In Silico and In Vitro Studies of Mycotoxins and Their Cocktails; Their Toxicity and Its Mitigation by Silibinin Pre-Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Silico Prediction of Physicochemical, Pharmacological and Toxicological Properties
2.2. Verification of the In Silico Prediction
2.2.1. Acute Toxicity of Pure Mycotoxins
2.2.2. Genotoxicity of Single Mycotoxins
2.2.3. P-gp Substrate Probability of Single Mycotoxins
2.3. Influence of the Mycotoxins Properties in Mixtures
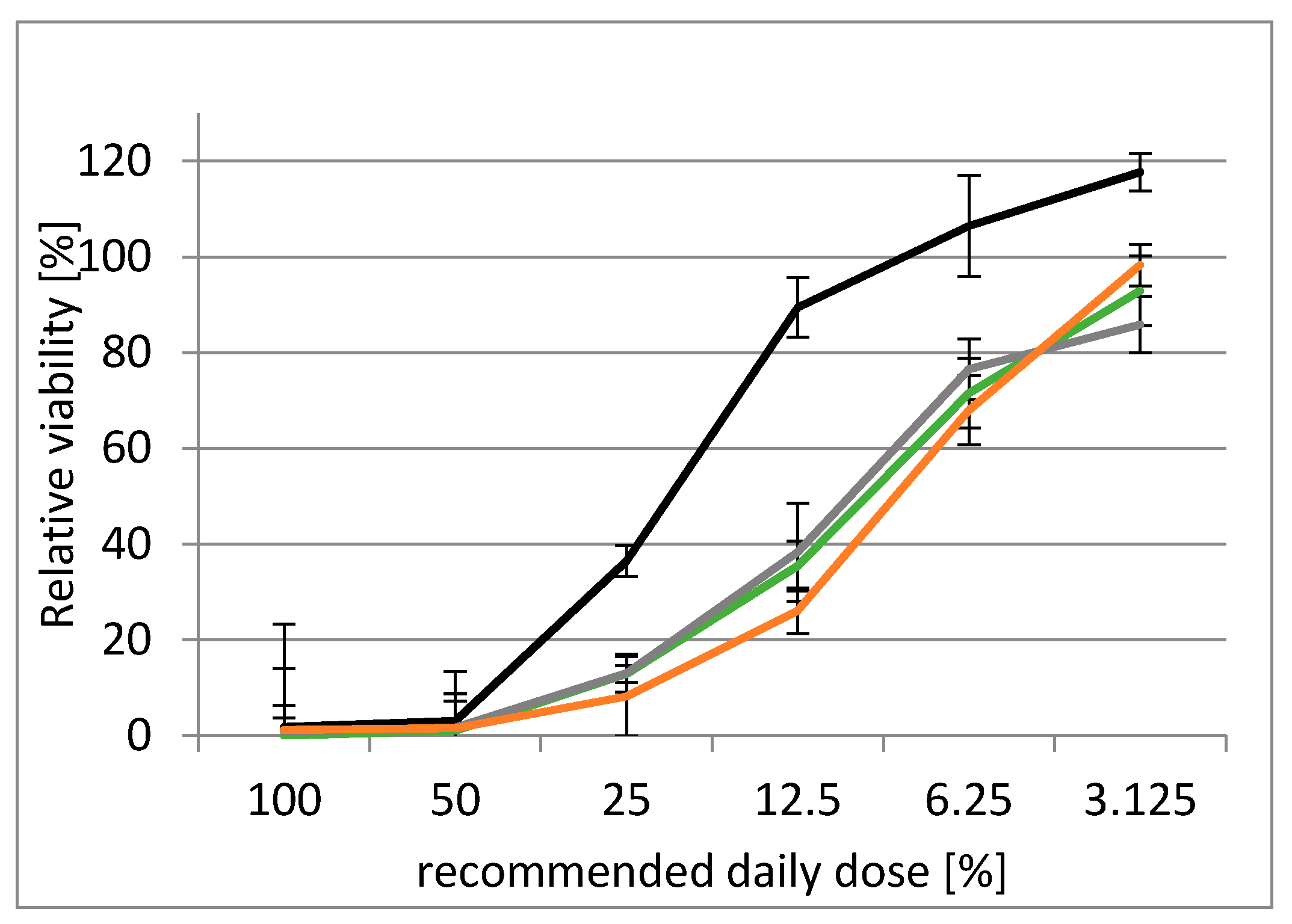
2.3.1. Determination of Acute Toxicity of Milk Thistle-Based Dietary Supplement
2.3.2. Acute Toxicity of Mycotoxins in Binary Mixtures
2.3.3. Suppression of Mycotoxins´ Acute Toxicity by Silibinin
2.3.4. Protective Effects of Silibinin Against Mycotoxin Genotoxicity
2.4. Bioavailability of Mycotoxins and Silibinin
3. Conclusions
4. Materials and Methods
4.1. Reagents and Instrumentations
4.2. Cell lines and Cell Cultures
4.3. Milk Thistle-based Dietary Supplement
4.4. In Silico Toxicity Analysis
4.5. Cytotoxicity Assay
4.6. P-gp Substrate Determination
4.7. Caco-2/RAW 264.7 Co-Culture System
4.8. U-HPLC-MS Determination of Mycotoxins and Silibinin
4.9. Combination Effect of Mycotoxins in Binary Mixtures
4.10. Genotoxicity Assay
4.11. Data Processing and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCB1 | ATP-binding cassette sub family member 1 |
| AFB1 | aflatoxin B1 |
| AME | alternariol-9-methyl ether |
| AOH | alternariol |
| ASBT | intestinal bile acid transporter |
| BEA | beauvericin |
| BF-2 | a fibroblast cell line originally established from the caudal fin of Lepomis macrochirus (Bluegill) |
| C-28/I2 | immortalized human chondrocyte |
| Caco-2 | Caucasian colon adenocarcinoma |
| CI | combination index |
| CHO-K1 | Chinese hamster ovary |
| DAS | diacetoxyscirpenol |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DON | deoxynivalenol |
| EMEM | Minimum Essential Medium |
| ENN A | enniatin A |
| ENN A1 | enniatin A1 |
| ENN B | enniatin B |
| ENN B1 | enniatin B1 |
| ENNs | enniatins |
| FB1 | fumonisin B1 |
| FBS | fetal bovine serum |
| HEK-293T | human embryonic kidney 293 |
| HepG2 | hepatocellular carcinoma epithelial |
| HT-2 | HT-2 toxin |
| HT29 | human Caucasian colon adenocarcinoma |
| IC50 | the mycotoxin concentration that halved the cellular viability |
| LMP | low melting point |
| LogP | logarithmic values of octanol–water partition coefficient |
| LOQ | limit of quantitation |
| MCF7 | human Caucasian breast adenocarcinoma |
| MDA-MD-231 | human breast adenocarcinoma |
| MDCK | Madin–Darby canine kidney |
| MDR1 | multidrug resistance protein 1 |
| MPA | mycophenolic acid |
| NADH | 1,4-Dihydronicotinamide adenine dinucleotide |
| OTA | ochratoxin A |
| PBS | phosphate buffer saline |
| PepT1 | intestinal peptide transporter 1 |
| P-gp | P-glycoprotein |
| RAW | mouse macrophage |
| ROS | reactive oxygen species |
| RSD | relative standard deviation |
| SF-9 | the lepidopteran (S. frugiperda) cells obtained from the envelope of pupal ovaries |
| T-2 | T-2 toxin |
| TEN | tentoxin |
| TNF-α | tumor necrosis factor alpha |
| V79 | lung fibroblasts from male Chinese hamster |
| Vero | mammalian kidney epithelial |
| ZEA | zearalenone |
References
- Fernández-Blanco, C.; Elmo, L.; Waldner, T.; Ruiz, M.J. Cytotoxic Effects Induced by Patulin, Deoxynivalenol and Toxin T2 Individually and in Combination in Hepatic Cells (HepG2). Food Chem. Toxicol. 2018, 120, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, O.; Loghin, F.; Filip, L.; Cozma, A.; Miere, D.; Mañes, J.; Banc, R. Occurence of Fusarium Mycotoxins in Wheat from Europe—A Review. Acta Univ. Cibiniensis Ser. E Food Technol. 2015, 19, 35–60. [Google Scholar] [CrossRef] [Green Version]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and Molecular Mechanisms for Immune Modulation by Deoxynivalenol and Other Trichothecenes: Unraveling a Paradox. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Liu, S.; Wu, W.; Zhang, H. Comparison of Anorectic Potencies of Type a Trichothecenes T-2 Toxin, HT-2 Toxin, Diacetoxyscirpenol, and Neosolaniol. Toxins 2018, 10, 179. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Qiu, M.; Sun, L.; Wang, X.; Li, C.; Xu, D.; Gooneratne, R. Cytotoxicity of T-2 and Modified T-2 Toxins: Induction of JAK/STAT Pathway in RAW264.7 Cells by Hepatopancreas and Muscle Extracts of Shrimp Fed with T-2 Toxin. Toxicol. Res. 2017, 6, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Creppy, E.E. Update of Survey, Regulation and Toxic Effects of Mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria Mycotoxins: Occurrence, Toxicity and Toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Luz, C.; Saladino, F.; Luciano, F.B.; Mañes, J.; Meca, G. Occurrence, Toxicity, Bioaccessibility and Mitigation Strategies of Beauvericin, a Minor Fusarium Mycotoxin. Food Chem. Toxicol. 2017, 107, 430–439. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Mañes, J.; Berrada, H.; Juan, C. Development and Validation of a LC-ESI-MS/MS Method for the Determination of Alternaria Toxins Alternariol, Alternariol Methyl-Ether and Tentoxin in Tomato and Tomato-Based Products. Toxins 2016, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- Zambonin, C.G.; Monaci, L.; Aresta, A. Solid-Phase Microextraction-High Performance Liquid Chromatography and Diode Array Detection for the Determination of Mycophenolic Acid in Cheese. Food Chem. 2002, 78, 249–254. [Google Scholar] [CrossRef]
- Fontaine, K.; Passeró, E.; Vallone, L.; Hymery, N.; Coton, M.; Jany, J.L.; Mounier, J.Ô.; Coton, E. Occurrence of Roquefortine C, Mycophenolic Acid and Aflatoxin M1 Mycotoxins in Blue-Veined Cheeses. Food Control 2015, 47, 634–640. [Google Scholar] [CrossRef]
- De Angelis, E.; Monaci, L.; Mackie, A.; Salt, L.; Visconti, A. Reprint of “Bioaccessibility of T-2 and HT-2 Toxins in Mycotoxin Contaminated Bread Models Submitted to in Vitro Human Digestion”. Innov. Food Sci. Emerg. Technol. 2013, 25, 88–96. [Google Scholar] [CrossRef]
- Weidner, M.; Hüwel, S.; Ebert, F.; Schwerdtle, T.; Galla, H.J.; Humpf, H.U. Influence of T-2 and HT-2 Toxin on the Blood-Brain Barrier In Vitro: New Experimental Hints for Neurotoxic Effects. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Franzova, P.; Juan-García, A.; Font, G. Toxicological Interactions between the Mycotoxins Beauvericin, Deoxynivalenol and T-2 Toxin in CHO-K1 Cells in Vitro. Toxicon 2011, 58, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Beier, R.C.; Shen, J.; Smet, D.D.; De Saeger, S.; Zhang, S. T-2 Toxin, a Trichothecene Mycotoxin: Review of Toxicity, Metabolism, and Analytical Methods. J. Agric. Food Chem. 2011, 59, 3441–3453. [Google Scholar] [CrossRef] [PubMed]
- Hassanane, M.; ESA, A.; S, E.-F.; MA, A.; A, H. Mutagenicity of the Mycotoxin Diacetoxyscirpenol on Somatic and Germ Cells of Mice. Mycotoxin Res. 2000, 16, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Brundage, M.A.; Hamilton, P.B. 4β, 15-Diacetoxyscirpenol Induces Cytotoxicity and Alterations in Phagocytic and Fc-Receptor Expression Functions in Chicken Macrophages In Vitro. Immunopharmacol. Immunotoxicol. 1998, 20, 541–553. [Google Scholar] [CrossRef]
- Zhou, H.; George, S.; Hay, C.; Lee, J.; Qian, H.; Sun, X. Individual and Combined Effects of Aflatoxin B1, Deoxynivalenol and Zearalenone on HepG2 and RAW 264.7 Cell Lines. Food Chem. Toxicol. 2017, 103, 18–27. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.J. Interaction Effects of Enniatin B, Deoxinivalenol and Alternariol in Caco-2 Cells. Toxicol. Lett. 2016, 241, 38–48. [Google Scholar] [CrossRef]
- Wang, H.W.; Wang, J.Q.; Zheng, B.Q.; Li, S.L.; Zhang, Y.D.; Li, F.D.; Zheng, N. Cytotoxicity Induced by Ochratoxin A, Zearalenone, and α-Zearalenol: Effects of Individual and Combined Treatment. Food Chem. Toxicol. 2014, 71, 217–224. [Google Scholar] [CrossRef]
- Bouaziz, C.; Sharaf el dein, O.; El Golli, E.; Abid-Essefi, S.; Brenner, C.; Lemaire, C.; Bacha, H. Different Apoptotic Pathways Induced by Zearalenone, T-2 Toxin and Ochratoxin A in Human Hepatoma Cells. Toxicology 2008, 254, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative Study of Cytotoxicity and Oxidative Stress Induced by Deoxynivalenol, Zearalenone or Fumonisin B1 in Human Intestinal Cell Line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Macáková, P.; Juan-García, A.; Font, G. Cytotoxic Effects of Mycotoxin Combinations in Mammalian Kidney Cells. Food Chem. Toxicol. 2011, 49, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.; Juan-García, A.; Font, G.; Ruiz, M.J. Reactive Oxygen Species Induced by Beauvericin, Patulin and Zearalenone in CHO-K1 Cells. Toxicol. Vitr. 2009, 23, 1504–1509. [Google Scholar] [CrossRef]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A Review of the Mycotoxin Enniatin B. Front. Public Health 2017, 5, 304. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Juan-García, A.; Juan, C.; Font, G.; Ruiz, M.J. Alternariol Induce Toxicity via Cell Death and Mitochondrial Damage on Caco-2 Cells. Food Chem. Toxicol. 2016, 88, 32–39. [Google Scholar] [CrossRef]
- Frizzell, C.; Ndossi, D.; Kalayou, S.; Eriksen, G.S.; Verhaegen, S.; Sørlie, M.; Elliott, C.T.; Ropstad, E.; Connolly, L. An in Vitro Investigation of Endocrine Disrupting Effects of the Mycotoxin Alternariol. Toxicol. Appl. Pharmacol. 2013, 271, 64–71. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Schmit, C.; Burkhardt, B.; Altemöller, M.; Podlech, J.; Metzler, M. Glucuronidation of the Mycotoxins Alternariol and Alternariol-9-Methyl Ether in Vitro: Chemical Structures of Glucuronides and Activities of Human UDP-Glucuronosyltransferase Isoforms. Mycotoxin Res. 2009, 25, 3–10. [Google Scholar] [CrossRef]
- Gomis, J.-M.; Haraux, F.; Santolini, J.; André, F.; Sigalat, C.; Minoletti, C. An Insight into the Mechanism of Inhibition and Reactivation of the F 1 -ATPases by Tentoxin. Biochemistry 2002, 41, 6008–6018. [Google Scholar] [CrossRef]
- Wu, T.Y.; Fridley, B.L.; Jenkins, G.D.; Batzler, A.; Wang, L.; Weinshilboum, R.M. Mycophenolic Acid Response Biomarkers: A Cell Line Model System-Based Genome-Wide Screen. Int. Immunopharmacol. 2011, 11, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Liu, Z.; Huang, H.; Liu, H.; Li, L. Effects of Mycophenolic Acid on Endothelial Cells. Int. Immunopharmacol. 2005, 5, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.; Clark, R.F. Health Effects of Mycotoxins: A Toxicological Overview. J. Toxicol. Clin. Toxicol. 2004, 42, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; George, S.; Li, C.; Gurusamy, S.; Sun, X.; Gong, Z.; Qian, H. Combined Toxicity of Prevalent Mycotoxins Studied in Fish Cell Line and Zebrafish Larvae Revealed That Type of Interactions Is Dose-Dependent. Aquat. Toxicol. 2017, 193, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Speijers, G.J.A.; Speijers, M.H.M. Combined Toxic Effects of Mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Castell-Auví, A.; Motilva, M.J.; Macià, A.; Torrell, H.; Bladé, C.; Pinent, M.; Arola, L.; Ardévol, A. Organotypic Co-Culture System to Study Plant Extract Bioactivity on Hepatocytes. Food Chem. 2010, 122, 775–781. [Google Scholar] [CrossRef]
- Surai, P. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Kim, J.; Sharma, R.P. Silymarin Protects against Liver Damage in BALB/c Mice Exposed to Fumonisin B1despite Increasing Accumulation of Free Sphingoid Bases. Toxicol. Sci. 2004, 80, 335–342. [Google Scholar] [CrossRef]
- Naseer, O.; Khan, J.A.; Khan, M.S.; Omer, M.O.; Chishti, G.A.; Sohail, M.L.; Saleem, M.U. Comparative Efficacy of Silymarin and Choline Chloride (Liver Tonics) in Preventing the Effects of Aflatoxin B1in Bovine Calves. Pol. J. Vet. Sci. 2016, 19, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Sozmen, M.; Devrim, A.K.; Tunca, R.; Bayezit, M.; Dag, S.; Essiz, D. Protective Effects of Silymarin on Fumonisin B1-Induced Hepatotoxicity in Mice. J. Vet. Sci. 2014, 15, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Al-Anati, L.; Essid, E.; Reinehr, R.; Petzinger, E. Silibinin Protects OTA-Mediated TNF-α Release from Perfused Rat Livers and Isolated Rat Kupffer Cells. Mol. Nutr. Food Res. 2009, 53, 460–466. [Google Scholar] [CrossRef]
- Essid, E.; Dernawi, Y.; Petzinger, E. Apoptosis Induction by OTA and TNF-?? In Cultured Primary Rat Hepatocytes and Prevention by Silibinin. Toxins 2012, 4, 1139–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essid, E.; Petzinger, E. Silibinin Pretreatment Protects against Ochratoxin A-Mediated Apoptosis in Primary Rat Hepatocytes. Mycotoxin Res. 2011, 27, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Duca, R.; Mabondzo, A.; Bravin, F.; Delaforge, M. In Vitro Co-Culture Models to Evaluate Acute Cytotoxicity of Individual and Combined Mycotoxin Exposures on Caco-2, THP-1 and HepaRG Human Cell Lines. Chem. Biol. Interact. 2018, 281, 51–59. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Marín, S.; Rojas-García, A.E.; Sanchis, V.; Ramos, A.J. UPLC-MS/MS Analysis of Ochratoxin A Metabolites Produced by Caco-2 and HepG2 Cells in a Co-Culture System. Food Chem. Toxicol. 2017, 109, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Arias, C.A.; Crespo-Sempere, A.; Marín, S.; Sanchis, V.; Ramos, A.J. Modulation of the Xenobiotic Transformation System and Inflammatory Response by Ochratoxin A Exposure Using a Co-Culture System of Caco-2 and HepG2 Cells. Food Chem. Toxicol. 2015, 86, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenclova, M.; Novakova, A.; Viktorova, J.; Jonatova, P.; Dzuman, Z.; Ruml, T.; Kren, V.; Hajslova, J.; Vitek, L.; Stranska-Zachariasova, M. Poor Chemical and Microbiological Quality of the Commercial Milk Thistle-Based Dietary Supplements May Account for Their Reported Unsatisfactory and Non-Reproducible Clinical Outcomes. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Veprikova, Z.; Zachariasova, M.; Dzuman, Z.; Zachariasova, A.; Fenclova, M.; Slavikova, P.; Vaclavikova, M.; Mastovska, K.; Hengst, D.; Hajslova, J. Mycotoxins in Plant-Based Dietary Supplements: Hidden Health Risk for Consumers. J. Agric. Food Chem. 2015, 63, 6633–6643. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of Alternaria Toxins in Feed and Food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of T-2 and HT-2 Toxin in Food and Feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the Risks for Public Health Related to the Presence of Zearalenone in Food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks to Human and Animal Health Related to the Presence of Beauvericin and Enniatins in Food and Feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to Human and Animal Health Related to the Presence of Deoxynivalenol and Its Acetylated and Modified Forms in Food and Feed. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risk to Human and Animal Health Related to the Presence of 4,15-Diacetoxyscirpenol in Food and Feed. EFSA J. 2018, 16. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.J. Role of Quercetin on Caco-2 Cells against Cytotoxic Effects of Alternariol and Alternariol Monomethyl Ether. Food Chem. Toxicol. 2016, 89, 60–66. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Sørensen, I.F.; Sørensen, J.L.; Søndergaard, T.E.; Purup, S. Cytotoxic and Apoptotic Effect of Mycotoxins in Human Small Intestinal Cells. J. Anim. Sci. 2016, 94. [Google Scholar] [CrossRef]
- Tatay, E.; Meca, G.; Font, G.; Ruiz, M.J. Interactive Effects of Zearalenone and Its Metabolites on Cytotoxicity and Metabolization in Ovarian CHO-K1 Cells. Toxicol. In Vitro 2014, 28, 95–103. [Google Scholar] [CrossRef]
- Prosperini, A.; Font, G.; Ruiz, M.J. Interaction Effects of Fusarium Enniatins (A, A1, B and B1) Combinations on in Vitro Cytotoxicity of Caco-2 Cells. Toxicol. Vitr. 2014, 28, 88–94. [Google Scholar] [CrossRef]
- Behm, C.; Fllmann, W.; Degen, G.H. Cytotoxic Potency of Mycotoxins in Cultures of V79 Lung Fibroblast Cells. J. Toxicol. Environ. Health Part Curr. Issues 2012, 75, 1226–1231. [Google Scholar] [CrossRef]
- Lautraite, S.; Rio, B.; Guinard, J.; Parent-Massin, D. In Vitro Effects of Diacetoxyscirpenol (DAS) on Human and Rat Granulo-Monocytic Progenitors. Mycopathologia 1997, 140, 59–64. [Google Scholar] [CrossRef]
- Jha, S.N.; Jaiswal, P.; Grewal, M.K.; Gupta, M.; Bhardwaj, R. Detection of Adulterants and Contaminants in Liquid Foods—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1662–1684. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M. Mycotoxins Except Fusarium Toxins in Foods; Elsevier Ltd.: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Kosaka, Y.; Murakami, M.; Matsuo, H.; Matsusaka, N.; Taniguchi, K.; Sasaki, Y.F. Detection of Nivalenol Genotoxicity in Cultured Cells and Multiple Mouse Organs by the Alkaline Single-Cell Gel Electrophoresis Assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 415, 191–200. [Google Scholar] [CrossRef]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemöller, M.; Podlech, J.; Marko, D. Alternariol Acts as a Topoisomerase Poison, Preferentially Affecting the IIα Isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, C.; Fehr, M.; Schwarz, C.; Baechler, S.; Domnanich, K.; Böttler, U.; Pahlke, G.; Marko, D. Modulation of the Cellular Redox Status by the Alternaria Toxins Alternariol and Alternariol Monomethyl Ether. Toxicol. Lett. 2013, 216, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bony, S.; Carcelen, M.; Olivier, L.; Devaux, A. Genotoxicity Assessment of Deoxynivalenol in the Caco-2 Cell Line Model Using the Comet Assay. Toxicol. Lett. 2006, 166, 67–76. [Google Scholar] [CrossRef]
- Takakura, N.; Nesslany, F.; Fessard, V.; Le Hegarat, L. Absence of in Vitro Genotoxicity Potential of the Mycotoxin Deoxynivalenol in Bacteria and in Human TK6 and HepaRG Cell Lines. Food Chem. Toxicol. 2014, 66, 113–121. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Y.; Sun, J.; Mu, P.; Deng, Y. MiR449a/SIRT1/PGC-1α Is Necessary for Mitochondrial Biogenesis Induced by T-2 Toxin. Front. Pharmacol. 2018, 8, 954. [Google Scholar] [CrossRef]
- Klarić, M.Š.; Daraboš, D.; Rozgaj, R.; Kašuba, V.; Pepeljnjak, S. Beauvericin and Ochratoxin A Genotoxicity Evaluated Using the Alkaline Comet Assay: Single and Combined Genotoxic Action. Arch. Toxicol. 2010, 84, 641–650. [Google Scholar] [CrossRef]
- Prosperini, A.; Juan-García, A.; Font, G.; Ruiz, M.J. Beauvericin-Induced Cytotoxicity via ROS Production and Mitochondrial Damage in Caco-2 Cells. Toxicol. Lett. 2013, 222, 204–211. [Google Scholar] [CrossRef]
- Dornetshuber, R.; Heffeter, P.; Lemmens-Gruber, R.; Elbling, L.; Marko, D.; Micksche, M.; Berger, W. Oxidative Stress and DNA Interactions Are Not Involved in Enniatin- and Beauvericin-Mediated Apoptosis Induction. Mol. Nutr. Food Res. 2009, 53, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Mallebrera, B.; Juan-Garcia, A.; Font, G.; Ruiz, M.J. Mechanisms of Beauvericin Toxicity and Antioxidant Cellular Defense. Toxicol. Lett. 2016, 246, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Juan-García, A.; Font, G.; Ruiz, M.J. Reactive Oxygen Species Involvement in Apoptosis and Mitochondrial Damage in Caco-2 Cells Induced by Enniatins A, A1, B and B1. Toxicol. Lett. 2013, 222, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Lee, H.; Yoo, Y.S.; Hah, D.Y.; Kim, C.H.; Kim, E.; Kim, J.S. Evaluation of Oxidative DNA Damage Using an Alkaline Single Cell Gel Electrophoresis (SCGE) Comet Assay, and the Protective Effects of N-Acetylcysteine Amide on Zearalenone-Induced Cytotoxicity in Chang Liver Cells. Toxicol. Res. 2013, 29, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatay, E.; Font, G.; Ruiz, M.J. Cytotoxic Effects of Zearalenone and Its Metabolites and Antioxidant Cell Defense in CHO-K1 Cells. Food Chem. Toxicol. 2016, 96, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Jiang, L.P.; Chen, M.; Geng, C.Y.; Yang, G.; Ji, F.; Zhong, L.F.; Liu, X.F. Genotoxic Effects Induced by Zearalenone in a Human Embryonic Kidney Cell Line. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 755, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, C.; Gehrke, H.; Kropat, C.; Schwarz, C.; Bächler, S.; Fehr, M.; Pahlke, G.; Marko, D. Role of Topoisomerase Inhibition and DNA Repair Mechanisms in the Genotoxicity of Alternariol and Altertoxin-II. World Mycotoxin J. 2013, 6, 233–244. [Google Scholar] [CrossRef]
- Viktorová, J.; Dobiasová, S.; Řehořová, K.; Biedermann, D.; Káňová, K.; Šeborová, K.; Václavíková, R.; Valentová, K.; Ruml, T.; Křen, V.; et al. Antioxidant, Anti-Inflammatory, and Multidrug Resistance Modulation Activity of Silychristin Derivatives. Antioxidants 2019, 8, 303. [Google Scholar] [CrossRef] [Green Version]
- Fromm, M.F. Importance of P-Glycoprotein at Blood-Tissue Barriers. Trends Pharmacol. Sci. 2004, 25, 423–429. [Google Scholar] [CrossRef]
- Meyer, M.R.; Wagmann, L.; Schneider-Daum, N.; Loretz, B.; De Souza Carvalho, C.; Lehr, C.M.; Maurer, H.H. P-Glycoprotein Interactions of Novel Psychoactive Substances—Stimulation of ATP Consumption and Transport across Caco-2 Monolayers. Biochem. Pharmacol. 2015, 94, 220–226. [Google Scholar] [CrossRef]
- Li, X.; Mu, P.; Wen, J.; Deng, Y. Carrier-Mediated and Energy-Dependent Uptake and Efflux of Deoxynivalenol in Mammalian Cells. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videmann, B.; Tep, J.; Cavret, S.; Lecoeur, S. Epithelial Transport of Deoxynivalenol: Involvement of Human P-Glycoprotein (ABCB1) and Multidrug Resistance-Associated Protein 2 (ABCC2). Food Chem. Toxicol. 2007, 45, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Prouillac, C.; Videmann, B.; Mazallon, M.; Lecoeur, S. Induction of Cells Differentiation and ABC Transporters Expression by a Myco-Estrogen, Zearalenone, in Human Choriocarcinoma Cell Line (BeWo). Toxicology 2009, 263, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Duca, R.-C.; Mabondzo, A.; Bravin, F.; Delaforge, M. In Vivo Effects of Zearalenone on the Expression of Proteins Involved in the Detoxification of Rat Xenobiotics. Environ. Toxicol. 2012, 27, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Koraichi, F.; Videmann, B.; Mazallon, M.; Benahmed, M.; Prouillac, C.; Lecoeur, S. Zearalenone Exposure Modulates the Expression of ABC Transporters and Nuclear Receptors in Pregnant Rats and Fetal Liver. Toxicol. Lett. 2012, 211, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Cheng, G.; Huang, L.; Chen, D.; Tao, Y.; Pan, Y.; Hao, H.; Wu, Q.; Wan, D.; et al. High Risk of Embryo-Fetal Toxicity: Placental Transfer of T-2 Toxin and Its Major Metabolite HT-2 Toxin in BeWo Cells. Toxicol. Sci. 2014, 137, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Entezari, M.; Mokhtari, M.J.; Hashemi, M. Evaluation of Silibinin on the Viability of MCF-7 Human Breast Adenocarcinoma and HUVEC ( Human Umbilical Vein Endothelial ) Cell Lines. Breast 2011, 3, 283–288. [Google Scholar]
- Wan, L.Y.M.; Turner, P.C.; El-Nezami, H. Individual and Combined Cytotoxic Effects of Fusarium Toxins (Deoxynivalenol, Nivalenol, Zearalenone and Fumonisins B1) on Swine Jejunal Epithelial Cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef]
- Thuvander, A.; Wikman, C.; Gadhasson, I. In Vitro Exposure of Human Lymphocytes to Trichothecenes: Individual Variation in Sensitivity and Effects of Combined Exposure on Lymphocyte Function. Food Chem. Toxicol. 1999, 37, 639–648. [Google Scholar] [CrossRef]
- Lin, X.; Shao, W.; Yu, F.; Xing, K.; Liu, H.; Zhang, F.; Goldring, M.B.; Lammi, M.J.; Guo, X. Individual and Combined Toxicity of T-2 Toxin and Deoxynivalenol on Human C-28/I2 and Rat Primary Chondrocytes. J. Appl. Toxicol. 2019, 39, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Klarić, M.Š.; Rumora, L.; Ljubanović, D.; Pepeljnjak, S. Cytotoxicity and Apoptosis Induced by Fumonisin B1, Beauvericin and Ochratoxin a in Porcine Kidney PK15 Cells: Effects of Individual and Combined Treatment. Arch. Toxicol. 2008, 82, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Juranova, J.; Aury-Landas, J.; Boumediene, K.; Bauge, C.; Biedermann, D.; Ulrichova, J.; Frankova, J. Modulation of Skin Inflammatory Response by Active Components of Silymarin. Mol. Basel Switz. 2018, 24, 123. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Li, L.; Chen, S.; Yu, Y.; Qi, M.; Tashiro, S.I.; Onodera, S.; Ikejima, T. Silibinin Induced-Autophagic and Apoptotic Death Is Associated with an Increase in Reactive Oxygen and Nitrogen Species in HeLa Cells. Free Radic. Res. 2011, 45, 1307–1324. [Google Scholar] [CrossRef]
- Kim, T.H.; Woo, J.S.; Kim, Y.K.; Kim, K.H. Silibinin Induces Cell Death through Reactive Oxygen Species-Dependent Downregulation of Notch-1/ERK/Akt Signaling in Human Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2014, 349, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.Y.; Kuča, K.; Dohnal, V.; Zhang, X.J.; Yuan, Z.H. Oxidative Stress-Mediated Cytotoxicity and Metabolism of T-2 Toxin and Deoxynivalenol in Animals and Humans: An Update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef]
- Duan, W.; Jin, X.; Li, Q.; Tashiro, S.; Onodera, S.; Ikejima, T. Silibinin Induced Autophagic and Apoptotic Cell Death in HT1080 Cells Through a Reactive Oxygen Species Pathway. J. Pharmacol. Sci. 2010, 113, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahhab, M.A.; El-Nekeety, A.A.; Salman, A.S.; Abdel-Aziem, S.H.; Mehaya, F.M.; Hassan, N.S. Protective Capabilities of Silymarin and Inulin Nanoparticles against Hepatic Oxidative Stress, Genotoxicity and Cytotoxicity of Deoxynivalenol in Rats. Toxicon 2018, 142, 1–13. [Google Scholar] [CrossRef]
- Toğay, V.A.; Sevimli, T.S.; Sevimli, M.; Çelik, D.A.; Özçelik, N. DNA Damage in Rats with Streptozotocin-Induced Diabetes; Protective Effect of Silibinin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 825, 15–18. [Google Scholar] [CrossRef]
- Fernandes Veloso Borges, F.; Ribeiro E Silva, C.; Moreira Goes, W.; Ribeiro Godoy, F.; Craveiro Franco, F.; Hollanda Véras, J.; Luiz Cardoso Bailão, E.F.; De Melo E Silva, D.; Gomes Cardoso, C.; Divino Da Cruz, A.; et al. Protective Effects of Silymarin and Silibinin against DNA Damage in Human Blood Cells. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, P.; Kumar, A.; Ali, M.; Mishra, K.P. Radioprotection of Plasmid and Cellular DNA and Swiss Mice by Silibinin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 695, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Contreras, Y.; Mendoza-Lozano, R.P.; Martínez-Alcaraz, E.R.; Martínez-Alfaro, M.; Gallegos-Corona, M.A.; Ramírez-Morales, M.A.; Vázquez-Guevara, M.A. Silymarin and Dimercaptosuccinic Acid Ameliorate Lead-Induced Nephrotoxicity and Genotoxicity in Rats. Hum. Exp. Toxicol. 2016, 35, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Bordin, K.; Saladino, F.; Fernández-Blanco, C.; Ruiz, M.J.; Mañes, J.; Fernández-Franzón, M.; Meca, G.; Luciano, F.B. Reaction of Zearalenone and α-Zearalenol with Allyl Isothiocyanate, Characterization of Reaction Products, Their Bioaccessibility and Bioavailability in Vitro. Food Chem. 2017, 217, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Jakimiuk, E.; Zielonka, Ł.; Obremski, K.; Gajęcki, M. The Biotransformation of Chosen Mycotoxins. Pol Viet Sci. 2009, 12, 293–303. [Google Scholar]
- Kabak, B.; Ozbey, F. Assessment of the Bioaccessibility of Aflatoxins from Various Food Matrices Using an in Vitro Digestion Model, and the Efficacy of Probiotic Bacteria in Reducing Bioaccessibility. J. Food Compos. Anal. 2012, 27, 21–31. [Google Scholar] [CrossRef]
- Gratz, S.W.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.J.; Duncan, G.; MacDonald, S.; Lloyd, A.; Tarbin, J. Masked Trichothecene and Zearalenone Mycotoxins Withstand Digestion and Absorption in the Upper GI Tract but Are Efficiently Hydrolyzed by Human Gut Microbiota in Vitro. Mol. Nutr. Food Res. 2017, 61, 1600680. [Google Scholar] [CrossRef]
- Videmann, B.; Mazallon, M.; Tep, J.; Lecoeur, S. Metabolism and Transfer of the Mycotoxin Zearalenone in Human Intestinal Caco-2 Cells. Food Chem. Toxicol. 2008, 46, 3279–3286. [Google Scholar] [CrossRef]
- Meca, G.; Mañes, J.; Font, G.; Ruiz, M.J. Study of the potential toxicity of enniatins A, A 1, B, B 1 by evaluation of duodenal and colonic bioavailability applying an invitro method by Caco-2 cells. Toxicon. 2012, 59, 1–11. [Google Scholar] [CrossRef]
- Burkhardt, B.; Pfeiffer, E.; Metzler, M. Absorption and Metabolism of the Mycotoxins Alternariol and Alternariol-9-Methyl Ether in Caco-2 Cells in Vitro. Mycotoxin Res. 2009, 25, 149–157. [Google Scholar] [CrossRef]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [Green Version]
- Fæste, C.K.; Ivanova, L.; Uhlig, S. In vitro metabolism of the mycotoxin enniatin B in different species and cytochrome P450 enzyme phenotyping by chemical inhibitors. Drug Metab. Dispos. 2011, 39, 1768–1776. [Google Scholar] [CrossRef] [Green Version]
- Delaforge, M.; Andre, F.; Jaouen, M.; Dolgos, H.; Benech, H.; Gomis, J.M.; Noel, J.P.; Cavelier, F.; Verducci, J.; Aubagnac, J.L.; et al. Metabolism of tentoxin by hepatic cytochrome P-450 3A isozymes. Eur. J. Biochem. 1997, 250, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. Assay Guid. Man. Internet 2013, 114, 785–796. [Google Scholar] [CrossRef]
- Ferruzza, S.; Scarino, M.L.; Gambling, L.; Natella, F.; Sambuy, Y. Biphasic Effect of Iron on Human Intestinal Caco-2 Cells: Early Effect on Tight Junction Permeability with Delayed Onset of Oxidative Cytotoxic Damage. Cell. Mol. Biol. 2003, 49, 89–99. [Google Scholar] [PubMed]
- Stukonis, V.; Armonienė, R.; Lemežienė, N.; Kemešytė, V.; Statkevičiūtė, G. Identification of Fine-Leaved Species of Genus Festuca by Molecular Methods. Pak. J. Bot. 2015, 47, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- McKelvey-Martin, V.J.; Green, M.H.L.; Schmezer, P.; Pool-Zobel, B.L.; De Meo, M.P.; Collins, A. The Single Cell Gelelectrophores is Assay (comet Assay): A European Review. Mutat. Res. 1993, 288, 47–63. [Google Scholar] [CrossRef]



| Mycotoxins | Effects | References |
|---|---|---|
| T-2 and HT-2 | Inhibition of DNA, RNA, and protein synthesis. Induction of mutations and apoptosis. | [12,13,14,15] |
| DAS | Inhibition of DNA and protein synthesis. Suppression of macrophage phagocytic function. | [16,17] |
| DON | Inhibition of DNA, RNA, and protein synthesis. Decrease of the cell proliferation. | [18,19] |
| ZEA | Activation of the estrogen receptor. Inhibition of DNA and protein synthesis. Triggering of lipid peroxidation and cell death. | [20,21,22] |
| BEA | Increase of the biological membrane. Loss of ionic homeostasis. Induction of lipid peroxidation. | [14,23,24] |
| ENNs | Increase of the membrane permeability for cations. | [25] |
| AOH and AME | Single and double strand DNA breaks. Decrease of the cell proliferation. | [26,27,28] |
| TEN | ATP hydrolysis and inhibition of ATP synthesis. | [29] |
| MPA | Inhibition of inosine 5′-monophosphate dehydrogenase. Blocking of the DNA synthesis and proliferation of both T and B lymphocytes. | [30,31] |
| Parameters | Fusarium Toxins | Alternaria Toxins | Penicillium Toxin | Silibinin | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| trichothecenes | others | |||||||||||||||
| DON | HT-2 | T-2 | DAS | ZEA | ENN-A | ENN-A1 | ENN-B | ENN-B1 | BEA | AME | AOH | TEN | MPA | |||
| octanol–water partition coefficient | logP | 1.5 | 1.2 | 2 | -0.4 | 4.1 | 4.7* | 4.4* | 3.9* | 4.1* | 5.9* | 3.9 | 3.8 | 0.5 | 3.8 | 2.1 |
| BBB (blood–brain barrier) permeability | logPS | −2.2 | −2.0 | −2.0 | −3.8 | −1.4 | −1.3 | −1.4 | −1.8 | −1.6 | −1.4 | −1.6 | −1.9 | −2.5 | −2.9 | −2.9 |
| logBB | 0.2 | 0.6 | 0.5 | −0.07 | 0.6 | 1.4 | 1.0 | 0.5 | 0.8 | 0.3 | −0.1 | −0.4 | −0.2 | −0.7 | −0.9 | |
| human serum affinity | LogKa (HSA) | 4.0* | 3.4* | 4.7 | ||||||||||||
| plasma protein binding | PPB (%) | 88.1 | 97.0* | 95.8 | 65.7 | 96.0a | 98.0 | |||||||||
| estrogen receptor binding probability | Log (RBA) > −3 | 0.0* | 0.0* | 0.0 | 0 | 0.9 | 0a | 0a | 0a | 0a | 0.9 | 1.0 | 0 | |||
| genotoxicity probability | CHO/CHL all loci composite | 0.8 | 0.8 | 0.7 | 0.8 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.9 | 0.1 | 0.2 | 0.2 |
| chromosomal aberration in vitro | 0.7 | 0.6 | 0.6 | 0.9 | 0.6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.6 | 0.8 | 0.4 | 0.6 | 0.5 | |
| chromosomal aberration in vivo | 0.6 | 0.5 | 0.5 | 0.8 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.6 | 0.7 | 0.4 | 0.2 | 0.5 | |
| carcinogenicity in mice | 0.4 | 0.3 | 0.3 | 0.4 | 0.7 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.4 | 0.2 | 0.3 | 0.2 | |
| LD50 for mouse | (mg/kg) | 0.8 | 0.8a | 0.9 | 1.2 | 3.3* | 3.0 | 3.0 | 3.0 | 3.0 | 3.7 | 3.4* | 3.0 | 3.2 | ||
| P-gp substrate | substrate probability | 0.8 | 0.9 | 0.9 | 0.8* | 0.2* | 1.0 | 1.0 | 0.9 | 0.9 | 1.0* | 0.1 | 0.8* | 0.4 | 0.2* | |
| Caco-2 permeability | Pe (10 −4 cm/s) | 7.2 | 7.0 | 7.0 | 0.2 | 7.8 | 6.0 | 6.0 | 6.1 | 6.1 | 5.8 | 8.6 | 8.4 | 6.0 | 4.7 | 5.1 |
| first pass metabolism | N | N | N | Y | N | N | N | Y/N | N | N | Y | Y | Y | Y | ||
| Mycotoxins (nM) | Cell lines | |||
|---|---|---|---|---|
| RAW 264.7 | Caco-2 | HepG2 | HEK293T | |
| T-2 | 3.57 ± 0.27 | 13.37 ± 1.07 | 11.38 ± 0.37 | 3.87 ± 0.27 |
| HT-2 | 5.07 ± 0.46 | 44.23 ± 2.26 | 47.44 ± 1.29 | 21.22 ± 1.6 |
| DAS | 3.66 ± 0.37 | 17.74 ± 0.66 | 13.4 ± 1.79 | 6.58 ± 0.36 |
| Tested Mixtures | Recommended Daily Dose (%) | |||||
|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | 3.13 | |
| Silymarin | a | a | a | a | a | a |
| Mycotoxins plus silymarin | a | a | a | a | a | a |
| Milk thistle-based dietary supplement | a | a | a | a | a | a,b |
| Mycotoxins | a | a | b | b | b | b |
| Mycotoxins | DAS | HT-2 | DON | ZEA | ENN A | ENN A1 | ENN B | ENN B1 | TEN | MPA | BEA | AOH | AME |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mixture of T-2 (3.87 nM) | 0.64 ± 0.01 Syn | 0.67 ± 0.04 Syn | 1.29 ± 0.13 Ant | 0.92 ± 0.01 Add | 0.86 ± 0.07 Syn | 1.27 ± 0.13 Ant | 1.11 ± 0.10 Ant | 0.88 ± 0.07 Syn | 0.92 ± 0.07 Add | 0.85 ± 0.05 Syn | 0.95 ± 0.07 Add | 1.00 ± 0.09 Add | 0.97 ± 0.07 Add |
| Mixture of DAS (6.58 nM) | 0.54 ± 0.03 Syn | 0.88 ± 0.17 Syn | 0.85 ± 0.07 Syn | 0.83 ± 0.05 Syn | 0.79 ± 0.04 Syn | 0.79 ± 0.08 Syn | 0.82 ± 0.06 Syn | 0.92 ± 0.17 Add | 0.83 ± 0.07 Syn | 0.96 ± 0.04 Add | 0.91 ± 0.07 Add | 0.90 ± 0.05 Add | |
| Mixture of HT-2 (21.22 nM) | 0.87 ± 0.04 Syn | 0.83 ± 0.03 Syn | 0.80 ± 0.06 Syn | 0.93 ± 0.01 Add | 0.87 ± 0.05 Syn | 0.90 ± 0.05 Add | 0.94 ± 0.04 Add | 0.90 ± 0.04 Add | 0.91 ± 0.04 Add | 0.92 ± 0.02 Add | 0.92 ± 0.06 Add |
| Silibinin Concentration (µM) | 109.00 | 54.5 | 27.3 | 13.6 | 6.8 | 3.4 | 1.7 | 0.9 |
|---|---|---|---|---|---|---|---|---|
| T-2 exposure | 1.00 ± 0.08 Add | 0.91 ± 0.04 Add | 1.41 ± 0.11 Ant | 1.82 ± 0.06 Ant | 1.46 ± 0.14 Ant | 0.99 ± 0.02 Add | ||
| HT-2 exposure | 1.08 ± 0.03 Add | 1.03 ± 0.04 Add | 1.06 ± 0.07 Add | 1.25 ± 0.08 Ant | 1.57 ± 0.15 Ant | 1.27 ± 0.11 Ant | 1.10 ± 0.02 Add | |
| DAS exposure | 1.14 ± 0.01 Ant | 1.15 ± 0.02 Ant | 1.48 ± 0.08 Ant | 1.56 ± 0.13 Ant | 1.50 ± 0.04 Ant | 1.21 ± 0.05 Ant | 1.23 ± 0.06 Ant | 1.00 ± 0.04 Add |
| Mycotoxins | Apical Medium | Caco-2 Cells | Basolateral Medium | Raw 264.7 Cells | Total |
|---|---|---|---|---|---|
| AME | 35.92 ± 1.53c | 0.49 ± 0.02ab | 17.68 ± 0.86bcd | 0.01 ± 0.00ab | 53.94 ± 1.59bc |
| AOH | 39.00 ± 22.02cd | 0.04 ± 0.02a | 59.97 ± 2.86h | 0.07 ± 0.02h | 99.08 ± 24.47e |
| BEA | 10.40 ± 3.89a | 10.36 ± 0.98d | 2.31 ± 0.24a | 0.57 ± 0.03c | 23.65 ± 3.33a |
| DAS | 29.61 ± 0.87abc | 0.02 ± 0.00a | 40.12 ± 0.50f | 0.02 ± 0.00a | 69.77 ± 0.99cd |
| DON | 73.33 ± 2.90f | 0.00 ± 0.00a | 21.48 ± 0.91cde | 0.01 ± 0.00a | 94.83 ± 2.14e |
| ENN-A | 15.08 ± 5.18ab | 10.76 ± 0.43d | 16.18 ± 3.17b | 0.29 ± 0.04b | 42.31 ± 5.00a |
| ENN-A1 | 9.52 ± 3.02a | 2.09 ± 0.28c | 13.06 ± 0.45b | 0.22 ± 0.03b | 24.89 ± 3.24a |
| ENN-B | 24.08 ± 3.20abc | 1.15 ± 0.25b | 25.49 ± 4.31e | 0.31 ± 0.03b | 51.02 ± 7.70bc |
| ENN-B1 | 10.46 ± 2.43a | 0.51 ± 0.03ab | 28.16 ± 3.37e | 0.60 ± 0.10c | 39.72 ± 4.74ab |
| HT-2 | 72.63±2.25f | 0.01 ± 0.00a | 49.18 ± 1.80g | 0.01 ± 0.00a | 121.83 ± 1.33f |
| MPA | 57.27 ± 0.79ef | 0.00 ± 0.00a | 39.77 ± 0.52f | 0.02 ± 0.00a | 97.07 ± 0.27e |
| T-2 | 11.63 ± 0.62a | 0.01 ± 0.00a | 28.13 ± 0.48e | 0.01 ± 0.00a | 39.77 ± 0.98b |
| TEN | 28.07 ± 0.62abc | 0.00 ± 0.00a | 7.25 ± 0.72ab | 0.00 ± 0.00a | 35.33 ± 0.10ab |
| ZEA | 32.23 ± 1.71bc | 0.19 ± 0.01ab | 54.24 ± 2.38gh | 0.05 ± 0.01a | 86.70 ± 0.67de |
| Silibinin | 44.34 ± 1.96cd | 0.00 ± 0.00a | 23.24 ± 1.79de | 0.00 ± 0.00a | 67.58 ± 2.95cd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.N.; Viktorova, J.; Augustynkova, K.; Jelenova, N.; Dobiasova, S.; Rehorova, K.; Fenclova, M.; Stranska-Zachariasova, M.; Vitek, L.; Hajslova, J.; et al. In Silico and In Vitro Studies of Mycotoxins and Their Cocktails; Their Toxicity and Its Mitigation by Silibinin Pre-Treatment. Toxins 2020, 12, 148. https://doi.org/10.3390/toxins12030148
Tran VN, Viktorova J, Augustynkova K, Jelenova N, Dobiasova S, Rehorova K, Fenclova M, Stranska-Zachariasova M, Vitek L, Hajslova J, et al. In Silico and In Vitro Studies of Mycotoxins and Their Cocktails; Their Toxicity and Its Mitigation by Silibinin Pre-Treatment. Toxins. 2020; 12(3):148. https://doi.org/10.3390/toxins12030148
Chicago/Turabian StyleTran, Van Nguyen, Jitka Viktorova, Katerina Augustynkova, Nikola Jelenova, Simona Dobiasova, Katerina Rehorova, Marie Fenclova, Milena Stranska-Zachariasova, Libor Vitek, Jana Hajslova, and et al. 2020. "In Silico and In Vitro Studies of Mycotoxins and Their Cocktails; Their Toxicity and Its Mitigation by Silibinin Pre-Treatment" Toxins 12, no. 3: 148. https://doi.org/10.3390/toxins12030148
APA StyleTran, V. N., Viktorova, J., Augustynkova, K., Jelenova, N., Dobiasova, S., Rehorova, K., Fenclova, M., Stranska-Zachariasova, M., Vitek, L., Hajslova, J., & Ruml, T. (2020). In Silico and In Vitro Studies of Mycotoxins and Their Cocktails; Their Toxicity and Its Mitigation by Silibinin Pre-Treatment. Toxins, 12(3), 148. https://doi.org/10.3390/toxins12030148








