In Vitro Toxicokinetics and Phase I Biotransformation of the Mycotoxin Penitrem A in Dogs
Abstract
1. Introduction
2. Results and Discussion
2.1. Mass Spectrometric Fragmentation of Penitrem A
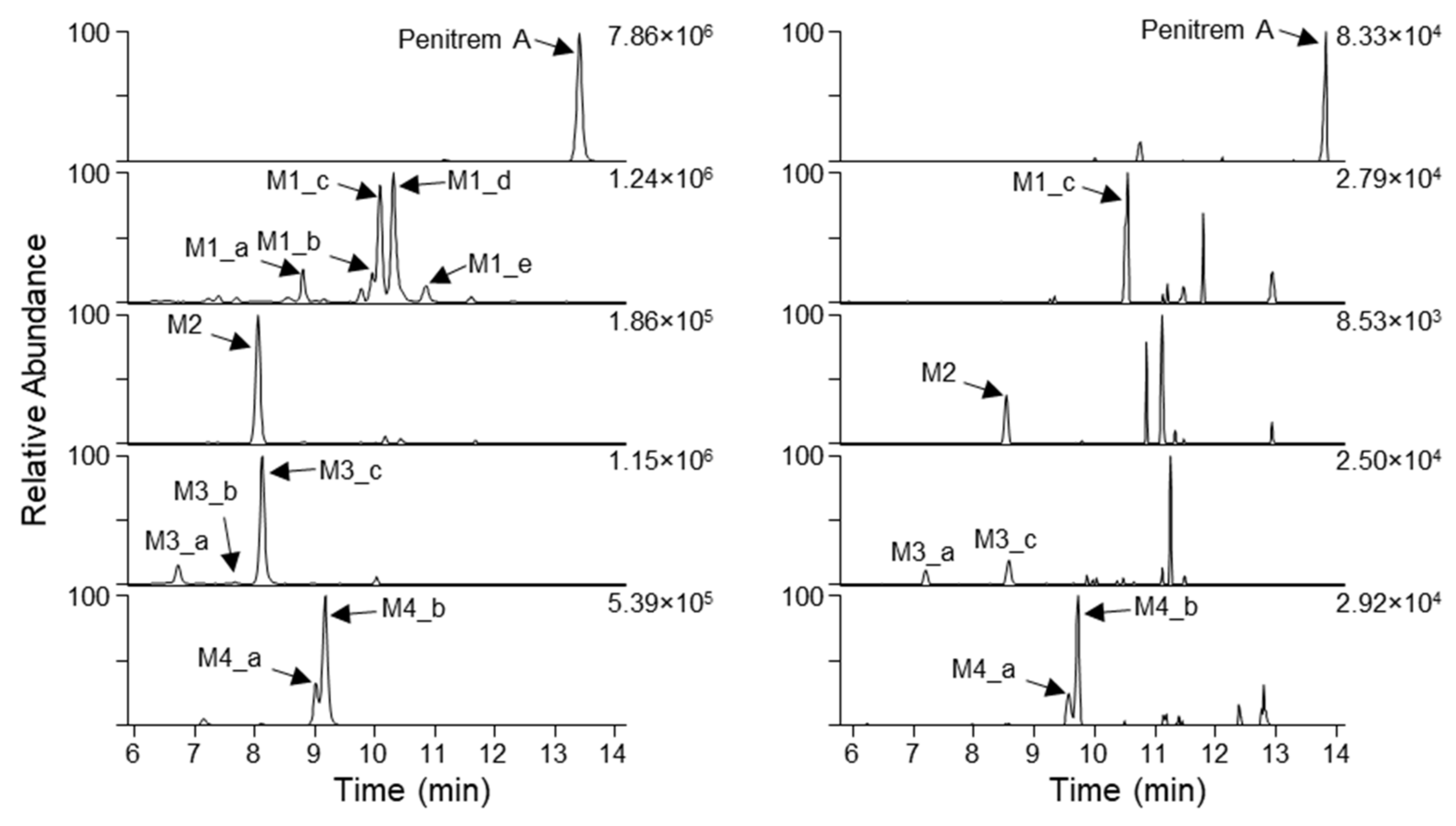
2.2. Analysis of Penitrem A Metabolites Produced by In Vitro Biotransformation
2.3. Detection of Penitrem A and Metabolites in Intoxicated Dogs
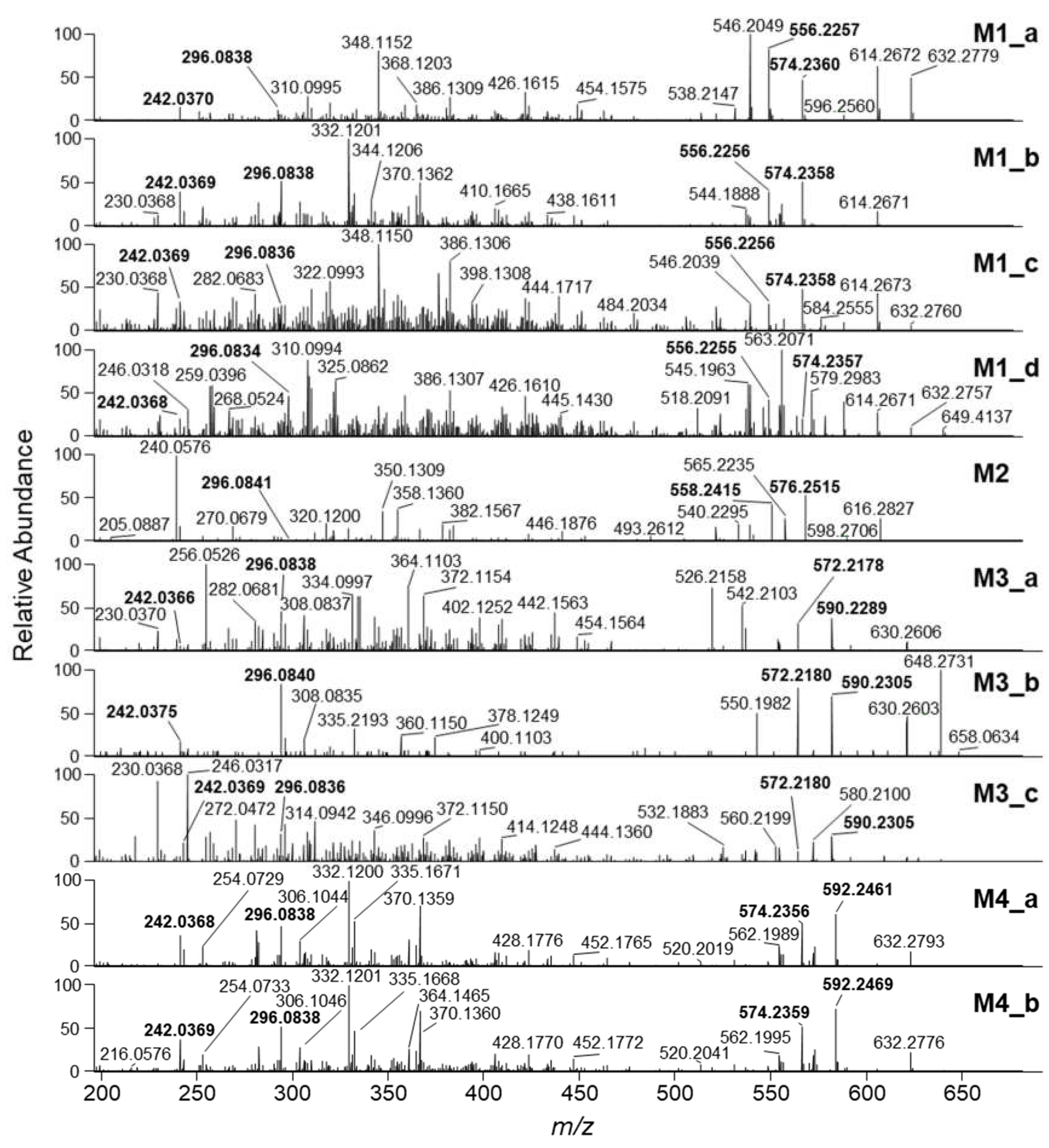
2.4. HRMS/MS Analysis of Putative Penitrem A Metabolites
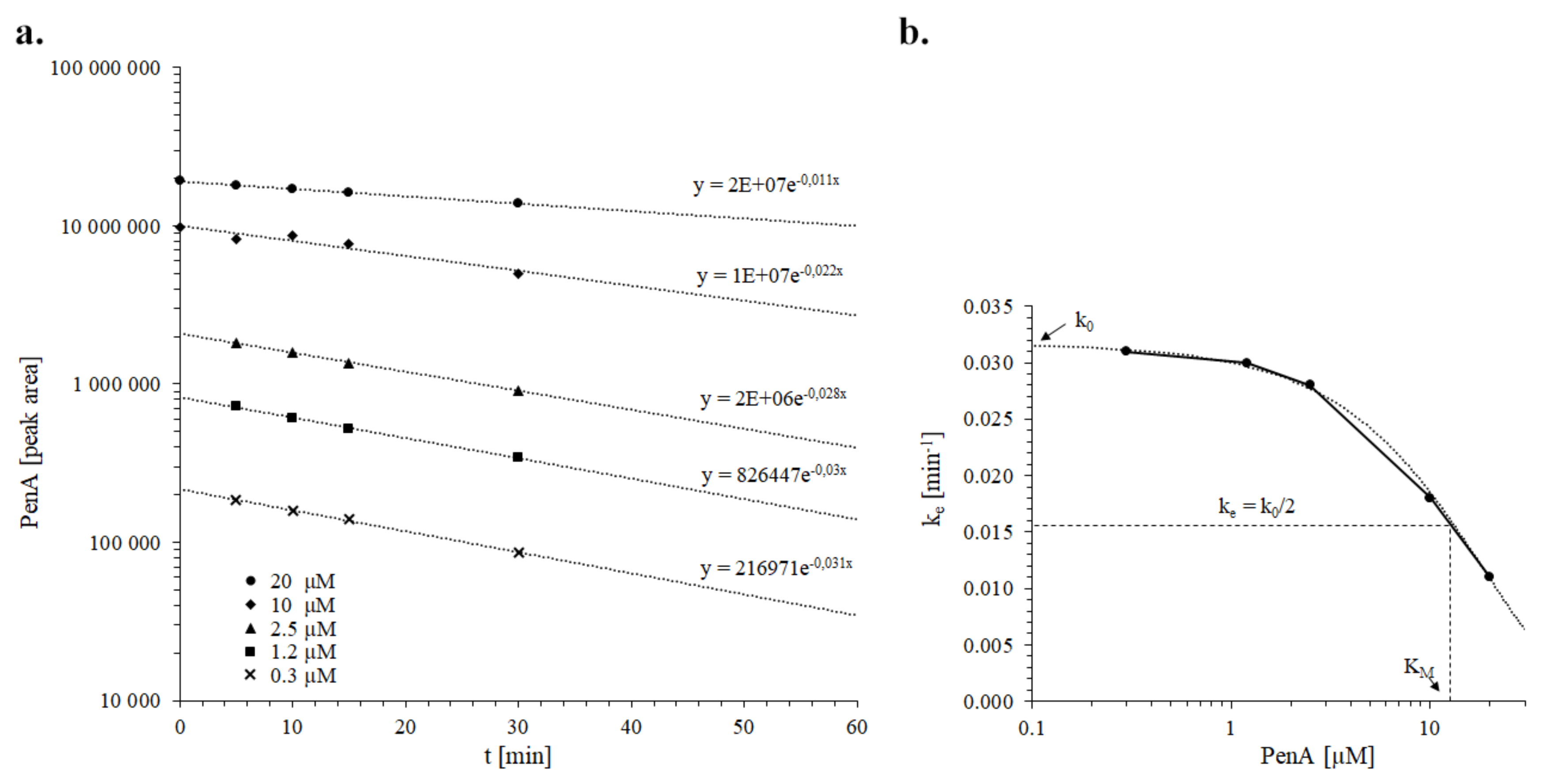
2.5. Kinetics of Penitrem A Depletion in Dog Liver Microsomes
2.6. Prediction of Toxicokinetic Parameters for Penitrem A in Dogs
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Microsomal Incubations
4.3. Determination of Toxicokinetic Parameters In Vitro and Upscaling to In Vivo
4.4. LC–ITMS
4.5. LC–HRMS and HRMS/MS
4.6. Preparation of Stomach Contents and Plasma Samples from Potentially Intoxicated Dogs
4.7. Anamnesis of the Penitrem A-Positive Dog
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richard, J.L.; Arp, L.H. Natural occurrence of the mycotoxin penitrem A in moldy cream cheese. Mycopathologia 1997, 67, 107–109. [Google Scholar]
- Cole, R.J.; Dorner, J.W.; Cox, R.H.; Raymond, L.W. Two classes of alkaloid mycotoxins produced by Penicillium crustosum Thom isolated from contaminated beer. J. Agric. Food Chem. 1983, 31, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.S.; Roberts, B.A.; Shreeve, B.J.; MacDonald, S.M.; Hayes, A.W. Tremorgenic toxins produced by soil fungi. Appl. Environ. Microbiol. 1979, 37, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, M.; Sala de Miguel, M.A.; Viera, J.B.; de Banchero, E.P. Tremorgenic mycotoxins produced by strains of Penicillium spp. isolated from toxic Poa huecu parodi. Mycopathologia 1992, 120, 177–182. [Google Scholar] [CrossRef]
- Kozák, L.; Szilágyi, Z.; Tóth, L.; Pócsi, I.; Molnár, I. Tremorgenic and neurotoxic paspaline-derived indole–diterpenes: Biosynthetic diversity, threats and applications. Appl. Microbiol. Biotechnol. 2019, 103, 1599–1616. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.S.; Fonnum, F.; Eriksen, G.S.; Rundberget, T.; Walaas, S.I.; Wigestrand, M.B. In vitro neuropharmacological evaluation of penitrem-induced tremorgenic syndromes: Importance of the GABAergic system. Neurochem. Int. 2011, 59, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, H.F.; Bogen, I.L.; Wigestrand, M.B.; Fonnum, F.; Walaas, S.I.; Moldes-Anaya, A. The fungal neurotoxin penitrem A induces the production of reactive oxygen species in human neutrophils at submicromolar concentrations. Toxicology 2017, 392, 64–70. [Google Scholar] [CrossRef]
- Sobotka, T.J.; Brodie, R.E.; Spaid, S.L. Neurobehavioral studies of tremorgenic mycotoxins verruculogen and penitrem A. Pharmacology 1978, 16, 287–294. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Moldes-Anaya, A.; Fæste, C.K. Penitrem A and analogues: Toxicokinetics, toxicodynamics including mechanism of action and clinical significance. World Mycotox. J. 2013, 6, 263–272. [Google Scholar] [CrossRef]
- Talcott, P.A. Tremorgens (Penitrem A and Roquefortine). In Small Animal Toxicology, 3rd ed.; Peterson, M.E., Talcott, P.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 677–682. [Google Scholar]
- Lewis, P.R.; Donoghue, M.B.; Hocking, A.D.; Cook, L.; Granger, L.V. Tremor syndrome associated with fungal toxin: Sequelae of food contamination. Med. J. Aust. 2005, 182, 582–584. [Google Scholar] [CrossRef]
- Hayes, A.W.; Presley, D.B.; Neville, J.A. Acute toxicity of penitrem A in dogs. Toxicol. Appl. Pharmacol. 1976, 35, 311–320. [Google Scholar] [CrossRef]
- Richard, J.L.; Bacchetti, P.; Arp, L.H. Moldy walnut toxicosis in a dog, caused by the mycotoxin, penitrem A. Mycopathologia 1981, 76, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.D.; Holds, K.; Tobin, N.F. Intoxication by tremorgenic mycotoxin (penitrem A) in a dog. Aust. Vet. J. 1988, 65, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.L. Acute penitrem A and roquefortine poisoning in a dog. Can. Vet. J. Rev. Vet. Can. 2002, 43, 372–374. [Google Scholar]
- Naude, T.W.; O’Brien, O.M.; Rundberget, T.; McGregor, A.D.; Roux, C.; Flåøyen, A. Tremorgenic neuromycotoxicosis in 2 dogs ascribed to the ingestion of penitrem A and possibly roquefortine in rice contaminated with Penicillium crustosum. J. S. Afr. Vet. Assoc. 2002, 73, 211–215. [Google Scholar] [CrossRef]
- Boysen, S.R.; Rozanski, E.A.; Chan, D.L.; Grobe, T.L.; Fallon, M.J.; Rush, J.E. Tremorgenic mycotoxicosis in four dogs from a single household. J. Am. Vet. Med. Assoc. 2002, 221, 1441–1444. [Google Scholar] [CrossRef]
- Young, K.L.; Villar, D.; Carson, T.L.; Ierman, P.M.; Moore, R.A.; Bottoff, M.R. Tremorgenic mycotoxin intoxication with penitrem A and roquefortine in two dogs. J. Am. Vet. Med. Assoc. 2003, 222, 52–53. [Google Scholar] [CrossRef]
- Munday, J.S.; Thompsom, D.; Finch, S.C.; Babu, J.V.; Wilkins, A.L.; Di Menna, M.E.; Miles, C.O. Presumptive tremorgenic mycotoxicoses in a dog in New Zealand, after eating mouldy walnuts. N. Z. Vet. J. 2008, 56, 145–147. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Jäderlund, K.H.; Moldes-Anaya, A.; Schönheit, J.; Bernhoft, A.; Jæger, G.; Rundberget, T.; Skaar, I. Poisoning of dogs with tremorgenic Penicillium toxins. Med. Mycol. 2010, 48, 188–196. [Google Scholar] [CrossRef]
- Evans, T.J.; Gupta, R.C. Tremorgenic Mycotoxins. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: New York, NY, USA, 2018; pp. 1033–1041. [Google Scholar]
- Chapman, E. Nursing a canine with presumptive tremorgenic mycotoxicosis following ingestion of mouldy dog food–a case report. Vet. Nurs. J. 2018, 33, 166–169. [Google Scholar] [CrossRef]
- Barker, A.K.; Stahl, C.; Ensley, S.M.; Jeffery, N.D.; Decus, D. Tremorgenic mycotoxicosis in dogs. Compend. Contin. Educ. Vet. 2013, 35, E2. [Google Scholar] [PubMed]
- Goda, A.A.; Naguib, K.M.; Mohamed, M.M.; Amra, H.A.; Nada, S.A.; Abdel-Ghaffar, A.R.B.; Gissendanner, C.R.; El Sayed, K.A. Astaxanthin and docosahexaenoic acid reverse the toxicity of the maxi-K (BK) channel antagonist mycotoxin penitrem A. Mar. Drugs 2016, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Rundberget, T.; Wilkins, A.L. Determination of Penicillium mycotoxins in foods and feeds using liquid chromatography–mass spectrometry. J. Chromatogr. A 2002, 964, 189–197. [Google Scholar] [CrossRef]
- Tor, E.R.; Puschner, B.; Filigenzi, M.S.; Tiwary, A.K.; Poppenga, R.H. LC-MS/MS screen for penitrem A and roquefortine C in serum and urine samples. Analyt. Chem. 2006, 78, 4624–4629. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.; Wilkins, A.L.; Rundberget, T.; Fæste, C.K. In vitro and in vivo hepatic metabolism of the fungal neurotoxin penitrem A. Drug Chem. Toxicol. 2009, 32, 26–37. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.; Rundberget, T.; Fæste, C.K.; Eriksen, G.S.; Bernhoft, A. Neurotoxicity of Penicillium crustosum secondary metabolites: Tremorgenic activity of orally administered penitrem A and thomitrem A and E in mice. Toxicon 2012, 60, 1428–1435. [Google Scholar] [CrossRef]
- Kalinina, S.A.; Jagels, A.; Hickert, S.; Mauriz Marques, L.M.; Cramer, B.; Humpf, H.U. Detection of the cytotoxic penitrems A–F in cheese from the European single market by HPLC–MS/MS. J. Agric. Food Chem. 2018, 66, 1264–1269. [Google Scholar] [CrossRef]
- Ito, K.; Houston, B. Prediction of human drug clearance from in vitro and preclinical data using physiologically based and empirical approaches. Pharm. Res. 2005, 22, 103–112. [Google Scholar] [CrossRef]
- Iwatsubo, T.; Hirota, N.; Ooie, T.; Suzuki, H.; Shimada, N.; Chiba, K.; Ishizaki, T.; Green, C.E.; Tyson, C.A.; Sugiyama, Y. Prediction of in vivo drug metabolism in the human liver from in vitro metabolism data. Pharmacol. Ther. 1997, 73, 147–171. [Google Scholar] [CrossRef]
- Obach, R.S.; Reed-Hagen, A.E. Measurement of Michaelis constant for cytochrome P450-mediated biotransformation reactions using a substrate depletion approach. Drug Met. Disp. 2002, 30, 831–837. [Google Scholar] [CrossRef]
- Lindstedt, S.L.; Schaeffer, P.J. Use of allometry in predicting anatomical and physiological parameters of mammals. Lab. Anim. 2002, 36, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Jones, R.D.O.; Ballard, P.G.; Griffiths, H.H. Determination of microsome and hepatocyte scaling factors for in vitro/in vivo extrapolation in the rat and dog. Xenobiotica 2008, 38, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Baxter, J.G.; Listin, T.E.; Silber, B.M.; Jones, B.C.; MacIntyre, F.; Ranve, D.J.; Wastall, P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J. Pharmacol. Exp. Ther. 1997, 283, 46–58. [Google Scholar] [PubMed]
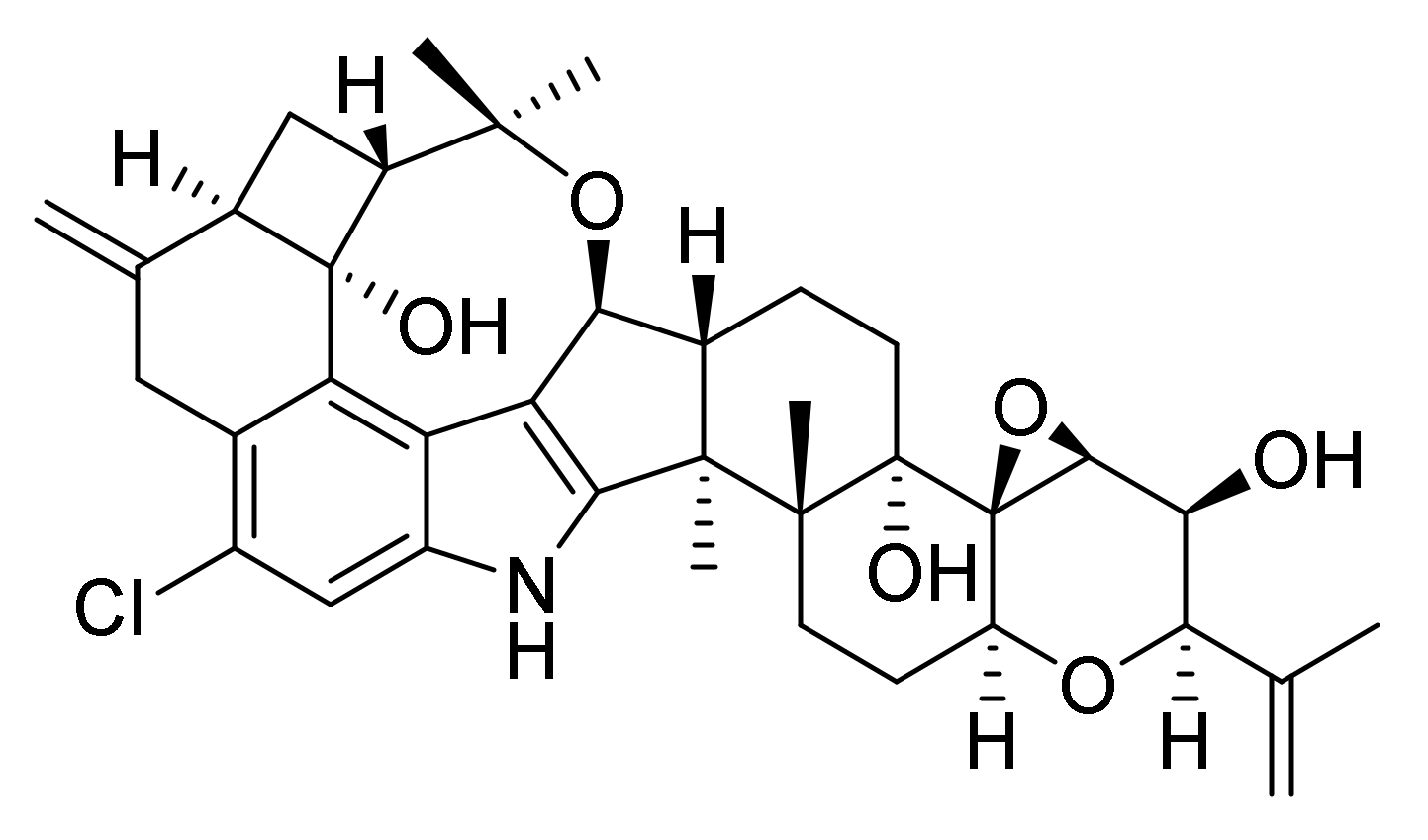
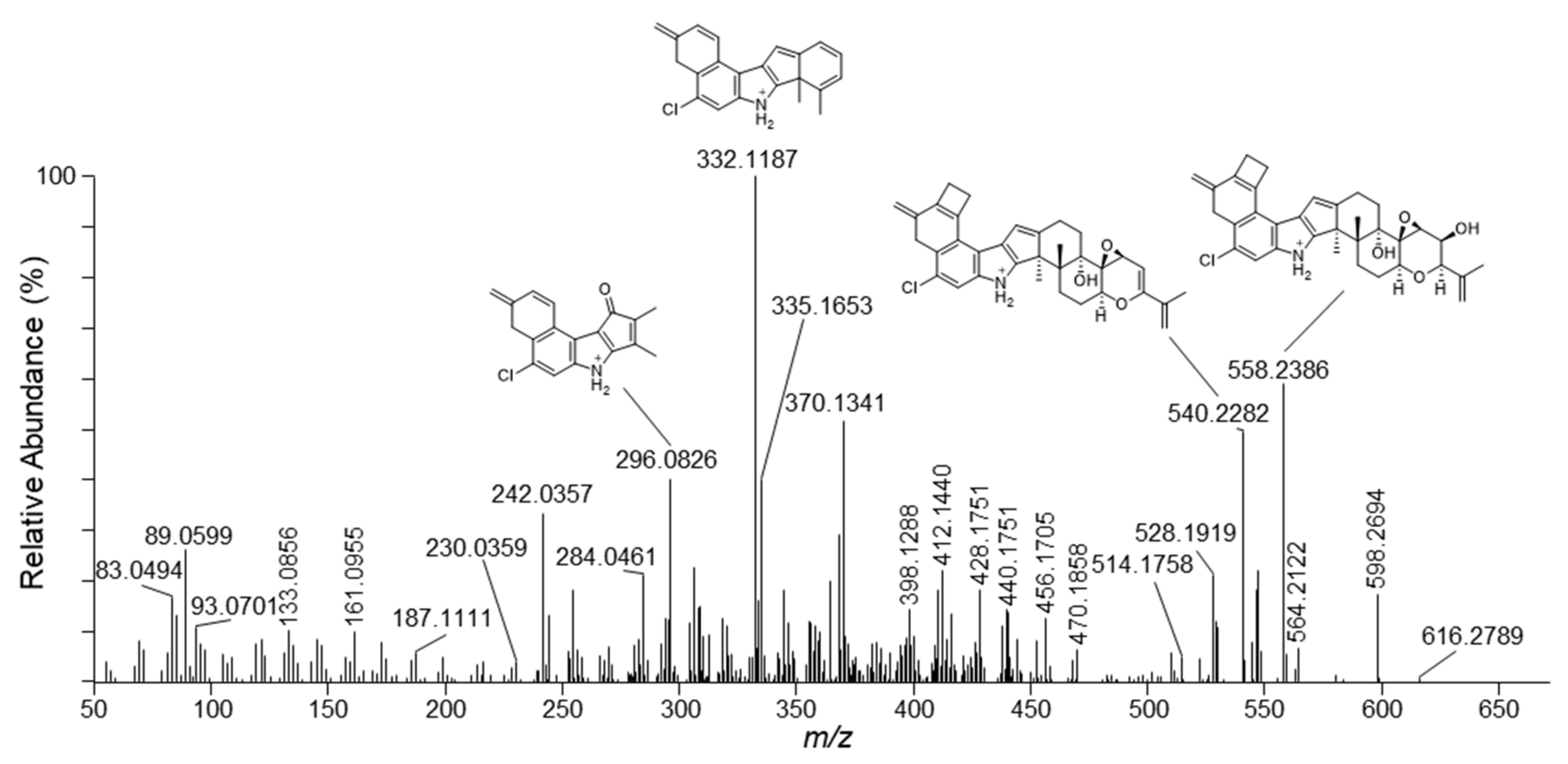
| m/z | Formula | Δm (ppm) | RDBeq 1 | Neutral Loss |
|---|---|---|---|---|
| 616.2789 | C37H43NO5Cl | −5.7 | 16.5 | H2O |
| 598.2694 | C37H41NO4Cl | −4.1 | 17.5 | 2×H2O |
| 564.2122 | C32H35NO6Cl | −4.5 | 15.5 | C5H10 |
| 558.2386 | C34H37NO4Cl | −3.5 | 16.5 | H2O + C3H6(O) 2 |
| 540.2282 | C34H35NO3Cl | −3.2 | 17.5 | 2×H2O + C3H6(O) 2 |
| 528.1919 | C32H31NO4Cl | −3.2 | 17.5 | 2×H2O + C5H10 |
| 428.1751 | C28H27NOCl | −5.8 | 15.5 | 2×H2O + C3H6(O) 2 + C6H8O2 |
| 412.1440 | C27H23NOCl | −5.5 | 16.5 | 2×H2O + C3H6(O) 2 + C6H8O2 + CH4 |
| 370.1341 | C25H21NCl | −4.3 | 15.5 | |
| 332.1187 | C22H19NCl | −4.1 | 13.5 | |
| 296.0826 | C18H15NOCl | −3.6 | 11.5 | |
| 242.0357 | C14H9NOCl | −4.2 | 10.5 |
| Metabolite ID | tR (min) 1 | m/z | Ion | Formula | Δm (ppm) | RDBeq 2 |
|---|---|---|---|---|---|---|
| Penitrem A | 13.42 | 634.2927 | [M+H]+ | C37H45NO6Cl | −0.46 | 16 |
| M1_a | 8.81 | 650.2875 | [M+H]+ | C37H45NO7Cl | −0.58 | 16 |
| M1_b | 9.97 | 650.2876 | [M+H]+ | C37H45NO7Cl | −0.44 | 16 |
| M1_c | 10.09 | 650.2858 | [M+H]+ | C37H45NO7Cl | −3.5 | 16 |
| M1_d | 10.31 | 650.2865 | [M+H]+ | C37H45NO7Cl | −2.2 | 16 |
| M1_e | 10.87 | 650.2868 | [M+H]+ | C37H45NO7Cl | −1.6 | 16 |
| M2 | 8.06 | 652.3046 | [M+H]+ | C37H47NO7Cl | 1.6 | 15 |
| M3_a | 6.73 | 666.2834 | [M+H]+ | C37H45NO8Cl | 0.81 | 16 |
| M3_b | 7.67 | 666.2831 | [M+H]+ | C37H45NO8Cl | 0.34 | 16 |
| M3_c | 8.13 | 666.2826 | [M+H]+ | C37H45NO8Cl | −0.39 | 16 |
| M4_a | 9.02 | 668.2984 | [M+H]+ | C37H47NO8Cl | −0.17 | 15 |
| M4_b | 9.18 | 668.2987 | [M+H]+ | C37H47NO8Cl | 0.28 | 15 |
| Parameter | Dog Liver Microsomes |
|---|---|
| ke (min−1) | 0.03 |
| t1/2,assay (min) | 23.1 |
| KM,assay (µM) | 12 |
| CLint,assay (L/h) | 1.8 × 10−3 |
| CLint (L/(h*kg)) | 1.6 |
| CLb (L/(h*kg)) | 0.9 |
| fmax (%) | 57 |
| Dog nr. | Sampling Date | Available Samples | Origin | Penitrem A |
|---|---|---|---|---|
| 1 | 14 February 2018 | Plasma, stomach contents | Fredrikstad 1 animal clinic | positive |
| 2 | 25 February 2018 | Plasma | Fredrikstad animal clinic | negative |
| 3 | 22 January 2018 | Serum | Fredrikstad animal clinic | negative |
| 4 | 1 April 2018 | Plasma | NMBU 2 small animal clinic | negative |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhlig, S.; Ivanova, L.; Voorspoels, P.; Fæste, C.K. In Vitro Toxicokinetics and Phase I Biotransformation of the Mycotoxin Penitrem A in Dogs. Toxins 2020, 12, 293. https://doi.org/10.3390/toxins12050293
Uhlig S, Ivanova L, Voorspoels P, Fæste CK. In Vitro Toxicokinetics and Phase I Biotransformation of the Mycotoxin Penitrem A in Dogs. Toxins. 2020; 12(5):293. https://doi.org/10.3390/toxins12050293
Chicago/Turabian StyleUhlig, Silvio, Lada Ivanova, Pauline Voorspoels, and Christiane Kruse Fæste. 2020. "In Vitro Toxicokinetics and Phase I Biotransformation of the Mycotoxin Penitrem A in Dogs" Toxins 12, no. 5: 293. https://doi.org/10.3390/toxins12050293
APA StyleUhlig, S., Ivanova, L., Voorspoels, P., & Fæste, C. K. (2020). In Vitro Toxicokinetics and Phase I Biotransformation of the Mycotoxin Penitrem A in Dogs. Toxins, 12(5), 293. https://doi.org/10.3390/toxins12050293







