Molecular Epidemiology of Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus in Wild, Captive and Laboratory Rats: Effect of Habitat on the Nasal S. aureus Population
Abstract
:1. Introduction
2. Results
2.1. Laboratory Rats and Wild Rats Are Colonized with S. aureus
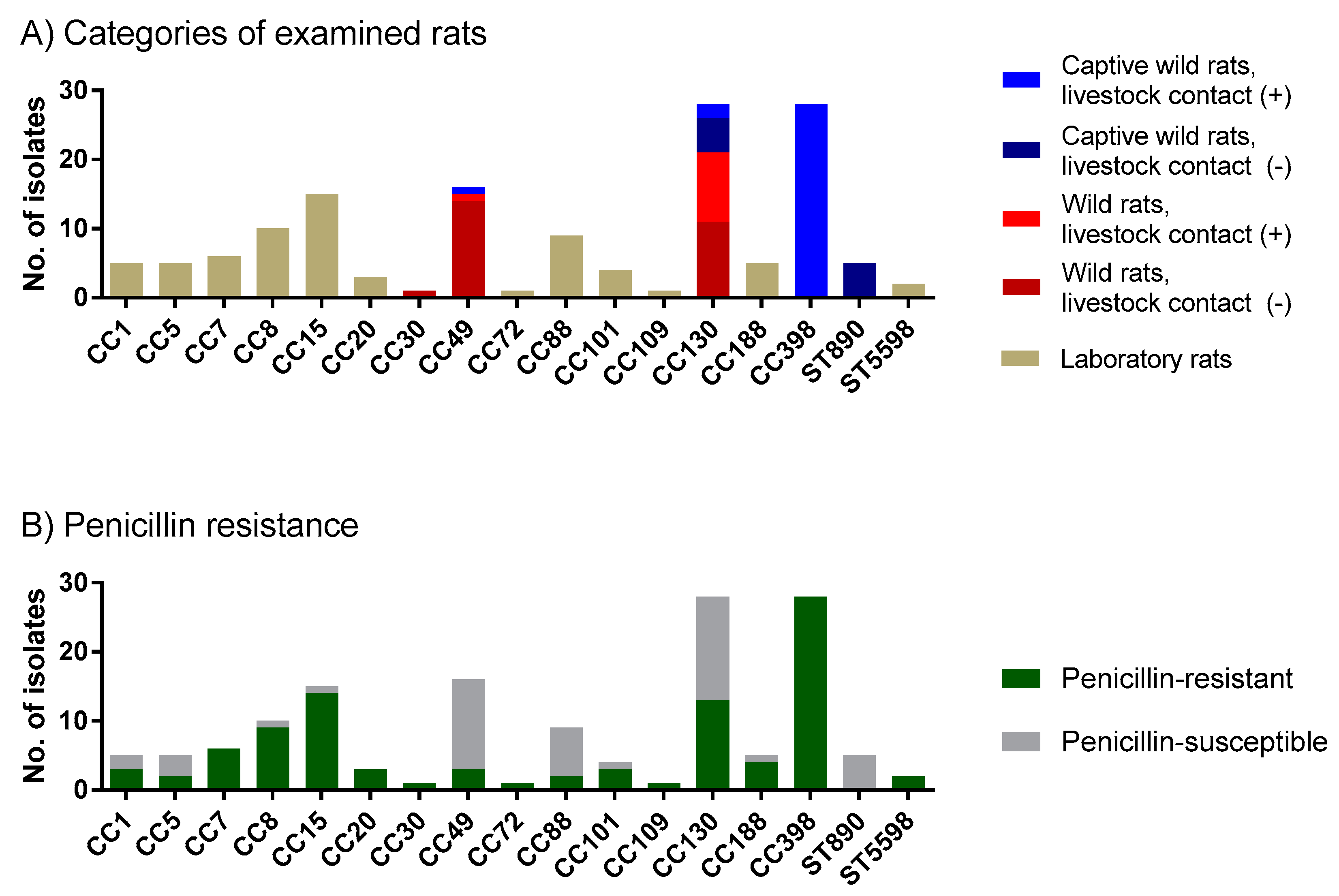
2.2. Wild Rats, Rats with Contact with Livestock and Laboratory Rats Carry Different S. aureus Clonal Complexes
2.3. Penicillin Resistance Was Low in Wild Rats but High in Rats with Livestock Contact
2.4. MRSA Was Detected among Wild Rats, but Not among Laboratory Rats
2.5. S. aureus Isolates from Rats Show Features of Host Adaptation
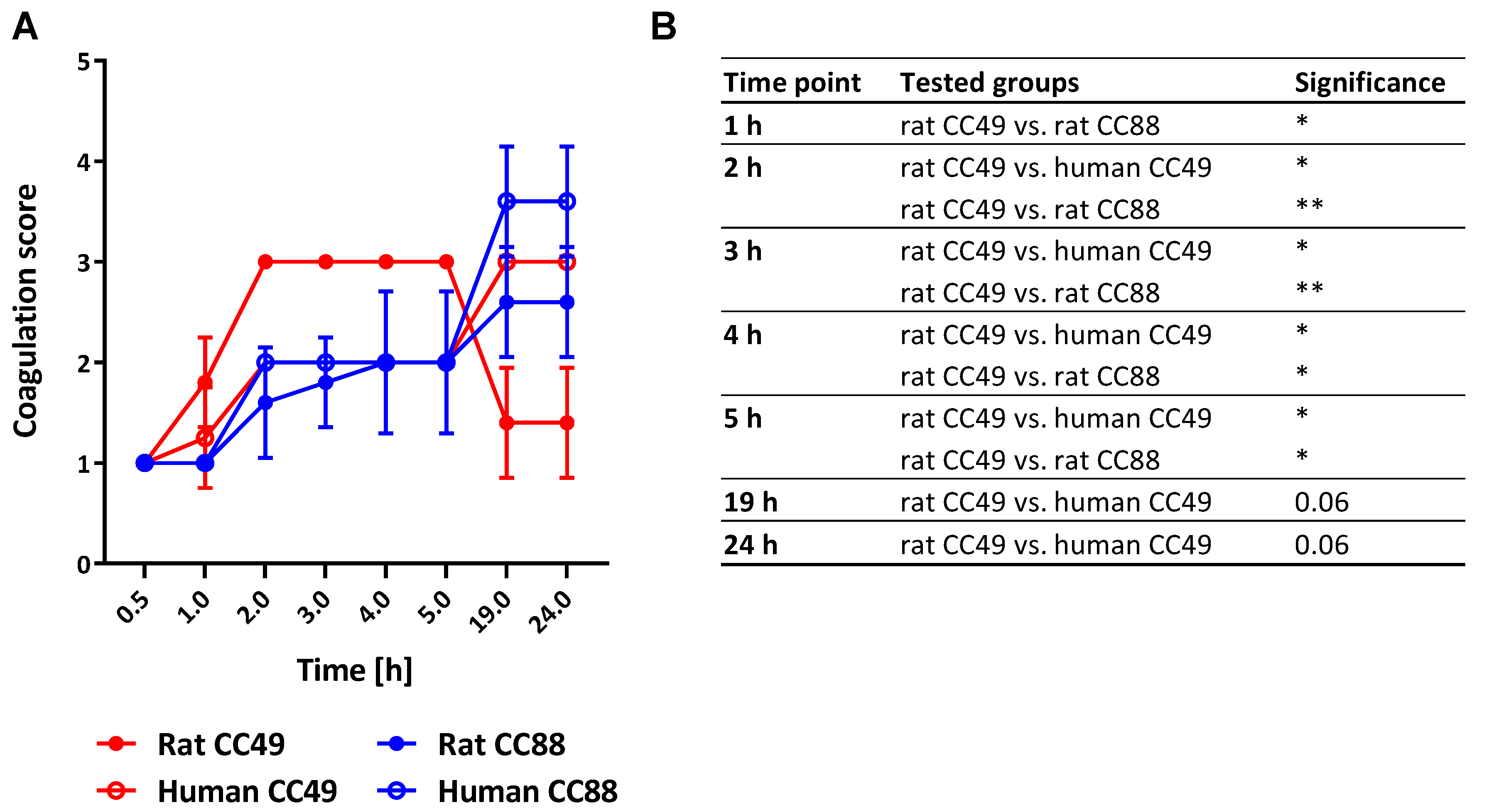
2.6. Both CC and Origin of S. aureus Determine Its Coagulation Behavior
3. Discussion
3.1. Wild Rats Are Predominantly Colonized with The S. aureus Lineages CC49 and CC130
3.2. Rats with Contact with Livestock Frequently Carry CC398
3.3. Laboratory Rats Carry Various Lineages, Mostly of Human Origin
3.4. Captive Wild Rats Maintain Their S. aureus Population
3.5. S. aureus Isolates from Rats Likely Adapt to Their Host by Eliminating MGEs Carrying Human-Specific Virulence Factors
3.6. Rat-derived CC49 Isolates Show Enhanced Procoagulatory Activity on Rat Plasma
3.7. Rats Carrying Human-Derived or LA-MRSA Present a Human Health Risk
4. Conclusions
5. Materials and Methods
5.1. Study Design and Ethics Statements
5.2. Sample Preparation and Screening for S. aureus
5.3. S. aureus Identification
5.4. Spa Genotyping and Multilocus Sequence Typing (MLST)
5.5. Virulence Gene Detection Using Multiplex PCR
5.6. MIC of Penicillin Against S. aureus Strains Using the Broth Microdilution Method
5.7. Antibiograms and mecA-D PCR
5.8. Coagulation Assay
5.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wertheim, H.F.L.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Kaspar, U.; Kriegeskorte, A.; Schubert, T.; Peters, G.; Rudack, C.; Pieper, D.H.; Wos-Oxley, M.; Becker, K. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ. Microbiol. 2016, 18, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Schaumburg, F.; Fegeler, C.; Friedrich, A.W.; Köck, R. Staphylococcus aureus from the German general population is highly diverse. Int. J. Med. Microbiol. 2017, 307, 21–27. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Redi, D.; Raffaelli, C.S.; Rossetti, B.; de Luca, A.; Montagnani, F. Staphylococcus aureus vaccine preclinical and clinical development: Current state of the art. New Microbiol. 2018, 41, 208–213. [Google Scholar]
- Harrison, E.M.; Weinert, L.A.; Holden, M.T.G.; Welch, J.J.; Wilson, K.; Morgan, F.J.E.; Harris, S.R.; Loeffler, A.; Boag, A.K.; Peacock, S.J.; et al. A shared population of epidemic methicillin-resistant Staphylococcus aureus 15 circulates in humans and companion animals. mBio 2014, 5, e00985-13. [Google Scholar] [CrossRef] [Green Version]
- Monecke, S.; Gavier-Widén, D.; Hotzel, H.; Peters, M.; Guenther, S.; Lazaris, A.; Loncaric, I.; Müller, E.; Reissig, A.; Ruppelt-Lorz, A.; et al. Diversity of Staphylococcus aureus Isolates in European Wildlife. PLoS ONE 2016, 11, e0168433. [Google Scholar] [CrossRef] [Green Version]
- Mrochen, D.M.; Schulz, D.; Fischer, S.; Jeske, K.; El Gohary, H.; Reil, D.; Imholt, C.; Trübe, P.; Suchomel, J.; Tricaud, E.; et al. Wild rodents and shrews are natural hosts of Staphylococcus aureus. Int. J. Med. Microbiol. 2018, 308, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Van Alen, S.; Ballhausen, B.; Peters, G.; Friedrich, A.W.; Mellmann, A.; Köck, R.; Becker, K. In the centre of an epidemic: Fifteen years of LA-MRSA CC398 at the University Hospital Münster. Vet. Microbiol. 2017, 200, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Schaumburg, F.; Mellmann, A.; Köksal, M.; Jurke, A.; Becker, K.; Friedrich, A.W. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS ONE 2013, 8, e55040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantosti, A. Methicillin-Resistant Staphylococcus aureus Associated with Animals and Its Relevance to Human Health. Front. Microbiol. 2012, 3, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graveland, H.; Duim, B.; van Duijkeren, E.; Heederik, D.; Wagenaar, J.A. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int. J. Med. Microbiol. 2011, 301, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Rothenburger, J.L.; Himsworth, C.G.; La Perle, K.M.D.; Leighton, F.A.; Nemeth, N.M.; Treuting, P.M.; Jardine, C.M. Pathology of wild Norway rats in Vancouver, Canada. J. Vet. Diagn. Investig. 2019, 31, 184–199. [Google Scholar] [CrossRef] [Green Version]
- Rothenburger, J.L.; Himsworth, C.G.; Nemeth, N.M.; Pearl, D.L.; Jardine, C.M. Environmental Factors Associated with the Carriage of Bacterial Pathogens in Norway Rats. Ecohealth 2018, 15, 82–95. [Google Scholar] [CrossRef]
- Rothenburger, J.L.; Rousseau, J.D.; Weese, J.S.; Jardine, C.M. Livestock-associated methicillin-resistant Staphylococcus aureus and Clostridium difficile in wild Norway rats (Rattus norvegicus) from Ontario swine farms. Can. J. Vet. Res. 2018, 82, 66–69. [Google Scholar]
- Himsworth, C.G.; Miller, R.R.; Montoya, V.; Hoang, L.; Romney, M.G.; Al-Rawahi, G.N.; Kerr, T.; Jardine, C.M.; Patrick, D.M.; Tang, P.; et al. Carriage of methicillin-resistant Staphylococcus aureus by wild urban Norway rats (Rattus norvegicus). PLoS ONE 2014, 9, e87983. [Google Scholar] [CrossRef]
- Lee, M.J.; Byers, K.A.; Donovan, C.M.; Zabek, E.; Stephen, C.; Patrick, D.M.; Himsworth, C.G. Methicillin-resistant Staphylococcus aureus in urban Norway rat (Rattus norvegicus) populations: Epidemiology and the impacts of kill-trapping. Zoonoses Public Health 2019, 66, 343–348. [Google Scholar] [CrossRef]
- Van de Giessen, A.W.; van Santen-Verheuvel, M.G.; Hengeveld, P.D.; Bosch, T.; Broens, E.M.; Reusken, C.B.E.M. Occurrence of methicillin-resistant Staphylococcus aureus in rats living on pig farms. Prev. Vet. Med. 2009, 91, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Bramble, M.; Morris, D.; Tolomeo, P.; Lautenbach, E. Potential role of pet animals in household transmission of methicillin-resistant Staphylococcus aureus: A narrative review. Vector Borne Zoonotic Dis. 2011, 11, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Aires Sousa, M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microbiol. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Sakwinska, O.; Giddey, M.; Moreillon, M.; Morisset, D.; Waldvogel, A.; Moreillon, P. Staphylococcus aureus host range and human-bovine host shift. Appl. Environ. Microbiol. 2011, 77, 5908–5915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, J.A.; Holden, M.T.G. Staphylococcus aureus: Superbug, super genome? Trends Microbiol. 2004, 12, 378–385. [Google Scholar] [CrossRef]
- Lindsay, J.A.; Moore, C.E.; Day, N.P.; Peacock, S.J.; Witney, A.A.; Stabler, R.A.; Husain, S.E.; Butcher, P.D.; Hinds, J. Microarrays Reveal that Each of the Ten Dominant Lineages of Staphylococcus aureus Has a Unique Combination of Surface-Associated and Regulatory Genes. J. Bacteriol. 2006, 188, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Wolz, C. Phages of Staphylococcus aureus and their impact on host evolution. Infect. Genet. Evol. 2014, 21, 593–601. [Google Scholar] [CrossRef]
- Richardson, E.J.; Bacigalupe, R.; Harrison, E.M.; Weinert, L.A.; Lycett, S.; Vrieling, M.; Robb, K.; Hoskisson, P.A.; Holden, M.T.G.; Feil, E.J.; et al. Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat. Ecol. Evol. 2018, 2, 1468–1478. [Google Scholar] [CrossRef]
- Viana, D.; Blanco, J.; Tormo-Más, M.Á.; Selva, L.; Guinane, C.M.; Baselga, R.; Corpa, J.M.; Lasa, Í.; Novick, R.P.; Fitzgerald, J.R.; et al. Adaptation of Staphylococcus aureus to ruminant and equine hosts involves SaPI-carried variants of von Willebrand factor-binding protein. Mol. Microbiol. 2010, 77, 1583–1594. [Google Scholar] [CrossRef]
- Cuny, C.; Abdelbary, M.; Layer, F.; Werner, G.; Witte, W. Prevalence of the immune evasion gene cluster in Staphylococcus aureus CC398. Vet. Microbiol. 2015, 177, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Viana, D.; Comos, M.; McAdam, P.R.; Ward, M.J.; Selva, L.; Guinane, C.M.; González-Muñoz, B.M.; Tristan, A.; Foster, S.J.; Fitzgerald, J.R.; et al. A single natural nucleotide mutation alters bacterial pathogen host tropism. Nat. Genet. 2015, 47, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Mrochen, D.M.; Grumann, D.; Schulz, D.; Gumz, J.; Trübe, P.; Pritchett-Corning, K.; Johnson, S.; Nicklas, W.; Kirsch, P.; Martelet, K.; et al. Global spread of mouse-adapted Staphylococcus aureus lineages CC1, CC15, and CC88 among mouse breeding facilities. Int. J. Med. Microbiol. 2018, 308, 598–606. [Google Scholar] [CrossRef]
- Lowder, B.V.; Guinane, C.M.; Ben Zakour, N.L.; Weinert, L.A.; Conway-Morris, A.; Cartwright, R.A.; Simpson, A.J.; Rambaut, A.; Nübel, U.; Fitzgerald, J.R. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2009, 106, 19545–19550. [Google Scholar] [CrossRef] [Green Version]
- Moodley, A.; Espinosa-Gongora, C.; Nielsen, S.S.; McCarthy, A.J.; Lindsay, J.A.; Guardabassi, L. Comparative host specificity of human- and pig- associated Staphylococcus aureus clonal lineages. PLoS ONE 2012, 7, e49344. [Google Scholar] [CrossRef]
- Walther, B.; Monecke, S.; Ruscher, C.; Friedrich, A.W.; Ehricht, R.; Slickers, P.; Soba, A.; Wleklinski, C.-G.; Wieler, L.H.; Lübke-Becker, A. Comparative molecular analysis substantiates zoonotic potential of equine methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2009, 47, 704–710. [Google Scholar] [CrossRef] [Green Version]
- Vincze, S.; Stamm, I.; Monecke, S.; Kopp, P.A.; Semmler, T.; Wieler, L.H.; Lübke-Becker, A.; Walther, B. Molecular analysis of human and canine Staphylococcus aureus strains reveals distinct extended-host-spectrum genotypes independent of their methicillin resistance. Appl. Environ. Microbiol. 2013, 79, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Astrup, L.B.; Skovgaard, K.; Rasmussen, R.S.; Iburg, T.M.; Agerholm, J.S.; Aalbæk, B.; Jensen, H.E.; Nielsen, O.L.; Johansen, F.F.; Heegaard, P.M.H.; et al. Staphylococcus aureus infected embolic stroke upregulates Orm1 and Cxcl2 in a rat model of septic stroke pathology. Neurol. Res. 2019, 41, 399–412. [Google Scholar] [CrossRef]
- Hanses, F.; Roux, C.; Dunman, P.M.; Salzberger, B.; Lee, J.C. Staphylococcus aureus gene expression in a rat model of infective endocarditis. Genome Med. 2014, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Power, M.E.; Olson, M.E.; Domingue, P.A.G.; Costerton, J.W. A rat model of Staphylococcus aureus chronic osteomyelitis that provides a suitable system for studying the human infection. J. Med. Microbiol. 1990, 33, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Trübe, P.; Hertlein, T.; Mrochen, D.M.; Schulz, D.; Jorde, I.; Krause, B.; Zeun, J.; Fischer, S.; Wolf, S.A.; Walther, B.; et al. Bringing together what belongs together: Optimizing murine infection models by using mouse-adapted Staphylococcus aureus strains. Int. J. Med. Microbiol. 2019, 309, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Emolo, C.; Holtfreter, S.; Wiles, S.; Kreiswirth, B.; Missiakas, D.; Schneewind, O. Staphylococcal Protein A Contributes to Persistent Colonization of Mice with Staphylococcus aureus. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtfreter, S.; Grumann, D.; Balau, V.; Barwich, A.; Kolata, J.; Goehler, A.; Weiss, S.; Holtfreter, B.; Bauerfeind, S.S.; Döring, P.; et al. Molecular Epidemiology of Staphylococcus aureus in the General Population in Northeast Germany: Results of the Study of Health in Pomerania (SHIP-TREND-0). J. Clin. Microbiol. 2016, 54, 2774–2785. [Google Scholar] [CrossRef] [Green Version]
- Schulz, D.; Grumann, D.; Trübe, P.; Pritchett-Corning, K.; Johnson, S.; Reppschläger, K.; Gumz, J.; Sundaramoorthy, N.; Michalik, S.; Berg, S.; et al. Laboratory Mice Are Frequently Colonized with Staphylococcus aureus and Mount a Systemic Immune Response-Note of Caution for In vivo Infection Experiments. Front. Cell. Infect. Microbiol. 2017, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.M.-L.; Lloyd, D.H.; Lindsay, J.A. Staphylococcus aureus host specificity: Comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology 2008, 154, 1949–1959. [Google Scholar] [CrossRef] [Green Version]
- Van Wamel, W.J.B.; Rooijakkers, S.H.M.; Ruyken, M.; van Kessel, K.P.M.; van Strijp, J.A.G. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Ripa, L.; Alcalá, L.; Simón, C.; Gómez, P.; Mama, O.M.; Rezusta, A.; Zarazaga, M.; Torres, C. Diversity of Staphylococcus aureus clones in wild mammals in Aragon, Spain, with detection of MRSA ST130-mecC in wild rabbits. J. Appl. Microbiol. 2019, 127, 284–291. [Google Scholar] [CrossRef]
- Ruiz-Ripa, L.; Gómez, P.; Alonso, C.A.; Camacho, M.C.; de La Puente, J.; Fernández-Fernández, R.; Ramiro, Y.; Quevedo, M.A.; Blanco, J.M.; Zarazaga, M.; et al. Detection of MRSA of Lineages CC130-mecC and CC398-mecA and Staphylococcus delphini-lnu (A) in Magpies and Cinereous Vultures in Spain. Microb. Ecol. 2019, 78, 409–415. [Google Scholar] [CrossRef]
- Ben Said, M.; Abbassi, M.S.; Gómez, P.; Ruiz-Ripa, L.; Sghaier, S.; El Fekih, O.; Hassen, A.; Torres, C. Genetic characterization of Staphylococcus aureus isolated from nasal samples of healthy ewes in Tunisia. High prevalence of CC130 and CC522 lineages. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51, 37–40. [Google Scholar] [CrossRef]
- Simpson, V.R.; Davison, N.J.; Kearns, A.M.; Pichon, B.; Hudson, L.O.; Koylass, M.; Blackett, T.; Butler, H.; Rasigade, J.P.; Whatmore, A.M. Association of a lukM-positive clone of Staphylococcus aureus with fatal exudative dermatitis in red squirrels (Sciurus vulgaris). Vet. Microbiol. 2013, 162, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Simpson, V.; Davison, N.; Hudson, L.; Whatmore, A.M. Staphylococcus aureus ST49 infection in red squirrels. Vet. Rec. 2010, 167, 69. [Google Scholar] [CrossRef] [PubMed]
- Mama, O.M.; Ruiz-Ripa, L.; Fernández-Fernández, R.; González-Barrio, D.; Ruiz-Fons, J.F.; Torres, C. High frequency of coagulase-positive staphylococci carriage in healthy wild boar with detection of MRSA of lineage ST398-t011. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef] [PubMed]
- Overesch, G.; Büttner, S.; Rossano, A.; Perreten, V. The increase of methicillin-resistant Staphylococcus aureus (MRSA) and the presence of an unusual sequence type ST49 in slaughter pigs in Switzerland. BMC Vet. Res. 2011, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haenni, M.; Châtre, P.; Dupieux-Chabert, C.; Métayer, V.; Bes, M.; Madec, J.-Y.; Laurent, F. Molecular Epidemiology of Methicillin-Resistant Staphylococcus aureus in Horses, Cats, and Dogs Over a 5-Year Period in France. Front. Microbiol. 2017, 8, 2493. [Google Scholar] [CrossRef] [PubMed]
- Deplano, A.; Vandendriessche, S.; Nonhoff, C.; Denis, O. Genetic diversity among methicillin-resistant Staphylococcus aureus isolates carrying the mecC gene in Belgium. J. Antimicrob. Chemother. 2014, 69, 1457–1460. [Google Scholar] [CrossRef] [Green Version]
- Witte, W. Selective pressure by antibiotic use in livestock. Int. J. Antimicrob. Agents 2000, 16, 19–24. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ammini, P.; Kumar, S.; Varela, M.F. The Food Production Environment and the Development of Antimicrobial Resistance in Human Pathogens of Animal Origin. Microorganisms 2017, 5, 11. [Google Scholar] [CrossRef]
- Kadlec, K.; Entorf, M.; Peters, T. Occurrence and Characteristics of Livestock-Associated Methicillin-Resistant Staphylococcus aureus in Quarter Milk Samples from Dairy Cows in Germany. Front. Microbiol. 2019, 10, 1295. [Google Scholar] [CrossRef] [Green Version]
- Köck, R.; Ballhausen, B.; Bischoff, M.; Cuny, C.; Eckmanns, T.; Fetsch, A.; Harmsen, D.; Goerge, T.; Oberheitmann, B.; Schwarz, S.; et al. The impact of zoonotic MRSA colonization and infection in Germany. Berl. Munch. Tierarztl. Wochenschr. 2014, 127, 384–398. [Google Scholar]
- Guinane, C.M.; Ben Zakour, N.L.; Tormo-Mas, M.A.; Weinert, L.A.; Lowder, B.V.; Cartwright, R.A.; Smyth, D.S.; Smyth, C.J.; Lindsay, J.A.; Gould, K.A.; et al. Evolutionary genomics of Staphylococcus aureus reveals insights into the origin and molecular basis of ruminant host adaptation. Genome Biol. Evol. 2010, 2, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messenger, A.M.; Barnes, A.N.; Gray, G.C. Reverse zoonotic disease transmission (zooanthroponosis): A systematic review of seldom-documented human biological threats to animals. PLoS ONE 2014, 9, e89055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, J.; Langella, P.; Cherbuy, C. The intestinal microbiota in the rat model: Major breakthroughs from new technologies. Anim. Health Res. Rev. 2012, 13, 54–63. [Google Scholar] [CrossRef]
- Mulcahy, M.E.; McLoughlin, R.M. Host-Bacterial Crosstalk Determines Staphylococcus aureus Nasal Colonization. Trends Microbiol. 2016, 24, 872–886. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.-S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- De Haas, C.J.C.; Veldkamp, K.E.; Peschel, A.; Weerkamp, F.; van Wamel, W.J.B.; Heezius, E.C.J.M.; Poppelier, M.J.J.G.; van Kessel, K.P.M.; van Strijp, J.A.G. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 2004, 199, 687–695. [Google Scholar] [CrossRef]
- Gladysheva, I.P.; Turner, R.B.; Sazonova, I.Y.; Liu, L.; Reed, G.L. Coevolutionary patterns in plasminogen activation. Proc. Natl. Acad. Sci. USA 2003, 100, 9168–9172. [Google Scholar] [CrossRef] [Green Version]
- Holtfreter, S.; Bröker, B.M. Staphylococcal superantigens: Do they play a role in sepsis? Arch. Immunol. Ther. Exp. 2005, 53, 13–27. [Google Scholar]
- Rooijakkers, S.H.M.; Ruyken, M.; Roos, A.; Daha, M.R.; Presanis, J.S.; Sim, R.B.; van Wamel, W.J.B.; van Kessel, K.P.M.; van Strijp, J.A.G. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 2005, 6, 920–927. [Google Scholar] [CrossRef]
- Hashimoto, M.; Watanabe, S.; Oiwa, K.; Ohta, Y.; Kishi, T.; Okamoto, T.; Giddings, J.C.; Yamamoto, J. Enhanced thrombolysis induced by argatroban or activated protein C in the presence or absence of staphylokinase, measured in an in vivo animal model using mesenteric arterioles. Haemostasis 2001, 31, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Baba, T.; Sekine, M.; Fukuda, M.; Hiramatsu, K. Beta-hemolysin promotes skin colonization by Staphylococcus aureus. J. Bacteriol. 2013, 195, 1194–1203. [Google Scholar] [CrossRef]
- Verkaik, N.J.; Benard, M.; Boelens, H.A.; de Vogel, C.P.; Nouwen, J.L.; Verbrugh, H.A.; Melles, D.C.; van Belkum, A.; van Wamel, W.J.B. Immune evasion cluster-positive bacteriophages are highly prevalent among human Staphylococcus aureus strains, but they are not essential in the first stages of nasal colonization. Clin. Microbiol. Infect. 2011, 17, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, N.P.; Markham, J.G. Staphylococci in man and animals. Distribution and characteristics of strains. J. Comp. Pathol. 1966, 76, 49–56. [Google Scholar] [CrossRef]
- Grumann, D.; Scharf, S.S.; Holtfreter, S.; Kohler, C.; Steil, L.; Engelmann, S.; Hecker, M.; Völker, U.; Bröker, B.M. Immune cell activation by enterotoxin gene cluster (egc)-encoded and non-egc superantigens from Staphylococcus aureus. J. Immunol. 2008, 181, 5054–5061. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, M.R.; Young, K.E.; Gibbon, L.G.; Webster, C.A.; Tranter, H.S.; Arbuthnott, J.P.; Todd, I. Stimulation of rat spleen cells by staphylococcal enterotoxins. FEMS Immunol. Med. Microbiol. 1993, 7, 169–174. [Google Scholar] [CrossRef]
- Van Loo, I.; Huijsdens, X.; Tiemersma, E.; de Neeling, A.; van de Sande-Bruinsma, N.; Beaujean, D.; Voss, A.; Kluytmans, J. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg. Infect. Dis. 2007, 13, 1834–1839. [Google Scholar] [CrossRef]
- Becker, K.; Ballhausen, B.; Kahl, B.C.; Köck, R. The clinical impact of livestock-associated methicillin-resistant Staphylococcus aureus of the clonal complex 398 for humans. Vet. Microbiol. 2017, 200, 33–38. [Google Scholar] [CrossRef]
- Gibbs, S.G.; Green, C.F.; Tarwater, P.M.; Mota, L.C.; Mena, K.D.; Scarpino, P.V. Isolation of antibiotic-resistant bacteria from the air plume downwind of a swine confined or concentrated animal feeding operation. Environ. Health Perspect. 2006, 114, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, S.G.; Green, C.F.; Tarwater, P.M.; Scarpino, P.V. Airborne antibiotic resistant and nonresistant bacteria and fungi recovered from two swine herd confined animal feeding operations. J. Occup. Environ. Hyg. 2004, 1, 699–706. [Google Scholar] [CrossRef]
- Himsworth, C.G.; Parsons, K.L.; Jardine, C.; Patrick, D.M. Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis. 2013, 13, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wobeser, G.; Campbell, G.D.; Dallaire, A.; McBurney, S. Tularemia, plague, yersiniosis, and Tyzzer’s disease in wild rodents and lagomorphs in Canada: A review. Can. Vet. J. 2009, 50, 1251–1256. [Google Scholar] [PubMed]
- Strand, T.M.; Lundkvist, Å. Rat-borne diseases at the horizon. A systematic review on infectious agents carried by rats in Europe 1995–2016. Infect. Ecol. Epidemiol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, K.D. Range of Movement and Activity of Common Rats (Rattus norvegicus) on Agricultural Land. J. Appl. Ecol. 1978, 15, 663–677. [Google Scholar] [CrossRef]
- Reid, R.A.; Reid, A.K. Route finding by rats in an open arena. Behav. Processes 2005, 68, 51–67. [Google Scholar] [CrossRef]
- Young, D.M.; Harris, H.W.; Charlebois, E.D.; Chambers, H.; Campbell, A.; Perdreau-Remington, F.; Lee, C.; Mankani, M.; Mackersie, R.; Schecter, W.P. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch. Surg. 2004, 139, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Luedicke, C.; Slickers, P.; Ehricht, R.; Monecke, S. Molecular fingerprinting of Staphylococcus aureus from bone and joint infections. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 457–463. [Google Scholar] [CrossRef]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host adaptation and emergence of methicillin resistance in livestock. mBio 2012, 3, e00305-11. [Google Scholar] [CrossRef] [Green Version]
- Van Alen, S.; Ballhausen, B.; Kaspar, U.; Köck, R.; Becker, K. Prevalence and Genomic Structure of Bacteriophage phi3 in Human-Derived Livestock-Associated Methicillin-Resistant Staphylococcus aureus Isolates from 2000 to 2015. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [Green Version]
- Spahr, C.; Knauf-Witzens, T.; Vahlenkamp, T.; Ulrich, R.G.; Johne, R. Hepatitis E virus and related viruses in wild, domestic and zoo animals: A review. Zoonoses Public Health 2018, 65, 11–29. [Google Scholar] [CrossRef]
- Holtfreter, S.; Bauer, K.; Thomas, D.; Feig, C.; Lorenz, V.; Roschack, K.; Friebe, E.; Selleng, K.; Lövenich, S.; Greve, T.; et al. egc-Encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect. Immun. 2004, 72, 4061–4071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtfreter, S.; Grumann, D.; Schmudde, M.; Nguyen, H.T.T.; Eichler, P.; Strommenger, B.; Kopron, K.; Kolata, J.; Giedrys-Kalemba, S.; Steinmetz, I.; et al. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J. Clin. Microbiol. 2007, 45, 2669–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Völzke, H.; Alte, D.; Schmidt, C.O.; Radke, D.; Lorbeer, R.; Friedrich, N.; Aumann, N.; Lau, K.; Piontek, M.; Born, G.; et al. Cohort profile: The study of health in Pomerania. Int. J. Epidemiol. 2011, 40, 294–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Sparling, J.; Chow, B.L.; Elsayed, S.; Hussain, Z.; Church, D.L.; Gregson, D.B.; Louie, T.; Conly, J.M. New Quadriplex PCR Assay for Detection of Methicillin and Mupirocin Resistance and Simultaneous Discrimination of Staphylococcus aureus from Coagulase-Negative Staphylococci. J. Clin. Microbiol. 2004, 42, 4947–4955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strommenger, B.; Kettlitz, C.; Weniger, T.; Harmsen, D.; Friedrich, A.W.; Witte, W. Assignment of Staphylococcus Isolates to Groups by spa Typing, SmaI Macrorestriction Analysis, and Multilocus Sequence Typing. J. Clin. Microbiol. 2006, 44, 2533–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmsen, D.; Claus, H.; Witte, W.; Rothgänger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [Green Version]
- Goerke, C.; Pantucek, R.; Holtfreter, S.; Schulte, B.; Zink, M.; Grumann, D.; Bröker, B.M.; Doskar, J.; Wolz, C. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J. Bacteriol. 2009, 191, 3462–3468. [Google Scholar] [CrossRef] [Green Version]
- Jean, B.; Mpw, P.; Eliopoulos, G.M.; Jenkins, S.G. M100: Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Schwendener, S.; Cotting, K.; Perreten, V. Novel methicillin resistance gene mecD in clinical Macrococcus caseolyticus strains from bovine and canine sources. Sci. Rep. 2017, 7, 43797. [Google Scholar] [CrossRef]
- Cuny, C.; Layer, F.; Strommenger, B.; Witte, W. Rare occurrence of methicillin-resistant Staphylococcus aureus CC130 with a novel mecA homologue in humans in Germany. PLoS ONE 2011, 6, e24360. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.; van Alen, S.; Idelevich, E.A.; Schleimer, N.; Seggewiß, J.; Mellmann, A.; Kaspar, U.; Peters, G. Plasmid-Encoded Transferable mecB-Mediated Methicillin Resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018, 24, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperber, W.H.; Tatini, S.R. Interpretation of the Tube Coagulase Test for Identification of Staphylococcus aureus. Appl. Microbiol. 1975, 29, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Category 1 | Country | State 2 | No. (Rats) | S. aureus+ (%) | MRSA (%) 3 | PenR (%) 4 |
|---|---|---|---|---|---|---|
| Free-living wild | GER | BW | 17 | 1 (5.9) | 1 (5.9) | 1 (100.0) |
| GER | MV | 18 | 4 (22.2) | 0 (0.0) | 1 (25.0) | |
| GER | NRW_1 | 49 | 4 (8.2) | 1 (2.0) | 3 (75.0) | |
| NRW_2 | 32 | 10 (31.3) | 0 (0.0) | 9 (90.0) | ||
| CZE | MSR | 29 | 18 (62.1) | 0 (0.0) | 1 (5.6) | |
| Total | 145 | 37 (25.5) | 2 (1.4) | 15 (40.5) | ||
| Captivewild | GER | BB | 72 | 14 (19.4) | 5 (6.9) | 5 (35.7) |
| GER | BE | 35 | 27 (77.1) | 1 (2.9) | 25 (92.6) | |
| GER | NRW | 81 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Total | 188 | 41 (21.8) | 6 (3.2) | 30 (73.2) | ||
| Laboratory | GER | BW | 20 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| GER | HE | 40 | 7 (17.5) | 0 (0.0) | 5 (71.4) | |
| GER | MV | 21 | 7 (33.3) | 0 (0.0) | 7 (100.0) | |
| GER | NRW | 33 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Total | 114 | 14 (12.3) | 0 (0.0) | 12 (85.7) |
| Category 1 | Strain ID | Habitat 2 | Country 3 | State 3 | Strain | Species | Year | spa Type | CC | mec Genes 4 | MRSA agar | CefR | Oxa-MIC (µg/mL) | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Free-living wild | KS/17/175 | town | GER | NRW_1 | NA | Rattus norvegicus | 2016 | t685 | CC30 | mecA | + | + | Oxa ≤ 0.25 | MRSA 5 |
| KS/17/378 | zoo, pest animal | GER | BW | NA | R. norvegicus | 2012 | t843 | CC130 | mecC | + | + | Oxa ≤ 0.25 | MRSA 5 | |
| Captive wild | KS/17/19 | livestock farm | GER | BB | Neufels | R. rattus | 2016 | t011 | CC398 | mecA | + | + | Oxa ≥ 4 | MRSA |
| KS/17/20 | livestock farm | GER | BB | Neufels | R. rattus | 2016 | t011 | CC398 | mecA | + | + | Oxa ≥ 4 | MRSA | |
| KS/17/21 | livestock farm | GER | BB | Neufels | R. rattus | 2016 | t011 | CC398 | mecA | + | + | Oxa ≥ 4 | MRSA | |
| KS/17/22 | livestock farm | GER | BB | Neufels | R. rattus | 2016 | t011 | CC398 | mecA | + | + | Oxa ≥ 4 | MRSA | |
| KS/17/46 | livestock farm | GER | BB | Neufels | R. rattus | 2016 | t011 | CC398 | mecA | + | + | Oxa ≥ 4 | MRSA | |
| KS/17/390 | livestock farm | GER | BE | Neufels | R. rattus | 2017 | t843 | CC130 | mecC | + | + | Oxa ≤ 0.25 | MRSA5 |
| Rat | Human | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | Total | % | No. | Total | % | p Value 2 | ||
| Phage-carried IEC genes | CC7 | 6 | 6 | 100.0 | 10 | 10 | 100.0 | n.s. |
| CC8 | 0 | 10 | 0.0 | 9 | 10 | 90.0 | p < 0.001 | |
| CC49 | 0 | 14 | 0.0 | 3 | 4 | 75.0 | p < 0.001 | |
| CC88 | 3 | 9 | 33.3 | 10 | 10 | 100.0 | p < 0.01 | |
| CC130 | 0 | 28 | 0.0 | 0 | 9 | 0.0 | n.s. | |
| CC398 | 0 | 15 | 0.0 | 5 | 7 | 71.4 | p < 0.001 | |
| MGE-carried SAg genes | CC7 | 3 | 6 | 50.0 | 9 | 10 | 90.0 | n.s. |
| CC8 | 0 | 10 | 0.0 | 7 | 10 | 70.0 | p < 0.01 | |
| CC49 | 0 | 14 | 0.0 | 0 | 4 | 0.0 | n.s. | |
| CC88 | 0 | 9 | 0.0 | 5 | 10 | 50.0 | p < 0.05 | |
| CC130 | 0 | 28 | 0.0 | 0 | 9 | 0.0 | n.s. | |
| CC398 | 0 | 15 | 0.0 | 0 | 7 | 0.0 | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raafat, D.; Mrochen, D.M.; Al’Sholui, F.; Heuser, E.; Ryll, R.; Pritchett-Corning, K.R.; Jacob, J.; Walther, B.; Matuschka, F.-R.; Richter, D.; et al. Molecular Epidemiology of Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus in Wild, Captive and Laboratory Rats: Effect of Habitat on the Nasal S. aureus Population. Toxins 2020, 12, 80. https://doi.org/10.3390/toxins12020080
Raafat D, Mrochen DM, Al’Sholui F, Heuser E, Ryll R, Pritchett-Corning KR, Jacob J, Walther B, Matuschka F-R, Richter D, et al. Molecular Epidemiology of Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus in Wild, Captive and Laboratory Rats: Effect of Habitat on the Nasal S. aureus Population. Toxins. 2020; 12(2):80. https://doi.org/10.3390/toxins12020080
Chicago/Turabian StyleRaafat, Dina, Daniel M. Mrochen, Fawaz Al’Sholui, Elisa Heuser, René Ryll, Kathleen R. Pritchett-Corning, Jens Jacob, Bernd Walther, Franz-Rainer Matuschka, Dania Richter, and et al. 2020. "Molecular Epidemiology of Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus in Wild, Captive and Laboratory Rats: Effect of Habitat on the Nasal S. aureus Population" Toxins 12, no. 2: 80. https://doi.org/10.3390/toxins12020080
APA StyleRaafat, D., Mrochen, D. M., Al’Sholui, F., Heuser, E., Ryll, R., Pritchett-Corning, K. R., Jacob, J., Walther, B., Matuschka, F.-R., Richter, D., Westerhüs, U., Pikula, J., van den Brandt, J., Nicklas, W., Monecke, S., Strommenger, B., van Alen, S., Becker, K., Ulrich, R. G., & Holtfreter, S. (2020). Molecular Epidemiology of Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus in Wild, Captive and Laboratory Rats: Effect of Habitat on the Nasal S. aureus Population. Toxins, 12(2), 80. https://doi.org/10.3390/toxins12020080






