacy of bovine vaccination against Escherichia coli O157:H7—A systematic review and meta-analysis. Zoonoses Public Health 2013, 60, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, J.M.; Amezcua, M.R.; Rajic, A.; Waddell, L. Pre-harvest Interventions to Reduce the Shedding of E. coli O157 in the Faeces of Weaned Domestic Ruminants: A Systematic Review. Zoonoses Public Health 2007, 54, 260–277. [Google Scholar] [CrossRef]
- Callaway, T.R.; Elder, R.O.; Keen, J.E.; Anderson, R.C.; Nisbet, D.J. Forage Feeding to Reduce Preharvest Escherichia coli Populations in Cattle, a Review. J. Dairy Sci. 2003, 86, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Ellis-Iversen, J.; Smith, R.P.; Winden, S.V.; Paiba, G.A.; Watson, E.; Snow, L.C.; Cook, A.J.C. Farm practices to control E. coli O157 in young cattle—A randomised controlled trial. Vet. Res. 2008, 39, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Berry, E.D.; Wells, J.E. Soil Solarization Reduces Escherichia coli O157:H7 and Total Escherichia coli on Cattle Feedlot Pen Surfaces. J. Food Prot. 2012, 75, 7–13. [Google Scholar] [CrossRef]
- E. coli O157:H7 and STEC. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education/get-answers/food-safety-fact-sheets/foodborne-illness-and-disease/escherichia-coli-o157h7/ct_index (accessed on 20 January 2020).
- Werber, D.; Mason, B.W.; Evans, M.R.; Salmon, R.L. Preventing household transmission of Shiga toxin-producing Escherichia coli O157 infection: Promptly separating siblings might be the key. Clin. Infect. Dis. 2008, 46, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Shiga Toxin-Producing Escherichia coli: Guidance, Data and Analysis—GOV.UK. Available online: https://www.gov.uk/government/collections/vero-cytotoxin-producing-escherichia-coli-vtec-guidance-data-and-analysis (accessed on 20 January 2020).
- Mor, M.; Ashkenazi, S. The dilemma of antimicrobial treatment of Shiga toxin-producing Escherichia coli. Pediatr. Infect. Dis. J. 2014, 33, 979–981. [Google Scholar] [CrossRef]
- Bennish, M.L.; Khan, W.A.; Begum, M.; Bridges, E.A.; Ahmed, S.; Saha, D.; Salam, M.A.; Acheson, D.; Ryan, E.T. Low risk of hemolytic uremic syndrome after early effective antimicrobial therapy for Shigella dysenteriae type 1 infection in Bangladesh. Clin. Infect. Dis. 2006, 42, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.; Jelacic, S.; Habeeb, R.L.; Watkins, S.L.; Tarr, P.I. The Risk of the Hemolytic–Uremic Syndrome after Antibiotic Treatment of Escherichia coli O157:H7 Infections. N. Engl. J. Med. 2000, 342, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, K.; Ida, O.; Kimoto, K.; Takatorige, T.; Nakanishi, N.; Tatara, K. Effect of early fosfomycin treatment on prevention of hemolytic uremic syndrome accompanying Escherichia coli O157:H7 infection. Clin. Nephrol. 1999, 52, 357–362. [Google Scholar] [PubMed]
- Tajiri, H.; Nishi, J.; Ushijima, K.; Shimizu, T.; Ishige, T.; Shimizu, M.; Tanaka, H.; Brooks, S. A role for fosfomycin treatment in children for prevention of haemolytic–uraemic syndrome accompanying Shiga toxin-producing Escherichia coli infection. Int. J. Antimicrob. Agents 2015, 5, 586–589. [Google Scholar] [CrossRef]
- Nitschke, M.; Sayk, F.; Härtel, C.; Roseland, R.T.; Hauswaldt, S.; Steinhoff, J.; Fellermann, K.; Derad, I.; Wellhöner, P.; Büning, J.; et al. Association Between Azithromycin Therapy and Duration of Bacterial Shedding Among Patients With Shiga Toxin–Producing Enteroaggregative Escherichia coli O104:H4. JAMA 2012, 307, 1046–1052. [Google Scholar] [CrossRef] [Green Version]
- Menne, J.; Nitschke, M.; Stingele, R.; Abu-Tair, M.; Beneke, J.; Bramstedt, J.; Bremer, J.P.; Brunkhorst, R.; Busch, V.; Dengler, R.; et al. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: Case-control study. BMJ 2012, 345, e4565. [Google Scholar] [CrossRef] [Green Version]
- Panos, G.Z.; Betsi, G.I.; Falagas, M.E. Systematic review: Are antibiotics detrimental or beneficial for the treatment of patients with Escherichia coli O157:H7 infection? Aliment. Pharmacol. Ther. 2006, 24, 731–742. [Google Scholar] [CrossRef]
- Safdar, N.; Said, A.; Gangnon, R.E.; Maki, D.G. Risk of Hemolytic Uremic Syndrome After Antibiotic Treatment of Escherichia coli O157:H7 Enteritis: A Meta-analysis. JAMA 2002, 288, 996–1001. [Google Scholar] [CrossRef]
- Freedman, S.B.; Xie, J.; Neufeld, M.S.; Hamilton, W.L.; Hartling, L.; Tarr, P.I.; Nettel-Aguirre, A.; Chuck, A.; Lee, B.; Johnson, D.; et al. Shiga Toxin–Producing Escherichia coli Infection, Antibiotics, and Risk of Developing Hemolytic Uremic Syndrome: A Meta-analysis. Clin. Infect. Dis. 2016, 62, 1251–1258. [Google Scholar] [CrossRef] [Green Version]
- Proulx, F.; Turgeon, J.P.; Delage, G.; Lafleur, L.; Chicoine, L. Randomized, controlled trial of antibiotic therapy for Escherichia coli O157:H7 enteritis. J. Pediatr. 1992, 121, 299–303. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Chen, J.; Walker, C.M.; Gonzales, E.; Cleary, T.G. Rifaximin Does Not Induce Toxin Production or Phage-Mediated Lysis of Shiga Toxin-Producing Escherichia coli. Antimicrob. Agents Chemother. 2007, 51, 2837–2841. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; McDaniel, A.D.; Wolf, L.E.; Keusch, G.T.; Waldor, M.K.; Acheson, D.W.K. Quinolone Antibiotics Induce Shiga Toxin-Encoding Bacteriophages, Toxin Production, and Death in Mice. J. Infect. Dis. 2000, 181, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Donohou-Rolfe, A.; Krautz-Peterson, G.; Sevo, M.; Parry, N.; Abeijon, C.; Tzipori, S. Gnotobiotic piglet infection model for evaluating the safe use of antibiotics against Escherichia coli O157:H7 infection. J. Infect. Dis. 2009, 199, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grif, K.; Dierich, M.P.; Karch, H.; Allerberger, F. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Wilker, P.R.; Reiter, P.L.; Hedican, E.B.; Bender, J.B.; Hedberg, C.W. Antibiotic treatment of Escherichia coli O157 infection and the risk of hemolytic uremic syndrome, Minnesota. Pediatr. Infect. Dis. J. 2012, 31, 37–41. [Google Scholar] [CrossRef]
- Agger, M.; Scheutz, F.; Villumsen, S.; Mølbak, K.; Petersen, A.M. Antibiotic treatment of verocytotoxin-producing Escherichia coli (VTEC) infection: A systematic review and a proposal. J. Antimicrob. Chemother. 2015, 70, 2440–2446. [Google Scholar] [CrossRef] [Green Version]
- HCSP. Gastroentérites à Escherichia coli Entérohémorragique. Conduite à Tenir; Haut Conseil de la Santé Publique: Paris, France, 2015; Available online: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=494 (accessed on 20 January 2020).
- Garashi, T.; Ito, S.; Sako, M.; Saitoh, A.; Hataya, H.; Mizuguchi, M.; Morishima, T.; Ohnishi, K.; Kawamura, N.; Kitayama, H.; et al. Guidelines for the management and investigation of hemolytic uremic syndrome. Clin. Exp. Nephrol. 2014, 18, 525–557. [Google Scholar] [CrossRef]
- Shiga Toxin-Producing Escherichia coli (STEC): Symptoms, How to Avoid, How to Treat. Available online: https://www.gov.uk/government/publications/vero-cytotoxin-producing-escherichia-coli-symptoms-how-to-avoid-how-to-treat/vero-cytotoxin-producing-escherichia-coli-symptoms-how-to-avoid-how-to-treat (accessed on 20 January 2020).
- Shane, A.L.; Mody, R.K.; Crump, J.A.; Tarr, P.I.; Steiner, T.S.; Kotloff, K.; Langley, J.M.; Warren, C.A.; Cheng, A.C.; Cantey, J.; et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin. Infect. Dis. 2017, 65, e45–e80. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.M.; Griffin, P.M.; Jones, T.F.; Smith, K.E.; Scallan, E. Antimicrobial and antimotility agent use in persons with shiga toxin-producing Escherichia coli O157 infection in FoodNet Sites. Clin. Infect. Dis. 2011, 52, 1130–1132. [Google Scholar] [CrossRef] [Green Version]
- Grisaru, S.; Xie, J.; Samuel, S.; Hartling, L.; Tarr, P.I.; Schnadower, D.; Freedman, S.B.; Alberta Provincial Pediatric Enteric Infection Team. Associations Between Hydration Status, Intravenous Fluid Administration, and Outcomes of Patients Infected With Shiga Toxin-Producing Escherichia coli: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 68–76. [Google Scholar] [CrossRef]
- Ardissino, G.; Tel, F.; Possenti, I.; Testa, S.; Consonni, D.; Paglialonga, F.; Salardi, S.; Borsa-Ghiringhelli, N.; Salice, P.; Tedeschi, S.; et al. Early Volume Expansion and Outcomes of Hemolytic Uremic Syndrome. Pediatrics 2016, 137, e20152153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüth, S.; Fründt, T.W.; Rösch, T.; Schlee, C.; Lohse, A.W. Prevention of hemolytic uremic syndrome with daily bowel lavage in patients with Shiga toxin-producing enterohemorrhagic Escherichia coli O104:H4 infection. JAMA Intern. Med. 2014, 174, 1003–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2017, 41, 706–742. [Google Scholar] [CrossRef] [Green Version]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Mathew, R.O.; Nayer, A.; Asif, A. The endothelium as the common denominator in malignant hypertension and thrombotic microangiopathy. J. Am. Soc. Hypertens. JASH 2016, 10, 352–359. [Google Scholar] [CrossRef]
- Gómez-Lado, C.; Martinón-Torres, F.; Alvarez-Moreno, A.; Eirís-Puñal, J.; Carreira-Sande, N.; Rodriguez-Nuñez, A.; Castro-Gago, M. Reversible posterior leukoencephalopathy syndrome: An infrequent complication in the course of haemolytic-uremic syndrome. Rev. Neurol. 2007, 44, 475–478. [Google Scholar]
- Dyck, M.V.; Proesmans, W. Renoprotection by ACE inhibitors after severe hemolytic uremic syndrome. Pediatr. Nephrol. 2004, 19, 688–690. [Google Scholar] [CrossRef]
- Davis, T.K.; Neumayr, T.; Geile, K.; Doctor, A.; Hmeil, P. Citrate anticoagulation during continuous renal replacement therapy in pediatric critical care. Pediatr. Crit. Care Med. 2014, 15, 471–485. [Google Scholar] [CrossRef]
- Morabito, S.; Pistolesi, V.; Tritapepe, L.; Fiaccadori, E. Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 2173–2188. [Google Scholar] [CrossRef] [Green Version]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef]
- Goel, R.; Ness, P.M.; Takemoto, C.M.; Krishnamurti, L.; King, K.E.; Tobian, A.A.R. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood 2015, 125, 1470–1476. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, Y.; Baudel, J.-L.; Wynckel, A.; Galicier, L.; Azoulay, E.; Provôt, F.; Pène, F.; Mira, J.-P.; Presne, C.; Poullin, P.; et al. Are platelet transfusions harmful in acquired thrombotic thrombocytopenic purpura at the acute phase? Experience of the French thrombotic microangiopathies reference center. Am. J. Hematol. 2015, 90, E127–E129. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.M.; Coyle, T.E. Platelet transfusions and bleeding complications associated with plasma exchange catheter placement in patients with presumed thrombotic thrombocytopenic purpura. J. Clin. Apher. 2013, 28, 356–358. [Google Scholar] [CrossRef]
- Balestracci, A.; Martin, S.M.; Toledo, I.; Alvarado, C.; Wainsztein, R.E. Impact of platelet transfusions in children with post-diarrheal hemolytic uremic syndrome. Pediatr. Nephrol. 2013, 28, 919–925. [Google Scholar] [CrossRef]
- Beneke, J.; Sartison, A.; Kielstein, J.T.; Haller, H.; Nitschke, M.; Kunzendorf, U.; Loos, S.; Kemper, M.J.; Stahl, R.A.K.; Menne, J.; et al. Clinical and Laboratory Consequences of Platelet Transfusion in Shiga Toxin-Mediated Hemolytic Uremic Syndrome. Transfus. Med. Rev. 2017, 31, 51–55. [Google Scholar] [CrossRef]
- Weil, B.R.; Andreoli, S.P.; Billmire, D.F. Bleeding risk for surgical dialysis procedures in children with hemolytic uremic syndrome. Pediatr. Nephrol. 2010, 25, 1693–1698. [Google Scholar] [CrossRef]
- Cimolai, N.; Basalyga, S.; Mah, D.G.; Morrison, B.J.; Carter, J.E. A continuing assessment of risk factors for the development of Escherichia coli O157:H7-associated hemolytic uremic syndrome. Clin. Nephrol. 1994, 42, 85–89. [Google Scholar]
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Lo Vecchio, A.; Shamir, R.; Szajewska, H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: Update 2014. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [CrossRef]
- Loirat, C.; Sonsino, E.; Hinglais, N.; Jais, J.P.; Landais, P.; Fermanian, J. Treatment of the childhood haemolytic uraemic syndrome with plasma. A multicentre randomized controlled trial. The French Society of Paediatric Nephrology. Pediatr. Nephrol. 1988, 2, 279–285. [Google Scholar] [CrossRef]
- Rizzoni, G.; Claris-Appiani, A.; Edefonti, A.; Facchin, P.; Franchini, F.; Gusmano, R.; Imbasciati, E.; Pavanello, L.; Perfumo, F.; Remuzzi, G.; et al. Plasma infusion for hemolytic-uremic syndrome in children: Results of a multicenter controlled trial. J. Pediatr. 1988, 112, 284–290. [Google Scholar] [CrossRef]
- Rock, G.A.; Shumak, K.H.; Buskard, N.A.; Blanchette, V.S.; Kelton, J.G.; Nair, R.C.; Spasoff, R.A. Comparison of Plasma Exchange with Plasma Infusion in the Treatment of Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 1991, 325, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Dundas, S.; Murphy, J.; Soutar, R.L.; Jones, G.A.; Hutchinson, S.J.; Todd, W.T. Effectiveness of therapeutic plasma exchange in the 1996 Lanarkshire Escherichia coli O157:H7 outbreak. Lancet 1999, 354, 1327–1330. [Google Scholar] [CrossRef]
- Colic, E.; Dieperink, H.; Titlestad, K.; Tepel, M. Management of an acute outbreak of diarrhoea-associated haemolytic uraemic syndrome with early plasma exchange in adults from southern Denmark: An observational study. Lancet 2011, 378, 1089–1093. [Google Scholar] [CrossRef]
- Greinacher, A.; Friesecke, S.; Abel, P.; Dressel, A.; Stracke, S.; Fiene, M.; Ernst, F.; Selleng, K.; Weissenborn, K.; Schmidt, B.M.W.; et al. Treatment of severe neurological deficits with IgG depletion through immunoadsorption in patients with Escherichia coli O104:H4-associated haemolytic uraemic syndrome: A prospective trial. Lancet Lond. Engl. 2011, 378, 1166–1173. [Google Scholar] [CrossRef]
- Schwartz, J.; Padmanabhan, A.; Aqui, N.; Balogun, R.A.; Connelly-Smith, L.; Delaney, M.; Dunbar, N.M.; Witt, V.; Wu, Y.; Shaz, B.H. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J. Clin. Apher. 2016, 31, 149–162. [Google Scholar] [CrossRef]
- Lapeyraque, A.-L.; Malina, M.; Fremeaux-Bacchi, V.; Boppel, T.; Kirschfink, M.; Oualha, M.; Proulx, F.; Clermont, M.-J.; Le Deist, F.; Niaudet, P.; et al. Eculizumab in severe Shiga-toxin-associated HUS. N. Engl. J. Med. 2011, 364, 2561–2563. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Beutel, G.; Fleig, S.; Steinhoff, J.; Meyer, T.N.; Hafer, C.; Kuhlmann, U.; Bramstedt, J.; Panzer, U.; Vischedyk, M.; et al. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolytic–uraemic syndrome: An analysis of the German STEC-HUS registry. Nephrol. Dial. Transplant. 2012, 27, 3807–3815. [Google Scholar] [CrossRef] [Green Version]
- Delmas, Y.; Vendrely, B.; Clouzeau, B.; Bachir, H.; Bui, H.-N.; Lacraz, A.; Hélou, S.; Bordes, C.; Reffet, A.; Llanas, B.; et al. Outbreak of Escherichia coli O104:H4 haemolytic uraemic syndrome in France: Outcome with eculizumab. Nephrol. Dial. Transplant. 2014, 29, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Pape, L.; Hartmann, H.; Bange, F.C.; Suerbaum, S.; Bueltmann, E.; Ahlenstiel-Grunow, T. Eculizumab in Typical Hemolytic Uremic Syndrome (HUS) With Neurological Involvement. Medicine 2015, 94, e1000. [Google Scholar] [CrossRef]
- Watanabe-Takahashi, M.; Sato, T.; Dohi, T.; Noguchi, N.; Kano, F.; Murata, M.; Hamabata, T.; Natori, Y.; Nishikawa, K. An Orally Applicable Shiga Toxin Neutralizer Functions in the Intestine to Inhibit the Intracellular Transport of the Toxin. Infect. Immun. 2010, 78, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, K.; Watanabe, M.; Kita, E.; Igai, K.; Omata, K.; Yaffe, M.B.; Natori, Y. A multivalent peptide library approach identifies a novel Shiga toxin inhibitor that induces aberrant cellular transport of the toxin. FASEB J. 2006, 20, 2597–2599. [Google Scholar] [CrossRef] [PubMed]
- Tsutsuki, K.; Watanabe-Takahashi, M.; Takenaka, Y.; Kita, E.; Nishikawa, K. Identification of a Peptide-Based Neutralizer That Potently Inhibits Both Shiga Toxins 1 and 2 by Targeting Specific Receptor-Binding Regions. Infect. Immun. 2013, 81, 2133–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, K.; Matsuoka, K.; Kita, E.; Okabe, N.; Mizuguchi, M.; Hino, K.; Miyazawa, S.; Yamasaki, C.; Aoki, J.; Takashima, S.; et al. A therapeutic agent with oriented carbohydrates for treatment of infections by Shiga toxin-producing Escherichia coli O157:H7. Proc. Natl. Acad. Sci. USA 2002, 99, 7669–7674. [Google Scholar] [CrossRef] [Green Version]
- Kitov, P.I.; Sadowska, J.M.; Mulvey, G.; Armstrong, G.D.; Ling, H.; Pannu, N.S.; Read, R.J.; Bundle, D.R. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 2000, 403, 669–672. [Google Scholar] [CrossRef]
- Bernedo-Navarro, R.A.; Miyachiro, M.M.; Da, M.S.; Reis, C.F.; Conceição, R.A.; Gatti, M.S.; Yano, T. Peptides derived from phage display libraries as potential neutralizers of Shiga toxin-induced cytotoxicity in vitro and in vivo. J. Appl. Microbiol. 2014, 116, 1322–1333. [Google Scholar] [CrossRef]
- Li, T.; Tu, W.; Liu, Y.; Zhou, P.; Cai, K.; Li, Z.; Liu, X.; Ning, N.; Huang, J.; Wang, S.; et al. A potential therapeutic peptide-based neutralizer that potently inhibits Shiga toxin 2 in vitro and in vivo. Sci. Rep. 2016, 6, 21837. [Google Scholar] [CrossRef] [Green Version]
- Mulvey, G.L.; Marcato, P.; Kitov, P.I.; Sadowska, J.; Bundle, D.R.; Armstrong, G.D. Assessment in Mice of the Therapeutic Potential of Tailored, Multivalent Shiga Toxin Carbohydrate Ligands. J. Infect. Dis. 2003, 187, 640–649. [Google Scholar] [CrossRef]
- Paton, A.W.; Morona, R.; Paton, J.C. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat. Med. 2000, 6, 265–270. [Google Scholar] [CrossRef]
- Hostetter, S.J.; Helgerson, A.F.; Paton, J.C.; Paton, A.W.; Cornick, N.A. Therapeutic use of a receptor mimic probiotic reduces intestinal Shiga toxin levels in a piglet model of hemolytic uremic syndrome. BMC Res. Notes 2014, 7, 331. [Google Scholar] [CrossRef]
- Moxley, R.A.; Francis, D.H.; Tamura, M.; Marx, D.B.; Santiago-Mateo, K.; Zhao, M. Efficacy of Urtoxazumab (TMA-15 Humanized Monoclonal Antibody Specific for Shiga Toxin 2) Against Post-Diarrheal Neurological Sequelae Caused by Escherichia coli O157:H7 Infection in the Neonatal Gnotobiotic Piglet Model. Toxins 2017, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, E.L.; Contrini, M.M.; Glatstein, E.; González Ayala, S.; Santoro, R.; Allende, D.; Ezcurra, G.; Teplitz, E.; Koyama, T.; Matsumoto, Y.; et al. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 239–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitzan, M.; Poole, R.; Mehran, M.; Sicard, E.; Brockus, C.; Thuning-Roberson, C.; Rivière, M. Safety and pharmacokinetics of chimeric anti-Shiga toxin 1 and anti-Shiga toxin 2 monoclonal antibodies in healthy volunteers. Antimicrob. Agents Chemother. 2009, 53, 3081–3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, J.M.; Mukherjee, J.; Leysath, C.E.; Debatis, M.; Ofori, K.; Baldwin, K.; Boucher, C.; Peters, R.; Beamer, G.; Sheoran, A.; et al. A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with Shiga toxins 1 and 2. Infect. Immun. 2013, 81, 4592–4603. [Google Scholar] [CrossRef] [Green Version]
- Mejías, M.P.; Hiriart, Y.; Lauché, C.; Fernández-Brando, R.J.; Pardo, R.; Bruballa, A.; Ramos, M.V.; Goldbaum, F.A.; Palermo, M.S.; Zylberman, V.; et al. Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS). Sci. Rep. 2016, 6, 24913. [Google Scholar] [CrossRef] [Green Version]
- Gaston, M.A.; Pellino, C.A.; Weiss, A.A. Failure of Manganese to Protect from Shiga Toxin. PLoS ONE 2013, 8, e69823. [Google Scholar] [CrossRef] [Green Version]
- Perez, N.; Spizzirri, F.; Rahman, R.; Suarez, A.; Larrubia, C.; Lasarte, P. Steroids in the hemolytic uremic syndrome. Pediatr. Nephrol. 1998, 12, 101–104. [Google Scholar] [CrossRef]
- Loirat, C.; Beaufils, F.; Sonsino, E.; Schlegel, N.; Guesnu, M.; Pillion, G.; André, J.L.; Broyer, M.; Guyot, C.; Habib, R.; et al. Treatment of childhood hemolytic-uremic syndrome with urokinase. Cooperative controlled trial. Arch. Fr. Pediatr. 1984, 41, 15–19. [Google Scholar]
- Bergstein, J.M.; Edson, J.R.; Michael, A.F. Fibrinolytic treatment of the haemolytic-uraemic syndrome. Lancet 1972, 1, 448–449. [Google Scholar] [CrossRef]
- Van Damme-Lombaerts, R.; Proesmans, W.; Van Damme, B.; Eeckels, R.; Binda ki Muaka, P.; Mercieca, V.; Vlietinck, R.; Vermylen, J. Heparin plus dipyridamole in childhood hemolytic-uremic syndrome: A prospective, randomized study. J. Pediatr. 1988, 113, 913–918. [Google Scholar] [CrossRef]
- Cobeñas, C.J.; Alconcher, L.F.; Spizzirri, A.P.; Rahman, R.C. Long-term follow-up of Argentinean patients with hemolytic uremic syndrome who had not undergone dialysis. Pediatr. Nephrol. 2007, 22, 1343–1347. [Google Scholar] [CrossRef]
- Schieppati, A.; Ruggenenti, P.; Cornejo, R.P.; Ferrario, F.; Gregorini, G.; Zucchelli, P.; Rossi, E.; Remuzzi, G. Renal function at hospital admission as a prognostic factor in adult hemolytic uremic syndrome. The Italian Registry of Haemolytic Uremic Syndrome. J. Am. Soc. Nephrol. JASN 1992, 2, 1640–1644. [Google Scholar]
- Spinale, J.M.; Ruebner, R.L.; Copelovitch, L.; Kaplan, B.S. Long-term outcomes of Shiga toxin hemolytic uremic syndrome. Pediatr. Nephrol. 2013, 28, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.X.; Suri, R.S.; Barrowman, N.; Rehman, F.; Matsell, D.; Rosas-Arellano, M.P.; Salvadori, M.; Haynes, R.B.; Clark, W.F. Long-term Renal Prognosis of Diarrhea-Associated Hemolytic Uremic Syndrome: A Systematic Review, Meta-analysis, and Meta-regression. JAMA 2003, 290, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.X.; Salvadori, M.; Okell, J.M.; Thiessen-Philbrook, H.R.; Suri, R.S.; Filler, G.; Moist, L.; Matsell, D.; Clark, W.F. Albuminuria and estimated GFR 5 years after Escherichia coli O157 hemolytic uremic syndrome: An update. Am. J. Kidney Dis. 2008, 51, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Loos, S.; Aulbert, W.; Hoppe, B.; Ahlenstiel-Grunow, T.; Kranz, B.; Wahl, C.; Staude, H.; Humberg, A.; Benz, K.; Krause, M.; et al. Intermediate Follow-up of Pediatric Patients With Hemolytic Uremic Syndrome During the 2011 Outbreak Caused by E. coli O104:H4. Clin. Infect. Dis. 2017, 64, 1637–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derad, I.; Obermann, B.; Katalinic, A.; Eisemann, N.; Knobloch, J.K.-M.; Sayk, F.; Wellhöner, P.; Lehnert, H.; Solbach, W.; Süfke, S.; et al. Hypertension and mild chronic kidney disease persist following severe haemolytic uraemic syndrome caused by Shiga toxin-producing Escherichia coli O104:H4 in adults. Nephrol. Dial. Transplant. 2016, 31, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Repetto, H.A. Long-term course and mechanisms of progression of renal disease in hemolytic uremic syndrome. Kidney Int. Suppl. 2005, 68, S102–S106. [Google Scholar] [CrossRef] [Green Version]
- Caletti, M.G.; Gallo, G.; Gianantonio, C.A. Development of focal segmental sclerosis and hyalinosis in hemolytic uremic syndrome. Pediatr. Nephrol. 1996, 10, 687–692. [Google Scholar] [CrossRef]
- Lo, L.J.; Go, A.S.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordoñez, J.D.; Hsu, C. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009, 76, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Caletti, M.G.; Lejarraga, H.; Kelmansky, D.; Missoni, M. Two different therapeutic regimes in patients with sequelae of hemolytic-uremic syndrome. Pediatr. Nephrol. 2004, 19, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Caletti, M.G.; Missoni, M.; Vezzani, C.; Grignoli, M.; Piantanida, J.J.; Repetto, H.A.; Exeni, R.; Rasse, S.M. Effect of diet, enalapril, or losartan in post-diarrheal hemolytic uremic syndrome nephropathy. Pediatr. Nephrol. 2011, 26, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.; Valoti, E.; Piras, R.; Bresin, E.; Galbusera, M.; Tripodo, C.; Thaiss, F.; Remuzzi, G.; Noris, M. Two patients with history of STEC-HUS, posttransplant recurrence and complement gene mutations. Am. J. Transplant. 2013, 13, 2201–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassani, C.E.; Ferraris, J.; Gianantonio, C.A.; Ruiz, S.; Ramirez, J. Renal transplantation in patients with classical haemolytic-uraemic syndrome. Pediatr. Nephrol. 1991, 5, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, J.R.; Ramirez, J.A.; Ruiz, S.; Caletti, M.G.; Vallejo, G.; Piantanida, J.J.; Araujo, J.L.; Sojo, E.T. Shiga toxin-associated hemolytic uremic syndrome: Absence of recurrence after renal transplantation. Pediatr. Nephrol. 2002, 17, 809–814. [Google Scholar] [CrossRef]
- Loirat, C.; Niaudet, P. The risk of recurrence of hemolytic uremic syndrome after renal transplantation in children. Pediatr. Nephrol. 2003, 18, 1095–1101. [Google Scholar] [CrossRef]
- Schlieper, A.; Orrbine, E.; Wells, G.A.; Clulow, M.; McLaine, P.N.; Rowe, P.C. Neuropsychological sequelae of haemolytic uraemic syndrome. Investigators of the HUS Cognitive Study. Arch. Dis. Child. 1999, 80, 214–220. [Google Scholar] [CrossRef]
- Buder, K.; Latal, B.; Nef, S.; Neuhaus, T.J.; Laube, G.F.; Spartà, G. Neurodevelopmental long-term outcome in children after hemolytic uremic syndrome. Pediatr. Nephrol. 2015, 30, 503–513. [Google Scholar] [CrossRef]
- Schuppner, R.; Maehlmann, J.; Dirks, M.; Worthmann, H.; Tryc, A.B.; Sandorski, K.; Bahlmann, E.; Kielstein, J.T.; Giesemann, A.M.; Lanfermann, H.; et al. Neurological Sequelae in Adults After E coli O104: H4 Infection-Induced Hemolytic-Uremic Syndrome. Medicine 2016, 95, e2337. [Google Scholar] [CrossRef]
- Masumoto, K.; Nishimoto, Y.; Taguchi, T.; Tsutsumi, Y.; Kanemitsu, S.; Hara, T.; Suita, S. Colonic stricture secondary to hemolytic uremic syndrome caused by Escherichia coli O-157. Pediatr. Nephrol. 2005, 20, 1496–1499. [Google Scholar] [CrossRef]
- Spizzirri, F.D.; Rahman, R.C.; Bibiloni, N.; Ruscasso, J.D.; Amoreo, O.R. Childhood hemolytic uremic syndrome in Argentina: Long-term follow-up and prognostic features. Pediatr. Nephrol. 1997, 11, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Small, G.; Watson, A.R.; Evans, J.H.; Gallagher, J. Hemolytic uremic syndrome: Defining the need for long-term follow-up. Clin. Nephrol. 1999, 52, 352–356. [Google Scholar] [PubMed]
- Hüseman, D.; Gellermann, J.; Vollmer, I.; Ohde, I.; Devaux, S.; Ehrich, J.H.H.; Filler, G. Long-term prognosis of hemolytic uremic syndrome and effective renal plasma flow. Pediatr. Nephrol. 1999, 13, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.L.; Devoto, S.; Fayad, A.; Canepa, C.; Morrow, A.L.; Cleary, T.G. Association between severity of gastrointestinal prodrome and long-term prognosis in classic hemolytic-uremic syndrome. J. Pediatr. 1992, 120, 210–215. [Google Scholar] [CrossRef]
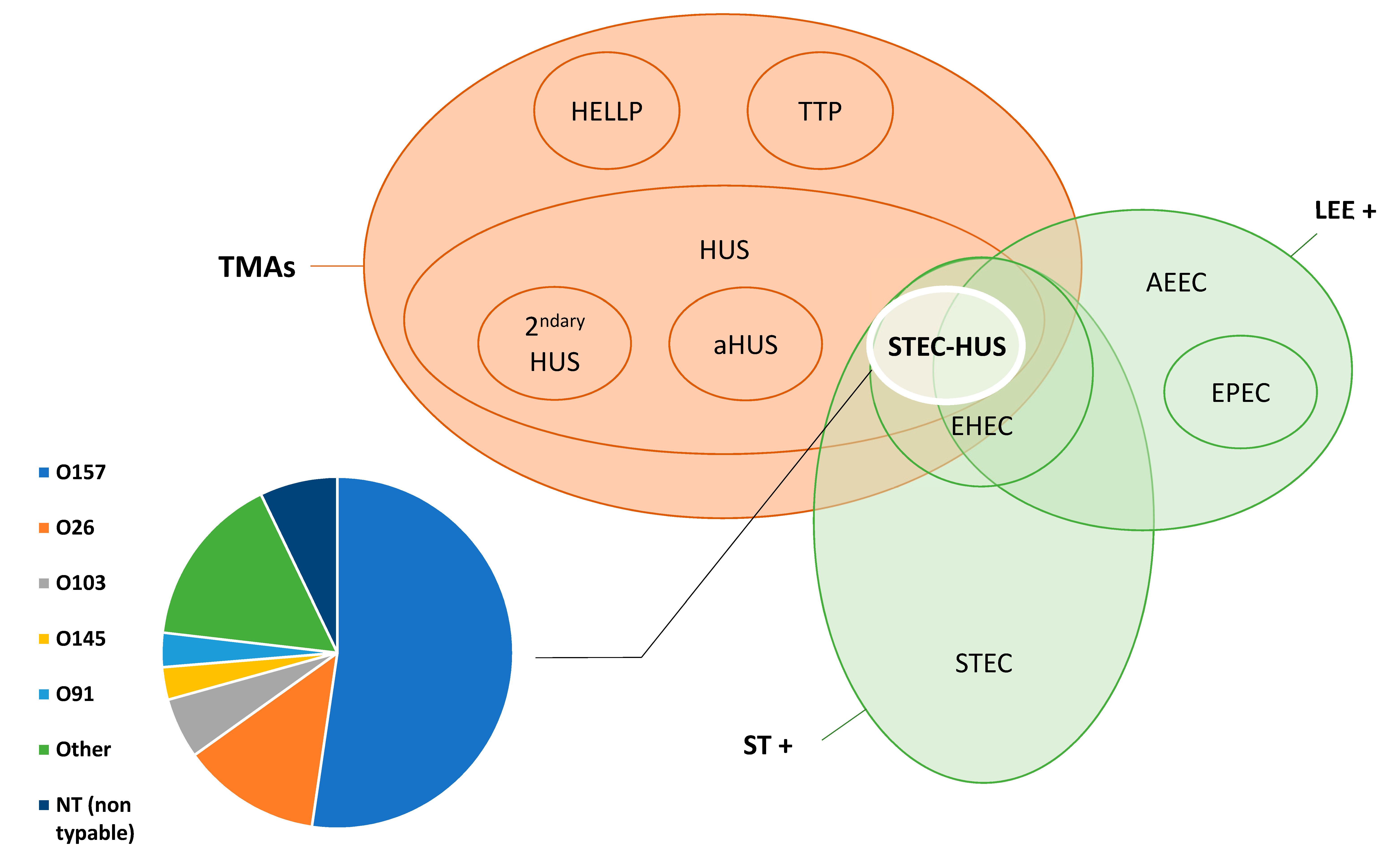


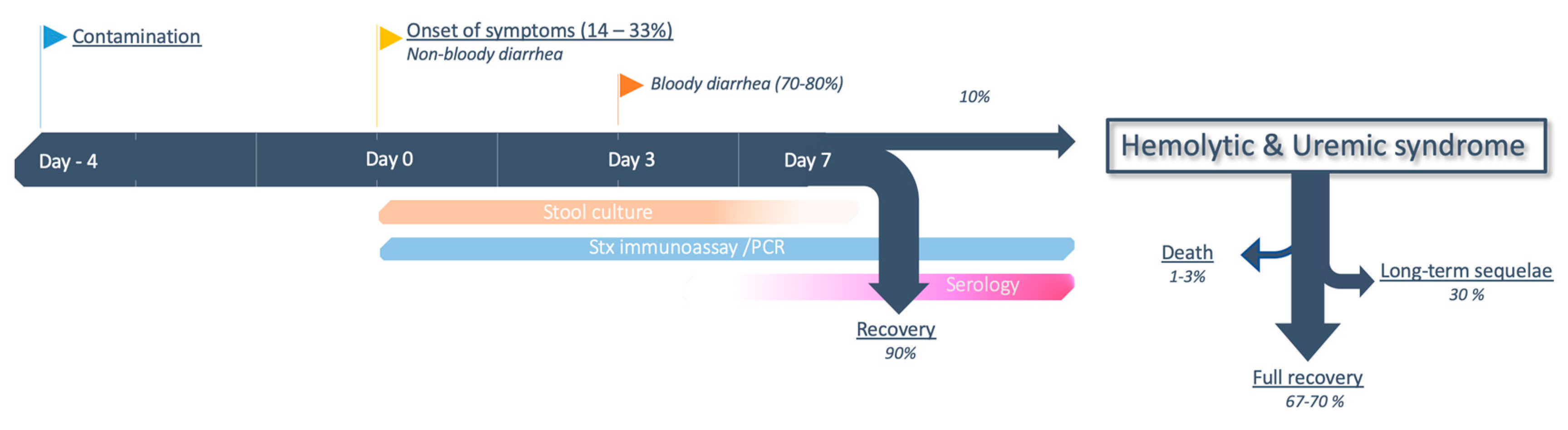
| STEC-HUS mainly occurs through large outbreaks |
| Despite sensational publications about large outbreaks, most STEC-HUS cases (≈75%) are actually sporadic, judging by nationwide studies [99] and surveillance networks [104]. |
| Ground beef is the cause of the majority of vehicle-born transmissions |
| Cattle are a major reservoir for E. coli. Ground beef was responsible for the first outbreaks reported [6,7] and currently represents around 33% of cases [91]. |
| E. coli is the only bacteria that produces Shiga toxin |
| Shigella dysenteriae type 1 produces a chromosomally encoded toxin almost identical to Stx1 [217]. In addition, Stx phages can occasionally be found in other gram-negative bacteria (Citrobacter, Salmonella). |
| Community-acquired nonbloody diarrhea does not suggest investigation for STEC |
| If digestive symptoms are the rule in STEC infections, the proportion of bloody diarrhea can vary between 65%–80%, and is usually lower in non-O157 infections [41,218]. Investigations for STEC can be ordered for community-acquired diarrheas irrespective of the presence of blood [219]. |
| Complement is involved in the pathophysiology of atypical HUS, not STEC-HUS |
| Although the breakthrough discovery of alternative complement pathway dysregulation in aHUS is not paralleled in STEC-HUS, recent publications highlighted a potential role in the pathophysiology of STEC-HUS [183], providing hope for potential clinical applications. |
| HUS with a negative stool culture is probably atypical |
| Stool culture sensitivity is insufficient to exclude STEC-HUS. The diagnostic strategy must include both culture and nonculture-based assays to detect Shiga toxins or the genes encoding it [219]. Additionally, by the time of HUS, enterohemorrhagic E. coli is less likely to be found in stool cultures [220]. |
| Antibiotics are detrimental during STEC infection |
| Antibiotics are not recommended for STEC infection. Nevertheless, an important distinction has to be made between antibiotics capable of triggering bacterial SOS response and the release of Stx (fluoroquinolones, B-lactams) and others (azithromycin, fosfomycin) which do not [33,221]. The potential beneficial effects of the latter agents are currently being evaluated. |
| O157:H7 is responsible for the majority of STEC infections throughout the world |
| A shift in epidemiology occurred in the 2000s, and thanks to new diagnostic techniques, non-0157 serotypes are now more commonly found than 0157:H7 in Europe and North America [41,50]. However, 0157:H7 is still responsible for the majority of cases in Latin America [93]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, A.; Cointe, A.; Mariani Kurkdjian, P.; Rafat, C.; Hertig, A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins 2020, 12, 67. https://doi.org/10.3390/toxins12020067
Joseph A, Cointe A, Mariani Kurkdjian P, Rafat C, Hertig A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins. 2020; 12(2):67. https://doi.org/10.3390/toxins12020067
Chicago/Turabian StyleJoseph, Adrien, Aurélie Cointe, Patricia Mariani Kurkdjian, Cédric Rafat, and Alexandre Hertig. 2020. "Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review" Toxins 12, no. 2: 67. https://doi.org/10.3390/toxins12020067
APA StyleJoseph, A., Cointe, A., Mariani Kurkdjian, P., Rafat, C., & Hertig, A. (2020). Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins, 12(2), 67. https://doi.org/10.3390/toxins12020067





