The Cytocidal Spectrum of Bacillus thuringiensis Toxins: From Insects to Human Cancer Cells
Abstract
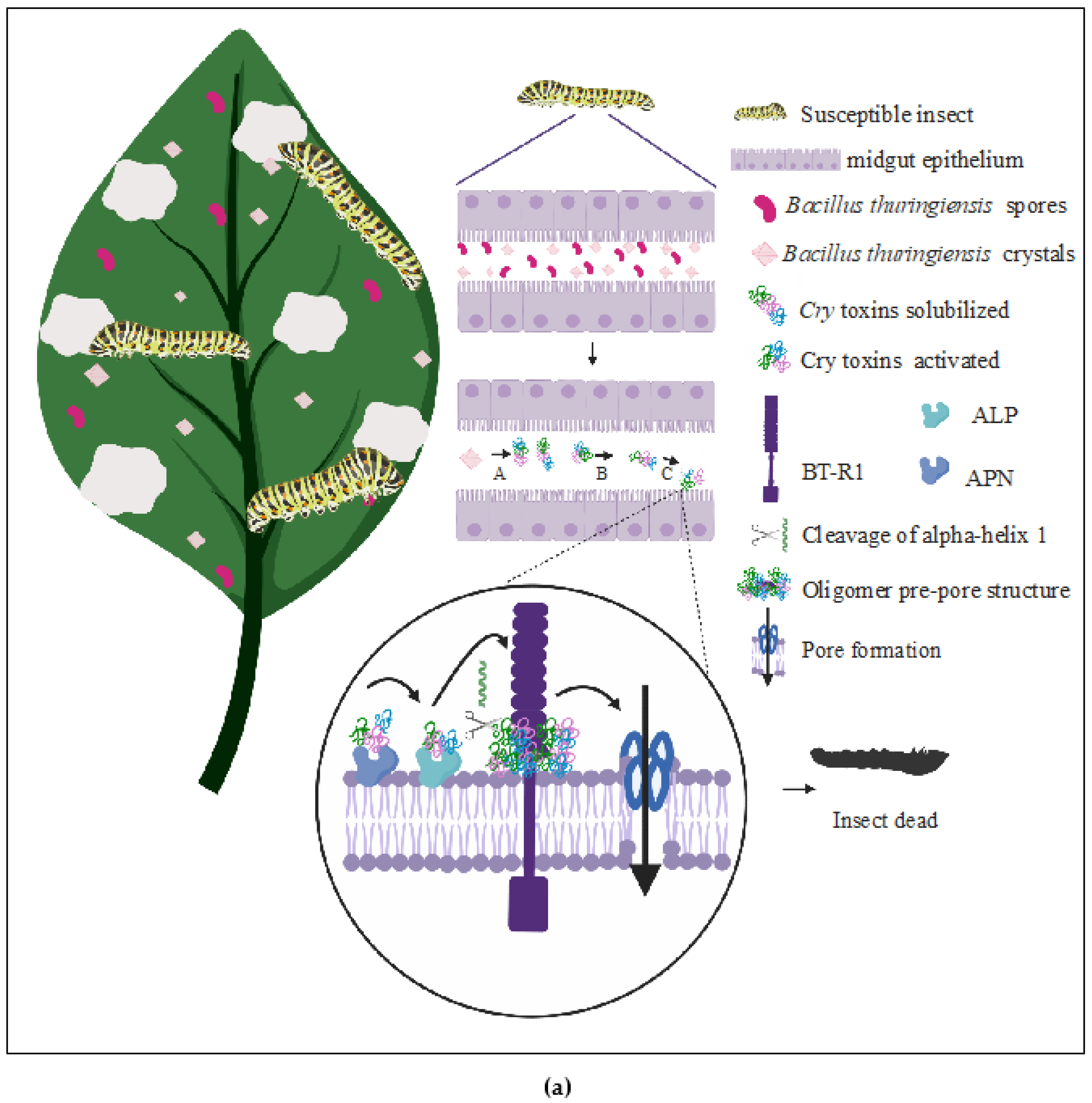
:1. Introduction
2. Leading Toxic Proteins of Bacillus thuringiensis and their Mechanism of Action
2.1. Cry Toxins
Mechanism of Action from Cry Toxins
(a) The Pore-Forming Model
(b) The Signaling Pathway Model
2.2. Cyt Family
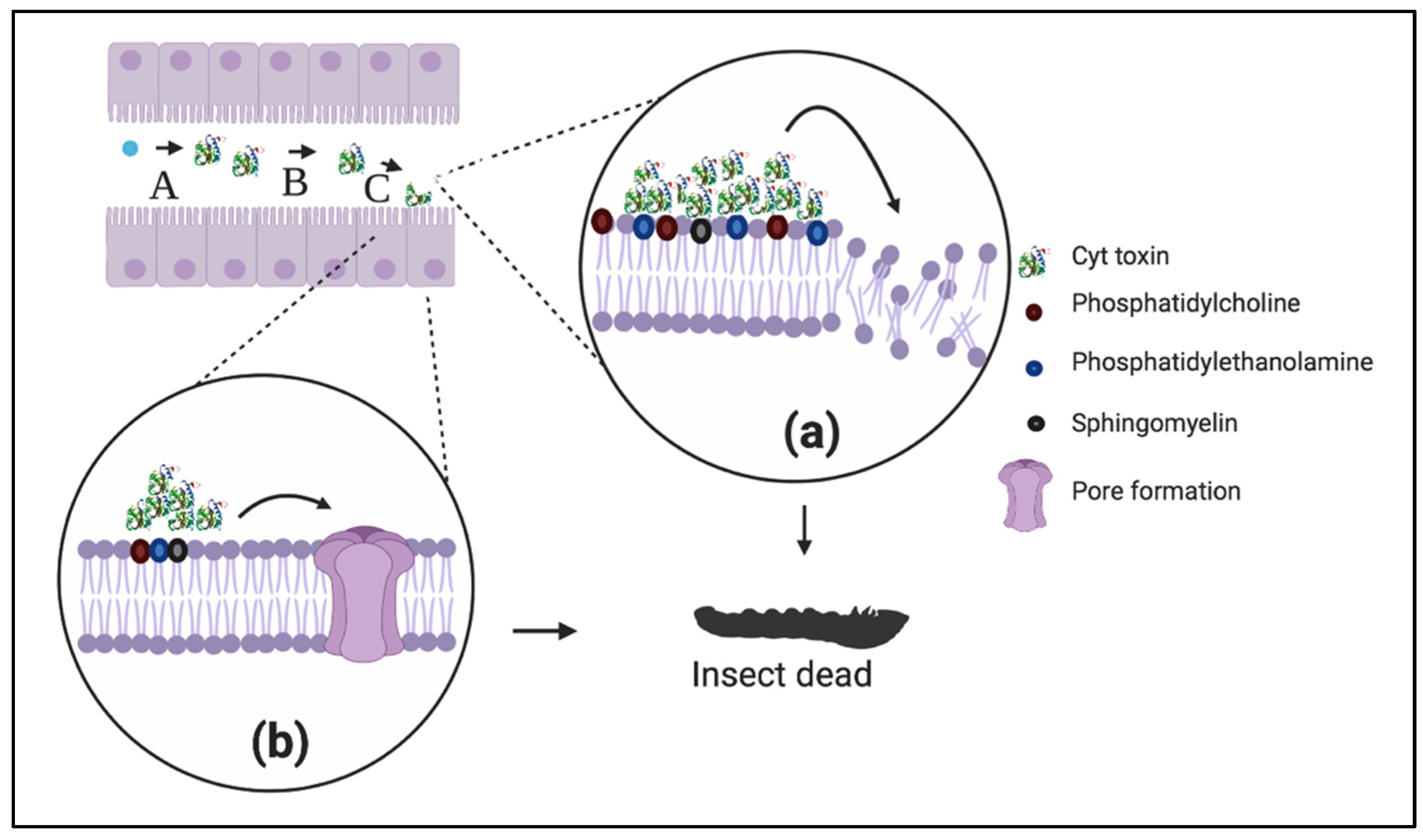
Cyt Proteins Mechanism of Action
(a) The Pore-Formation Model
(b) The Detergent-Effect Model
2.3. Parasporins
2.3.1. Parasporin Classification
PS1 Family
PS2 Family
PS3 Family
PS4 Family
PS5 Family
PS6 Family
2.3.2. Mechanism of Action of Parasporins
2.4. S-Layer Proteins
2.5. Toxins Secreted by Bt
2.5.1. Vip Family
2.5.2. Sip Toxins
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knowles, B.H.; Dow, J.A.T. The crystal delta-endotoxins of Bacillus thuringiensis—Models for their mechanism of action on the insect gut. Bioessays 1993, 15, 469–476. [Google Scholar] [CrossRef]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.F.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, J.Y.; Choi, J.Y.; Li, M.S.; Jin, B.R.; Je, Y.H. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Mol. Biol. 2007, 17, 547–559. [Google Scholar]
- Becker, N. Bacterial control of vector-mosquitoes and black flies. In Entomopathogenic Bacteria: From Laboratory to Field Application; Charles, J.F., Delécluse, A., Nielsen-LeRoux, C., Eds.; Kluwer Academic Publishers: Berlin, Germany, 2000; pp. 383–398. [Google Scholar] [CrossRef]
- Kotze, A.C.; Grady, J.O.; Gough, J.M.; Pearson, R.; Bagnall, N.H.; Kemp, D.H.; Akhurst, R.J. Toxicity of Bacillus Thuringiensis to Parasitic and Free-Living Life-Stages of Nematode Parasites of Livestock. Int. J. Parasitol. 2005, 35, 1013–1022. [Google Scholar] [CrossRef]
- Ohba, M.; Mizuki, E.; Uemori, A. Parasporin, a new anticancer protein group from Bacillus thuringiensis. Anticancer Res. 2009, 29, 427–433. [Google Scholar]
- Rubio, V.P.; Bravo, A.; Olmos, J. Identification of a Bacillus thuringiensis Surface Layer Protein with Cytotoxic Activity against MDA-MB-231 Breast Cancer Cells. J. Microbiol. Biotechnol. 2017, 27, 36–42. [Google Scholar] [CrossRef]
- Gang, G.; Lei, Z.; Zhou, Z.; Qiqi, M.; Jianping, L.; Chenguang, Z.; Lei, Z.; Ziniu, Y.; Ming, S. A new group of parasporal inclusions encoded by the S-layer gene of Bacillus thuringiensis. FEMS. Microbiol. Lett. 2008, 282, 1–7. [Google Scholar]
- Liu, L.; Boyd, S.D.; Bulla-Jr, L.A.; Winkler, D.D. The Defined Toxin-binding Region of the Cadherin G-protein Coupled Receptor, BT-R1, for the Active Cry1Ab Toxin of Bacillus thuringiensis. J. Proteomics Bioinform. 2018, 11, 201–210. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [Green Version]
- Bulla, L.A.; Kramer, K.J.; Cox, D.J.; Jones, B.L.; Davidson, L.I.; Lookhart, G.L. Purification and characterization of the entomocidal protoxin of Bacillus thuringiensis. J. Biol. Chem. 1981, 256, 3000–3004. [Google Scholar] [PubMed]
- Crickmore, N.; Zeigler, D.R.; Schnepf, E.; Rie, J.; Lereclus, D.; Baum, J.; Bravo, A.; Dean, D.H. Bacillus thuringiensis Toxin Nomenclature. Available online: http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/ (accessed on 3 April 2020).
- Höfte, H.; Whiteley, H.R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989, 5, 242–255. [Google Scholar]
- Van Frankenhuyzen, K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 2009, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Akiba, T.; Okumura, S. Parasporins 1 and 2: Their structure and activity. J. Invertebr. Pathol. 2016, 142, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sará, M.; Sleytr, U.B. S-Layer Proteins. J. Bacteriol. 2000, 182, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Peña, G.; Miranda-Rios, J.; de la Riva, G.; Pardo-López, L.; Soberón, M.; Bravo, A. A Bacillus thuringiensis S-layer protein involved in toxicity against Epilachna varivestis (Coleoptera: Coccinellidae). Appl. Environ. Microbiol. 2006, 72, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Donovan, W.P.; Engleman, J.T.; Donovan, J.C.; Baum, J.A.; Bunkers, G.J.; Chi, D.J.; Clinton, W.P.; English, L.; Heck, G.R.; Ilagan, O.M.; et al. Discovery and characterization of Sip1A: A novel secreted protein from Bacillus thuringiensis with activity against coleopteran larvae. Appl. Microbiol. Biotechnol. 2006, 72, 713–719. [Google Scholar] [CrossRef]
- Chakroun, M.; Banyuls, N.; Bel, Y.; Escriche, B.; Ferré, J. Bacterial Vegetative Insecticidal Proteins (Vip) from Entomopathogenic Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 329–350. [Google Scholar] [CrossRef] [Green Version]
- Sahin, B.; Gomis-Cebolla, J.; Güneş, H.; Ferré, J. Characterization of Bacillus thuringiensis isolates by their insecticidal activity and their production of Cry and Vip3 proteins. PLoS ONE 2018, 13, e0206813. [Google Scholar] [CrossRef]
- Christou, O.; Capell, T.; Kohli, A.; Gatehouse, J.A.; Gatehouse, A.M. Recent developments and future prospects in insect pest control in transgenic crop. Trend Plant Sci. 2006, 11, 302–308. [Google Scholar] [CrossRef]
- Pardo-Lopez, L.; Soberon, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Mendelson, M.; Kough, J.; Vaituzis, Z.; Matthews, K. Are Bt crops safe? Nat. Biotechnol. 2003, 21, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Romeis, J.; Meissle, M.; Bigler, F. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat. Biotechnol. 2006, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, J.Z.; Collins, H.L.; Earle, E.D.; Cao, J.; Shelton, A.M. A critical assessment of the effects of Bt transgenic pants on parasitoids. PLoS ONE 2008, 3, e2284. [Google Scholar]
- Melo, A.L.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Fischhoff, D.A.; Bowdish, K.S.; Perlak, F.J.; Marrone, P.G.; McCormick, S.M.; Niedermeyer, J.G.; Dean, D.A.; Kusano-Kretzmer, K.; Mayer, E.J.; Rochester, D.E.; et al. Insect tolerant transgenic tomato plants. Nat. Biotechnol. 1987, 5, 807–813. [Google Scholar] [CrossRef]
- Vaek, M.; Reynaerts, A.; Hofte, A. Transgenic plants protected from insects. Nature 1987, 325, 33–37. [Google Scholar] [CrossRef]
- Koch, M.S.; Ward, J.M.; Levine, S.L.; Baum, G.A.; Vicini, J.L.; Hammond, B.G. The food and environmental safety of Bt crops. Front. Plant. Sci. 2015, 6, 283–336. [Google Scholar] [CrossRef]
- Zhong, C.; Ellar, D.J.; Bishop, A.; Johnson, C.; Lin, S.; Hart, E.R. Characterization of a Bacillus thuringiensis delta endotoxin whic iIs toxic to insects in three orders. J. Invertebr. Pathol. 2000, 76, b131–b139. [Google Scholar] [CrossRef]
- Schnepf, H.E.; Whiteley, H.R. Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc. Natl. Acad. Sci. USA 1981, 78, 2893–2897. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.J.; Morgan, J.A.; Whipps, J.M.; Saunders, J.R. Plasmid Transfer between the Bacillus thuringiensis Subspecies kurstaki and tenebrionis in Laboratory Culture and Soil and in Lepidopteran and Coleopteran Larvae. Appl. Environ. Microbiol. 2000, 66, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenas, I.; Bravo, A.; Soberón, M.; Gómez, I. Role of alkaline phosphatase from Manduca sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. J. Biol. Chem. 2010, 285, 12497–12503. [Google Scholar] [CrossRef] [Green Version]
- Parker, M.W.; Feil, S.C. Pore forming protein toxins: From structure to function. Progress. Biophys. Mol. Biol. 2005, 88, 91–124. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Almazán, C.; Zavala, L.E.; Muñoz-Garay, C.; Jiménez-Juárez, N.; Pacheco, S.; Masson, L.; Soberón, M.; Bravo, A. Dominant Negative Mutants of Bacillus thuringiensis Cry1Ab Toxin Function as Anti-Toxins: Demonstration of the Role of Oligomerization in Toxicity. PLoS ONE 2009, 4, e5545. [Google Scholar] [CrossRef] [PubMed]
- De Maagd, R.A.; Bravo, A.; Crickmore, N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 2001, 17, 193–199. [Google Scholar] [CrossRef]
- Rajamohan, F.; Hussain, S.R.A.; Cotrill, J.A.; Gould, F.; Dean, D.H. Mutations at domain II, loop 3, of Bacillus thuringiensis CryIAa and CryIAb δ-endotoxins suggest loop 3 is involved in initial binding to lepidopteran midguts. J. Biol. Chem. 1996, 271, 25220–25226. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K.; Rajamohan, F.; Jenkins, J.L.; Curtiss, A.S.; Dean, D.H. Role of two arginine residues in domain II, loop 2 of Cry1Ab and Cry1Ac Bacillus thuringiensis δ-endotoxin in toxicity and binding to Manduca sexta and Lymantria dispar aminopeptidase N. Mol. Microbiol. 2000, 38, 289–298. [Google Scholar] [CrossRef] [Green Version]
- De Maagd, R.A.; Bravo, A.; Berry, C.; Crickmore, N.; Schnepf, H.E. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet. 2003, 37, 409–433. [Google Scholar] [CrossRef]
- Lakey, J.H.; van der Goot, F.G.; Pattus, F. All in the family: The toxic activity of pore-forming toxins. Toxicology 1994, 87, 85–108. [Google Scholar] [CrossRef]
- Hunt, S.; Green, J.; Artymiuk, P.J. Hemolysin E (HlyE, ClyA, SheA) and related toxins. Adv. Exp. Med. Biol. 2010, 677, 116–126. [Google Scholar]
- Kristan, K.C.; Viero, G.; Dalla-Serra, M.; Macek, P.; Anderluh, G. Molecular mechanism of pore formation by actinoporins. Toxicon 2009, 54, 1125–1134. [Google Scholar] [CrossRef]
- DuMont, A.L.; Torres, V.J. Cell targeting by the Staphylococcus aureus pore-forming toxins: It’s not just about lipids. Trends Microbiol. 2014, 22, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Iacovache, I.; Dal Peraro, M.; van der Goot, F.G. The Comprehensive Sourcebook of Bacterial Protein Toxins; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Hotze, E.M.; Le, H.M.; Sieber, J.R.; Bruxvoort, C.; Mclnerney, M.J.; Tweten, R.K. Identification and characterization of the first cholesterol-dependent cytolysins from Gram-negative bacteria. Infect. Immun. 2013, 81, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Gouaux, E. Channel-forming toxins: Tales of transformation. Curr. Opin. Struct. Biol. 1997, 7, 566–573. [Google Scholar] [CrossRef]
- Lesieur, C.; Vecsey-Semjn, B.; Abrami, L.; Fivaz, M.; van der Goot, F.G. Membrane insertion: The strategy of toxins. Mol. Membrane Biol. 1997, 14, 45–64. [Google Scholar] [CrossRef]
- Iacovache, I.; Bischofberger, M.; van der Goot, F.G. Structure and assembly of pore-forming proteins. Curr. Opin. Struct. Biol. 2010, 20, 241–246. [Google Scholar] [CrossRef]
- Dal Peraro, M.; van der Goot, G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Shepard, L.A.; Heuck, A.P.; Hamman, B.D.; Rossjohn, J.; Parker, M.W.; Ryan, K.R.; Johnson, A.E.; Tweten, R.K. Identification of a membrane- spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: An α-helical to β-sheet transition identified by fluorescence spectroscopy. Biochemistry 1998, 37, 14563–14574. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.C.; Yu, Z.; Sun, M. Structural Insights into Bacillus thuringiensis Cry, Cyt and Parasporin Toxins. Toxins 2014, 6, 2732–2770. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, M.; Oltean, D.I.; Gómez, I.; Pullikuth, A.K.; Soberón, M.; Bravo, A.; Gill, S.S. Heliothis virescens and Manduca sexta lipid rafts are involved in Cry1A toxin binding to the midgut epithelium and subsequent pore formation. J. Biol. Chem. 2002, 277, 13863–13872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, A.; Gómez, I.; Conde, J.; Muñoz-Garay, C.; Sánchez, J.; Miranda, R.; Zhuang, M.; Gill, S.S.; Soberón, M. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta 2004, 1667, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, A.; Soberón, M.; Gill, S.S. Bacillus thuringiensis Mechanisms and Use. Compr. Mol. Insect Sci. 2005, 175–205. [Google Scholar] [CrossRef]
- Pardo-López, L.; Gómez, I.; Rausell, C.; Sánchez, J.; Soberón, M.; Bravo, A. Structural changes of the Cry1Ac oligomeric pre-pore from Bacillus thuringiensis induced by N-acetylgalactosamine facilitates toxin membrane insertion. Biochemistry 2006, 45, 10329–10336. [Google Scholar]
- Pigott, C.R.; Ellar, D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef] [Green Version]
- Adang, M.J.; Crickmore, N.; Jurat-Fuentes, J.L. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. Adv. Insect Physiol. 2014, 47, 39–87. [Google Scholar]
- Gómez, I.; Oltean, D.I.; Gill, S.S.; Bravo, A.; Soberón, M. Mapping the epitope in cadherin-like receptors involved in Bacillus thuringiensis Cry1A toxin interaction using phage display. J. Biol. Chem. 2001, 276, 28906–28912. [Google Scholar] [CrossRef] [Green Version]
- Gomez, I.; Miranda-Rios, J.; Rudiño-Piñera, E.; Oltean, D.I.; Gill, S.S.; Bravo, A.; Soberón, M. Hydropathic complementarity determines interaction of epitope (869) HITDTNNK (876) in Manduca sexta Bt-R(1) receptor with loop 2 of domain II of Bacillus thuringiensis Cry1A toxins. J. Biol. Chem. 2002, 277, 30137–30143. [Google Scholar] [CrossRef] [Green Version]
- Gómez, I.; Dean, D.H.; Bravo, A.; Soberón, M. Molecular basis for Bacillus thuringiensis Cry1Ab toxin specificity: Two structural determinants in the Manduca sexta Bt-R1 receptor interact with loops α8 and 2 in domain II of Cy1Ab toxin. Biochemistry 2003, 42, 10482–10489. [Google Scholar]
- Dorsch, J.A.; Candas, M.; Griko, N.B.; Maaty, W.S.A.; Midboe, E.G.; Vadlamudi, R.K.; Bulla Jr, L.A. Cry1A toxins of Bacillus thuringiensis bind specifically to a region adjacent to the membrane-proximal extracellular domain of BT-R1 in Manduca sexta: Involvement of a cadherin in the entomopathogenicity of Bacillus thuringiensis. Insect Biochem. Mol. Biol. 2002, 32, 1025–1036. [Google Scholar] [CrossRef]
- Gómez, I.; Sánchez, J.; Miranda, R.; Bravo, A.; Soberón, M. Cadherin-like receptor binding facilitates proteolytic cleavage of helix alpha-1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS Lett. 2002, 513, 242–246. [Google Scholar] [CrossRef] [Green Version]
- Kuadkitkan, A.; Smith, D.R.; Berry, C. Investigation of the Cry4B-prohibitin interaction in Aedes aegypti cells. Curr. Microbiol. 2012, 65, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Grochulski, P.; Masson, L.; Borisova, S.; Pusztaicarey, M.; Schwartz, J.L.; Brousseau, R.; Cygler, M. Bacillus thuringiensis CrylA(a) insecticidal toxin: Crystal structure and channel formation. J. Mol. Biol. 1995, 254, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.; Gómez, I.; Arenas, I.; Saab-Rincón, G.; Rodríguez-Almazán, C.; Gill, S.S.; Bravo, A.; Soberón, M. Domain II loop 3 of Bacillus thuringiensis Cry1Ab toxin is involved in a “ping pong” binding mechanism with Manduca sexta aminopeptidase-N and cadherin receptors. J. Biol. Chem. 2009, 284, 32750–32757. [Google Scholar] [CrossRef] [Green Version]
- Ocelotl, J.; Sánchez, J.; Gómez, I.; Tabashnik, B.E.; Bravo, A.; Soberón, M. ABCC2 is associated with Bacillus thuringiensis Cry1Ac toxin oligomerization and membrane insertion in diamondback moth. Sci. Rep. 2017, 7, 2386. [Google Scholar] [CrossRef] [Green Version]
- Heckel, D.G. Learning the ABCs of Bt: ABC transporters and insect resistance to Bacillus thuringiensis provide clues to a crucial step in toxin mode of action. Pest. Biochem. Physiol. 2012, 104, 103–110. [Google Scholar] [CrossRef]
- Soberón, M.; Pardo-Lopez, L.; Lopez, I.; Gómez, I.; Tabashnik, B.E.; Bravo, A. Engineering modified Bt toxins to counter insect resistance. Science 2007, 318, 1640–1642. [Google Scholar]
- Porta, H.; Jimenez, G.; Cordoba, E.; Leon, P.; Soberón, M.; Bravo, A. Tobacco plants expressing the Cry1AbMod toxin suppress tolerance to Cry1Ab toxin of Manduca sexta cadherin-silenced larvae. Insect Biochem. Mol. Biol. 2011, 41, 513–519. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Huang, F.; Ghimire, M.N.; Leonard, B.R.; Siegfried, B.D.; Rangasamy, M.; Yang, Y.; Wu, Y.; Gahan, L.J.; Heckel, D.G.; et al. Efficacy of genetically modified Bt toxins against insects with different mechanisms of resistance. Nat. Biotechnol. 2011, 29, 1128–1131. [Google Scholar] [CrossRef]
- Wickham, T.J.; Davis, T.; Granados, R.R.; Shuler, M.L.; Wood, H.A. Screening of insect cell lines for the production of recombinant proteins and infectious virus in the baculovirus expression system. Biotechnol. Prog. 1992, 8, 391–396. [Google Scholar] [CrossRef]
- Castella, C.; Pauron, D.; Hilliou, F.; Trang, V.T.; Zucchini-Pascal, N.; Gallet, A.; Barbero, P. Transcriptomic analysis of Spodoptera frugiperda Sf9 cells resistant to Bacillus thuringiensis Cry1Ca toxin reveals that extracellular Ca2+, Mg2+ and production of cAMP are involved in toxicity. Biol. Open 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Kwa, M.S.G.; de Maagd, R.A.; Stiekema, W.J.; Vlak, J.M.; Bosh, D. Toxicity and binding properties of the Bacillus thuringiensis delta-endotoxin Cry1C to cultured insect cells. J. Invertebr. Pathol. 1998, 71, 121–127. [Google Scholar] [CrossRef]
- Zhang, X.; Candas, M.; Griko, N.B.; Taissing, R.; Bulla, L.A., Jr. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 2006, 103, 9897–9902. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Koni, P.A.; Ellar, D.J. Structure of the mosquitocidal delta-endotoxin CytB from Bacillus thuringiensis ssp. kyushuensis and implications for membrane pore formation. J. Mol. Biol. 1996, 257, 129–1522. [Google Scholar]
- Zhang, Q.; Hua, G.; Adang, M.J. Effects and mechanisms of Bacillus thuringiensis crystal toxins for mosquito larvae. Insect Sci. 2017, 24, 714–729. [Google Scholar] [CrossRef]
- Berry, C.; O’Neil, S.; Ben-Dov, E.; Jones, A.F.; Murphy, L.; Quail, M.A.; Holden, M.T.; Harris, D.; Zaritsky, A.; Parkhill, J. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2002, 68, 5082–5095. [Google Scholar] [CrossRef] [Green Version]
- Butko, P. Cytolytic toxin Cyt1A and its mechanism of membrane damage: Data and hypotheses. Appl. Environ. Microbiol. 2003, 69, 2415–2422. [Google Scholar] [CrossRef] [Green Version]
- Thomas, W.E.; Ellar, D.J. Bacillus thuringiensis var. israeliensis crystal delta-endotoxin: Effects on insect and mammalian cells in vitro and in vivo. J. Cell Sci. 1983, 60, 181–197. [Google Scholar]
- Chogule, N.P.; Li, H.; Liu, S.; Linz, L.B.; Narva, K.E.; Meade, T.; Bonning, B.C. Retargeting of the Bacillus thuringiensis toxin Cyt2Aa against hemipteran insect pests. Proc. Natl. Acad. Sci. USA 2013, 110, 8465–8470. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.C.; Lo, Y.C.; Lin, J.Y.; Liaw, Y.C. Crystal structures and electron micrographs of fungal volvatoxin A2. J. Mol. Biol. 2004, 343, 477–491. [Google Scholar] [CrossRef]
- Lin, J.Y.; Jeng, T.W.; Chen, C.C.; Shi, G.Y.; Tung, T.C. Isolation of a new cardiotoxic protein from the edible mushroom, Volvariella volvacea. Nature 1973, 246, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Manasherob, R.; Itsko, M.; Sela-Baranes, N.; Ben-Dov, E.; Berry, C.; Cohen, S.; Zaritsky, A. Cyt1Ca from Bacillus thuringiensis subsp. israelensis: Production in Escherichia coli and comparison of its biological activities with those of other Cyt-like proteins. Microbiology 2006, 152, 2651–2659. [Google Scholar] [CrossRef] [Green Version]
- Bravo, A.; Martínez de Castro, D.; Sánchez, J.; Cantón, P.E.; Mendoza, G.; Gómez, I.; Pacheco, S.; García-Gómez, B.I.; Onofre, J.; Ocelotl, J.; et al. Mechanism of action of Bacillus thuringiensis insecticidal toxins and their use in the control of insect pests. In Comprehensive Sourcebook of Bacterial Protein Toxins, 4th ed.; Alouf, J.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 858–873. [Google Scholar]
- Butko, P.; Huang, F.; Pusztai-Carey, M.; Surewicz, W.K. Membrane permeabilization induced by cytolytic delta-endotoxin CytA from Bacillus thuringiensis var. israelensis. Biochemistry 1996, 35, 11355–11360. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.C.; Dlécluse, A.; Walton, W.E. Cyt1Ab1 and Cyt2Ba1 from Bacillus thuringiensis subsp. medellin and B. thuringiensis subsp. israelensis synergize Bacillus sphaericus against Aedes aegypti and Resistant Culex quinquefasciatus (Diptera: Culicidae). Appl. Environ. Microbiol. 2001, 67, 3280–3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soberón, M.; Lopez-Díaz, J.A.; Bravo, A. Cyt toxins produced by Bacillus thuringiensis: A protein fold conserved in several pathogenic microorganisms. Peptides 2013, 41, 87–93. [Google Scholar]
- Pérez, C.; Fernández, L.E.; Sun, J.; Folch, J.L.; Gill, S.S.; Soberón, M.; Bravo, A. Bacillus thuringiensis subsp. israelensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 18303–18308. [Google Scholar]
- Zhang, B.H.; Liu, M.; Yang, Y.K.; Yuan, Z.M. Cytolytic Toxin Cyt1aa of Bacillus thuringiensis Synergizes the Mosquitocidal Toxin Mtx1 of Bacillus sphaericus. Biosci. Biotech. Bioch. 2006, 70, 2199–2204. [Google Scholar] [CrossRef] [Green Version]
- Torres-quintero, M.C.; Gómez, I.; Pacheco, S.; Sánchez, J.; Flores, H.; Osuna, J.; Mendoza, G.; Soberón, M.; Bravo, A. Engineering Bacillus thuringiensis Cyt1Aa toxin specificity from dipteran to lepidopteran toxicity. Sci. Rep. 2018, 8, 4989. [Google Scholar] [CrossRef]
- Ohba, M.; Aizawa, K. Insect toxicity of Bacillus thuringiensis isolated from soils of Japan. J. Invertebr. Pathol. 1986, 47, 12–20. [Google Scholar] [CrossRef]
- Chubika, T.; Girija, D.; Deepa, K.; Salini, S.; Meera, N.; Raghavamenon, A.; Divya, M.; Babu, T. A parasporin from Bacillus thuringiensis native to Peninsular India induces apoptosis in cancer cells through intrinsic pathway. J. Biosci. 2018, 43, 407–416. [Google Scholar] [CrossRef]
- Mizuki, E.; Ohba, M.; Akao, T.; Yamashita, S.; Saitoh, H.; Park, Y.S. Unique activity associated with non-insecticidal Bacillus thuringiensis parasporal inclusions: In vitro cell-killing action on human cancer cells. J. Appl. Microbiol. 1999, 86, 477–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, S.; Ohba, M.; Mizuki, E.; Crickmore, N.; Côté, J.C.; Nagamatsu, Y.; Kitada, S.; Sakai, H.; Harata, K.; Shin, T. Parasporin nomenclature. 2010. Available online: http://parasporin.fitc.pref.fukuoka.jp/list.html (accessed on 3 April 2020).
- Kim, H.S.; Yamashita, S.; Akao, T.; Saitoh, H.; Higuchi, K.; Park, Y.S.; Mizuki, E.; Ohba, M. In vitro cytotoxicity of non-Cyt inclusion proteins of a Bacillus thuringiensis isolate against human cells, including cancer cells. J. Appl. Microbiol. 2000, 89, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Akao, T.; Yamashita, S.; Katayama, H.; Maeda, M.; Saitoh, H.; Mizuki, E.; Ohba, M. Noninsecticidal parasporal proteins of a Bacillus thuringiensis serovar shandongiensis isolate exhibit a preferential cytotoxicity against human leukemic T cells. Biochem. Biophys. Res. Commun. 2000, 272, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Mizuki, E.; Park, Y.S.; Saitoh, H.; Yamashita, S.; Akao, T.; Higuchi, K.; Ohba, M. Parasporin, a human leukemic cell-recognizing parasporal protein of Bacillus thuringiensis. Clin. Diagn. Lab. Immunol. 2000, 7, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Akao, T.; Mizuki, E.; Saitoh, H.; Higuchi, K.; Park, Y.S.; Kim, H.S.; Ohba, M. Characterization of the anti-cancer-cell parasporal proteins of a Bacillus thuringiensis isolate. Can. J. Microbiol. 2000, 46, 913–919. [Google Scholar] [CrossRef]
- Lee, D.W.; Katayama, H.; Akao, T.; Maeda, M.; Tanaka, R.; Yamashita, S.; Saitoh, H.; Mizuki, E.; Ohba, M. A 28-kDa protein of the Bacillus thuringiensis serovar shandongiensis isolate 89-T-34-22 induces a human leukemic cell-specific cytotoxicity. Biochim. Biophys. Acta 2001, 1547, 57–63. [Google Scholar] [CrossRef]
- Yamagiwa, M.; Namba, A.; Akao, T.; Mizuki, E.; Ohba, M.; Sakai, H. Cytotoxicity of Bacillus thuringiensis crystal protein against mammalian cells. Mem. Fac. Eng. Okayama Univ. 2002, 36, 61–66. [Google Scholar]
- Namba, A.; Yamagiwa, M.; Amano, H.; Akao, T.; Mizuki, E.; Ohba, M.; Sakai, H. The cytotoxicity of Bacillus thuringiensis subsp. coreanensis A1519 strain against the human leukemic T cell. Biochim. Biophys. Acta 2003, 1622, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Akiba, T.; Abe, Y.; Kitada, S.; Kusaka, Y.; Ito, A.; Ichimatsu, T.; Katayama, H.; Akao, T.; Higuchi, K.; Mizuki, E.; et al. Crystallization of parasporin-2, a Bacillus thuringiensis crystal protein with selective cytocidal activity against human cells. Acta Cryst. 2004, D60, 2355–2357. [Google Scholar]
- Ito, A.; Sasaguri, Y.; Kitada, S.; Kusaka, Y.; Kuwano, K.; Masutomi, K.; Mizuki, E.; Akao, T.; Ohba, M. Selective cytocidal action of a crystal protein of Bacillus thuringiensis on human cancer cells. J. Biol. Chem. 2004, 279, 21282–21286. [Google Scholar] [CrossRef] [Green Version]
- Okumura, S.; Akao, T.; Higuchi, K.; Saitoh, H.; Mizuki, E.; Ohba, M.; Inouye, K. Bacillus thuringiensis serovar shandongiensis strain 89-T-34-22 produces multiple cytotoxic proteins with similar molecular masses against human cancer cell. Lett. Appl. Microbiol. 2004, 39, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Yamagiwa, M.; Akao, T.; Mizuki, E.; Ohba, M.; Sakai, H. A novel 29-kDa crystal protein from Bacillus thuringiensis induces caspase activation and cell death of Jurkat T cells. Biosci. Biotechnol. Biochem. 2005, 69, 2063–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, H.; Yokota, H.; Akao, T.; Nakamura, O.; Ohba, M.; Mekada, E.; Mizuki, E. Parasporin-1, a novel cytotoxic protein to human cells from non-insecticidal parasporal inclusions of Bacillus thuringiensis. J. Biochem. 2005, 137, 17–25. [Google Scholar] [CrossRef]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Wasano, N.; Yamashita, S.; Kusumoto, K.; Akao, T.; Mizuki, E.; Ohba, M.; Inouye, K. Identification of a Novel Cytotoxic Protein, Cry45Aa, from Bacillus thuringiensis A1470 and Its Selective Cytotoxic Activity against Various Mammalian Cell Lines. J. Agric. Food Chem. 2005, 53, 6313–6318. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Katayama, H.; Saitoh, H.; Akao, T.; Park, Y.S.; Mizuki, E.; Ohba, M.; Ito, A. Typical three-domain Cry proteins of Bacillus thuringiensis strain A1462 exhibit cytocidal activity on limited human cancer cells. J. Biochem. 2005, 138, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Saitoh, H.; Wasano, N.; Katayama, H.; Higuchi, K.; Mizuki, E.; Inouye, K. Efficient Solubilization, activation and purification of recombinant Cry45Aa of Bacillus thuringiensis expressed as an Escherichia coli inclusion body. Protein Expres. Purif. 2006, 47, 144–151. [Google Scholar] [CrossRef]
- Saitoh, H.; Okumura, S.; Ishikawa, T.; Akao, T.; Mizuki, E.; Ohba, M. Investigation of a novel Bacillus thuringiensis gene encoding a parasporal protein, parasporin-4, that preferentially kills leukaemic T cells. Biosci. Biotechnol. Biochem. 2006, 70, 2935–2941. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Kanagawa, R.; Kotani, Y.; Kimura, M.; Yamagiwa, M.; Yamane, Y.; Takebe, S.; Sakai, H. Parasporin-2Ab, a newly isolated cytotoxic crystal protein from Bacillus thuringiensis. Curr. Microbiol. 2007, 55, 278–283. [Google Scholar] [CrossRef]
- Inouye, K.; Okumura, S.; Mizuki, E. Parasporin-4, A Novel Cancer Cell-killing Protein Produced by Bacillus thuringiensis. Food Sci. Biotechnol. 2008, 17, 219–227. [Google Scholar]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Mizuki, E.; Inouye, K. Identification and characterization of a novel cytotoxic protein, parasporin-4, produced by Bacillus thuringiensis A1470 strain. Biotechnol. Annu. Rev. 2008, 14, 225–252. [Google Scholar]
- Uemori, A.; Ohgushi, A.; Yasutake, K.; Maeda, M.; Mizuki, E.; Ohba, M. Parasporin-1Ab, a Novel Bacillus thuringiensis Cytotoxin Preferentially Active on Human Cancer Cells In Vitro. Anticancer Res. 2008, 28, 91–95. [Google Scholar] [PubMed]
- Nagamatsu, Y.; Okamura, S.; Saitou, H.; Akao, T.; Mizuki, E. Three Cry toxins in two types from Bacillus thuringiensis strain M019 preferentially kill human hepatocyte cancer and uterus cervix cancer cells. Biosci. Biotechnol. Biochem. 2010, 74, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Begum, A.; Saga, M.; Hirao, A.; Mizuki, E.; Sakai, H.; Hayakawa, T. Parasporin 1Ac2, a Novel Cytotoxic Crystal Protein Isolated from Bacillus thuringiensis B0462 Strain. Curr. Microbiol. 2013, 66, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Ishikawa, T.; Saitoh, H.; Akao, T.; Mizuki, E. Identification of a second cytotoxic protein produced by Bacillus thuringiensis A1470. Biotechnol. Lett. 2013, 35, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Ekino, K.; Okumura, S.; Ishikawa, T.; Kitada, S.; Saitoh, H.; Akao, T.; Oka, T.; Nomura, Y.; Ohba, M.; Shin, T.; et al. Cloning and Characterization of a Unique Cytotoxic Protein Parasporin-5 Produced by Bacillus thuringiensis A1100 Strain. Toxins 2014, 6, 1882–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasutake, K.; Binh, N.D.; Kagoshima, K.; Uemori, A.; Ohgushi, A.; Maeda, M.; Mizuki, E.; Yu, Y.M.; Ohba, M. Occurrence of parasporin-producing Bacillus thuringiensis in Vietnam. Can. J. Microbiol. 2006, 52, 365–372. [Google Scholar] [CrossRef]
- Yasutake, K.; Uemori, A.; Binh, N.D.; Mizuki, E.; Ohba, M. Identification of parasporin genes in Vietnamese isolates of Bacillus thuringiensis. Zeitschrift fur Naturforschung C 2008, 63, 139–143. [Google Scholar] [CrossRef]
- Lenina, N.K.; Naveenkuma, A.; Sozhavendan, A.E.; Balakrishnan, N.; Balasubramani, V.; Udayasuriyan, V. Characterization of parasporin gene harboring Indian isolates of Bacillus thuringiensis. Biotech 2014, 4, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Nadarajah, V.D.; Ting, D.; Chan, K.K.; Mohamed, S.M.; Kanakeswary, K.; Lee, H.L. Selective cytotoxic activity against leukemic cell lines from mosquitocidal Bacillus thuringiensis parasporal inclusions. Southeast Asian J. Trop. Med. Public Health 2008, 39, 235–245. [Google Scholar]
- Zhu, L.; Li, C.; Wu, J.; Liang, J.; Shi, Y. Apoptosis of HL-60 cells induced by crystal proteins from Bacillus thuringiensis Bt9875. Wei Sheng Wu xue Bao 2008, 48, 690–694. [Google Scholar]
- Moazamian, E.; Bahador, N.; Azarpira, N.; Rasouli, M. Anti-cancer Parasporin Toxins of New Bacillus thuringiensis Against Human Colon (HCT-116) and Blood (CCRF-CEM) Cancer Cell Lines. Curr. Microbiol. 2018, 75, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Soud, M.; Al-Amri, M.Z.; Kumar, A.; Al-Sheikh, Y.A.; Ashour, A.E.; El-Kersh, T.A. Specific Cytotoxic Effects of Parasporal Crystal Proteins Isolated from Native Saudi Arabian Bacillus thuringiensis Strains against Cervical Cancer Cells. Molecules 2019, 24, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.C.; Mizuki, E.; Côte, J.C. Isolation and characterization of a novel Bacillus thuringiensis strain expressing a novel crystal protein with cytocidal activity against human cancer cells. J. Appl. Microbiol. 2007, 103, 65–79. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Granados, J.C.; Short, J.D.; Ammons, D.R.; Rampersad, J. Parasporins from a Caribbean Island: Evidence for a Globally dispersed Bacillus thuringiensis strain. Curr. Microbiol. 2011, 62, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Inouye, K.; Mizuki, E. Mode of action of parasporin-4, a cytocidal protein from Bacillus thuringiensis. Biochim. Biophys. Acta 2011, 1808, 1476–1482. [Google Scholar] [CrossRef] [Green Version]
- Katayama, H.; Kusaka, Y.; Mizuk, E. Parasporin-1 Receptor and Use Thereof. U.S. Patent 20110038880 2011. [Google Scholar]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Kitada, S.; Abe, Y.; Shimada, H.; Kusaka, Y.; Matsuo, Y.; Katayama, H.; Okumura, S.; Akao, T.; Mizuki, E.; Kuge, O.; et al. Cytocidal actions of parasporin-2, an anti-tumor crystal toxin from Bacillus thuringiensis. J. Biol. Chem. 2006, 281, 26350–26360. [Google Scholar] [CrossRef] [Green Version]
- Akiba, T.; Abe, Y.; Kitada, S.; Kusaka, Y.; Ito, A.; Ichimatsu, T.; Katayama, H.; Akao, T.; Higuchi, K.; Mizuki, E.; et al. Crystal structure of the parasporin-2 Bacillus thuringiensis toxin that recognizes cancer cells. J. Mol. Biol. 2009, 13, 121–133. [Google Scholar] [CrossRef]
- Abe, Y.; Inoue, H.; Ashida, H.; Maeda, Y.; Kinoshita, T.; Kitada, S. Glycan region of GPI anchored-protein is required for cytocidal oligomerization of an anticancer parasporin-2, Cry46Aa1 protein, from Bacillus thuringiensis strain 3. J. Invertebr. Pathol. 2017, 142, 71–81. [Google Scholar] [CrossRef]
- Brasseur, K.; Auger, P.; Asselin, E.; Parent, S.; Côte, J.C.; Sirois, M. Parasporin-2 from a New Bacillus thuringiensis 4R2 Strain Induces Caspases Activation and Apoptosis in Human Cancer Cells. PLoS ONE 2015, 10, e0135106. [Google Scholar] [CrossRef]
- Hazes, B. The (QxW)3 domain: A flexible lectin scaffold. Protein Sci. 1996, 5, 1490–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, V.; Domanska, B.; Elhigazi, A.; Afolabi, F.; West, M.J.; Crickmore, N. The human cancer cell active toxin Cry41Aa from Bacillus thuringiensis acts like its insecticidal counterparts. Biochem. J. 2017, 474, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Moniatte, M.; van der Goot, F.G.; Buckley, J.T.; Pattus, F.; van Dorsselaer, A. Characterisation of the heptameric pore-forming complex of the Aeromonas toxin aerolysin using MALDI-TOF mass spectrometry. FEBS Lett. 1996, 384, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Kitada, S.; Abe, Y.; Maeda, T.; Shimada, H. Parasporin-2 requires GPI-anchored proteins for the efficient cytocidal action to human hepatoma cells. Toxicology 2009, 264, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Sleytr, U.B.; Messner, P.; Pum, D.; Sára, M. Crystalline bacterial cell surface layers. Mol. Microbiol. 1993, 10, 911–916. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Shuster, B.; Egelseer, E.M.; Pum, D. S-layers: Principles and applications. FEMS Microbiol. Rev. 2014, 38, 823–864. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, W.; Yuan, M.; Tang, M.; Chen, J.; Pang, Y. Expression of vip1/vip2 genes in Escherichia coli and Bacillus thuringiensis and the analysis of their signal peptides. J. Appl. Microbiol. 2004, 97, 757–765. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, Y.; Shu, C.; Crickmore, N.; Wang, Q.; Du, L.; Song, F.; Zhang, J. Genomic sequencing identifies novel Bacillus thuringiensis Vip1/Vip2 binary and Cry8 toxins that have high toxicity to Scarabaeoidea larvae. Appl. Microbiol. Biotechnol. 2015, 99, 753–760. [Google Scholar] [CrossRef]
- Warren, G.W. Vegetative insecticidal proteins: Novel proteins for control of corn pests. In Advances in Insect Control, the Role of Transgenic Plants; Carozzi, N.B., Koziel, M., Eds.; Taylor & Francis Ltd.: London, UK, 1997; pp. 109–121. [Google Scholar] [CrossRef]
- Sattar, S.; Maiti, M.K. Molecular characterization of a novel vegetative insecticidal protein from Bacillus thuringiensis effective against sap-sucking insect pest. J. Microbiol. Biotechnol. 2011, 21, 937–946. [Google Scholar] [CrossRef] [Green Version]
- Jucovic, M.; Walters, F.S.; Warren, G.W.; Palekar, N.V.; Chen, J.S. From enzyme to zymogen: Engineering Vip2, an ADP-ribosyltransferase from Bacillus cereus, for conditional toxicity. Protein Eng. Des. Sel. 2008, 21, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Leuber, M.; Orlik, F.; Schiffler, B.; Sickmann, A.; Benz, R. Vegetative insecticidal protein (Vip1Ac) of Bacillus thuringiensis HD201: Evidence for oligomer and channel formation. Biochemistry 2006, 45, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Aktories, K.; Popoff, M.R.; Stiles, B.G. Binary bacterial toxins: Biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol. Mol. Biol. Rev. 2004, 68, 373–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bel, Y.; Banyuls, N.; Chakroun, M.; Escriche, B.; Ferré, J. Insights into the Structure of the Vip3Aa Insecticidal Protein by Protease Digestion Analysis. Toxins 2017, 4, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelkefi-Mesrati, L.; Boukedi, H.; Chakroun, M.; Kamoun, F.; Azzouz, H.; Tounsi, S.; Rouis, S.; Jaoua, S. Investigation of the steps involved in the difference of susceptibility of Ephesitia kuehniella and Spodoptera littoralis to the Bacillus thuringiensis Vip3Aa16 toxin. J. Invertebr. Path. 2011, 107, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, G.; Portillo, A.; Arias, E.; Ribas, R.M.; Olmos, J. New Combinations of Cry Genes From Bacillus Thuringiensis Strains Isolated From Northwestern Mexico. Int. Microbiol. 2012, 15, 211–218. [Google Scholar] [PubMed]
- Mendoza-Almanza, G.; Rocha-Zavaleta, L.; Aguilar-Zacarías, C.; Ayala-Luján, J.; Olmos, S.J. Cry1A Proteins are Cytotoxic to HeLa but not to SiHa Cervical Cancer Cells. Curr. Pharm. Biotechnol. 2019, 20, 1018. [Google Scholar] [CrossRef]


| Aedes spp. | Culex spp. | Anopheles spp. |
|---|---|---|
| Cyt1Aa, Cyt2Ba | Cyt1Aa, Cyt2Ba | Cyt1Aa, Cyt2Aa |
| Cry4Aa | Cry4Aa | Cry4Aa |
| Cry4Ba | Cry4Ba | Cry10Aa |
| Cry10Aa | Cry10Aa | Cry11Aa |
| Cry11Aa | Cry11Aa |
| PS | Bt Strain | Cry Gene | Protoxin (kDa) | Active Toxin (kDa) | Protease Activation | Main Cellular Target | EC50 [µg/mL] | Mechanism Action | Country | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| PS1Aa1 | A1190 | Cry31Aa1 | 81 | 15, 56 | Trypsin | HeLa | 0.12 | Apoptosis | Japan | [99] |
| PS1Aa2 | M15 | Cry31Aa2 | 83 | 55, 70 | Trypsin | Jurkat HepG2 | 0.02 0.02 | ND | Canada | [128] |
| PS1Aa3 | B195 | Cry31Aa3 | 81 | 56 | Trypsin | HeLa | 14.7 | ND | Japan | [116] |
| PS1Aa4 | Bt79-25 | Cry31Aa4 | 81 | NP | Proteinase K | ND | ND | ND | Vietnam | [122] |
| PS1Aa5 | Bt92-10 | Cry31Aa5 | 81 | NP | Proteinase K | ND | ND | ND | Vietnam | [122] |
| PS1Aa6 | M019, 64-1-94 | Cry31Aa6 | 70 | 15, 55 | Trypsin | HepG2 | 0.52 | ND | Japan, Caribbean | [117,129] |
| PS1Ab1 | B195 | Cry31Ab1 | 82 | 56 | Trypsin | HeLa | 14.7 | ND | Japan | [116] |
| PS1Ab2 | Bt31-5 | Cry31Ab2 | 82 | NP | Proteinase K | ND | ND | ND | Vietnam | [122] |
| PS1Ac1 | Bt87-29 | Cry31Ac1 | 87 | NP | Proteinase K | ND | ND | ND | Vietnam | [122] |
| PS1Ac2 | B0462 | Cry31Ac2 | 81 | 15, 60 | Proteinase K | HeLa | 2 | Apoptosis | Japan | [118] |
| PS1Ad1 | 64-1-94, M15, M019 | Cry31Ad1 | 73 | 14, 59 | Trypsin | HepG2 | 0.52 | ND | Caribbean, Canada, Japan | [117,128,129] |
| PS2Aa1 | A1547 | Cry46Aa1 | 37 | 30 | Proteinase K | HepG2 | 0.023 | Pore-forming | Japan, USA | [105] |
| PS2Aa2 | A1470 | Cry31Aa2 | 30 | 28 | Proteinase K | MOLT-4 | 0.041 | ND | Japan | [119] |
| PS2Ab1 | TK-E6 | Cry31Ab1 | 33 | 29 | Proteinase K | Jurkat | 0.545ng/ml | ND | Japan | [113] |
| PS3Aa1 | A1462 | Cry41Aa1 | 88 | 64 | Proteinase K | HL60 | 1.32 | ND | Japan | [100] |
| PS3Ab1 | A1462 | Cry41Ab1 | 88 | 64 | Proteinase K | HL60 | 1.25 | ND | Japan | [100] |
| PS4Aa1 | A1470 | Cry45Aa1 | 31 | 28 | Proteinase K | CaCo2 | 0.124 | Pore-forming | Japan | [111] |
| PS5Aa1 | A1100 | Cry64Aa1 | 33 | 30 | Proteinase K | TCS | 0.046 | ND | Japan | [120] |
| PS6Aa1 | M019, 64-1-94 | Cry63Aa1 | 85 | 14, 59 | Trypsin | HepG2 | 2.3 | ND | Japan, Caribbean | [117,129] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Almanza, G.; Esparza-Ibarra, E.L.; Ayala-Luján, J.L.; Mercado-Reyes, M.; Godina-González, S.; Hernández-Barrales, M.; Olmos-Soto, J. The Cytocidal Spectrum of Bacillus thuringiensis Toxins: From Insects to Human Cancer Cells. Toxins 2020, 12, 301. https://doi.org/10.3390/toxins12050301
Mendoza-Almanza G, Esparza-Ibarra EL, Ayala-Luján JL, Mercado-Reyes M, Godina-González S, Hernández-Barrales M, Olmos-Soto J. The Cytocidal Spectrum of Bacillus thuringiensis Toxins: From Insects to Human Cancer Cells. Toxins. 2020; 12(5):301. https://doi.org/10.3390/toxins12050301
Chicago/Turabian StyleMendoza-Almanza, Gretel, Edgar L. Esparza-Ibarra, Jorge L. Ayala-Luján, Marisa Mercado-Reyes, Susana Godina-González, Marisa Hernández-Barrales, and Jorge Olmos-Soto. 2020. "The Cytocidal Spectrum of Bacillus thuringiensis Toxins: From Insects to Human Cancer Cells" Toxins 12, no. 5: 301. https://doi.org/10.3390/toxins12050301
APA StyleMendoza-Almanza, G., Esparza-Ibarra, E. L., Ayala-Luján, J. L., Mercado-Reyes, M., Godina-González, S., Hernández-Barrales, M., & Olmos-Soto, J. (2020). The Cytocidal Spectrum of Bacillus thuringiensis Toxins: From Insects to Human Cancer Cells. Toxins, 12(5), 301. https://doi.org/10.3390/toxins12050301







