Pan-Genomic Analysis of Clostridium botulinum Group II (Non-Proteolytic C. botulinum) Associated with Foodborne Botulism and Isolated from the Environment
Abstract
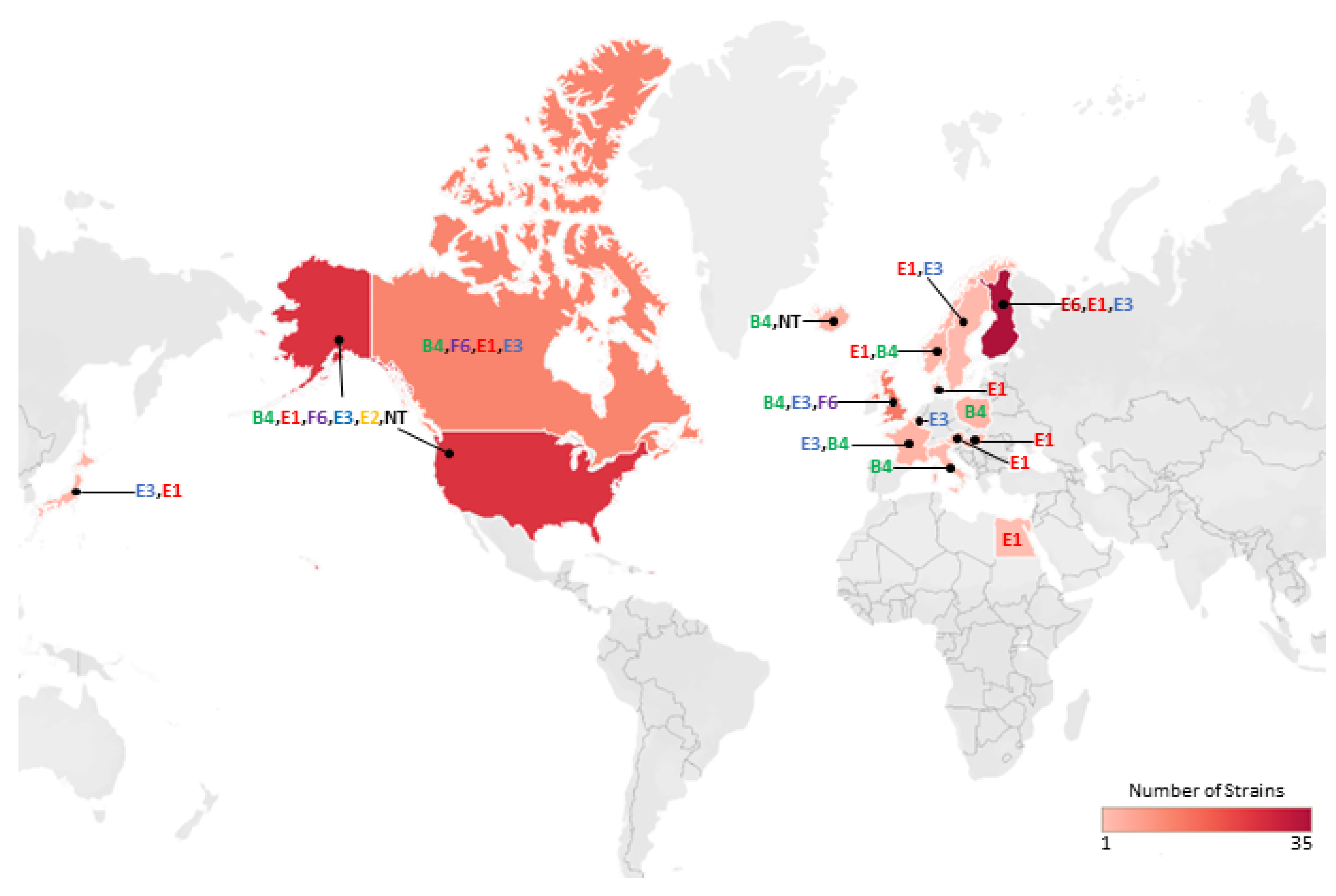
1. Introduction
2. Results and Discussion
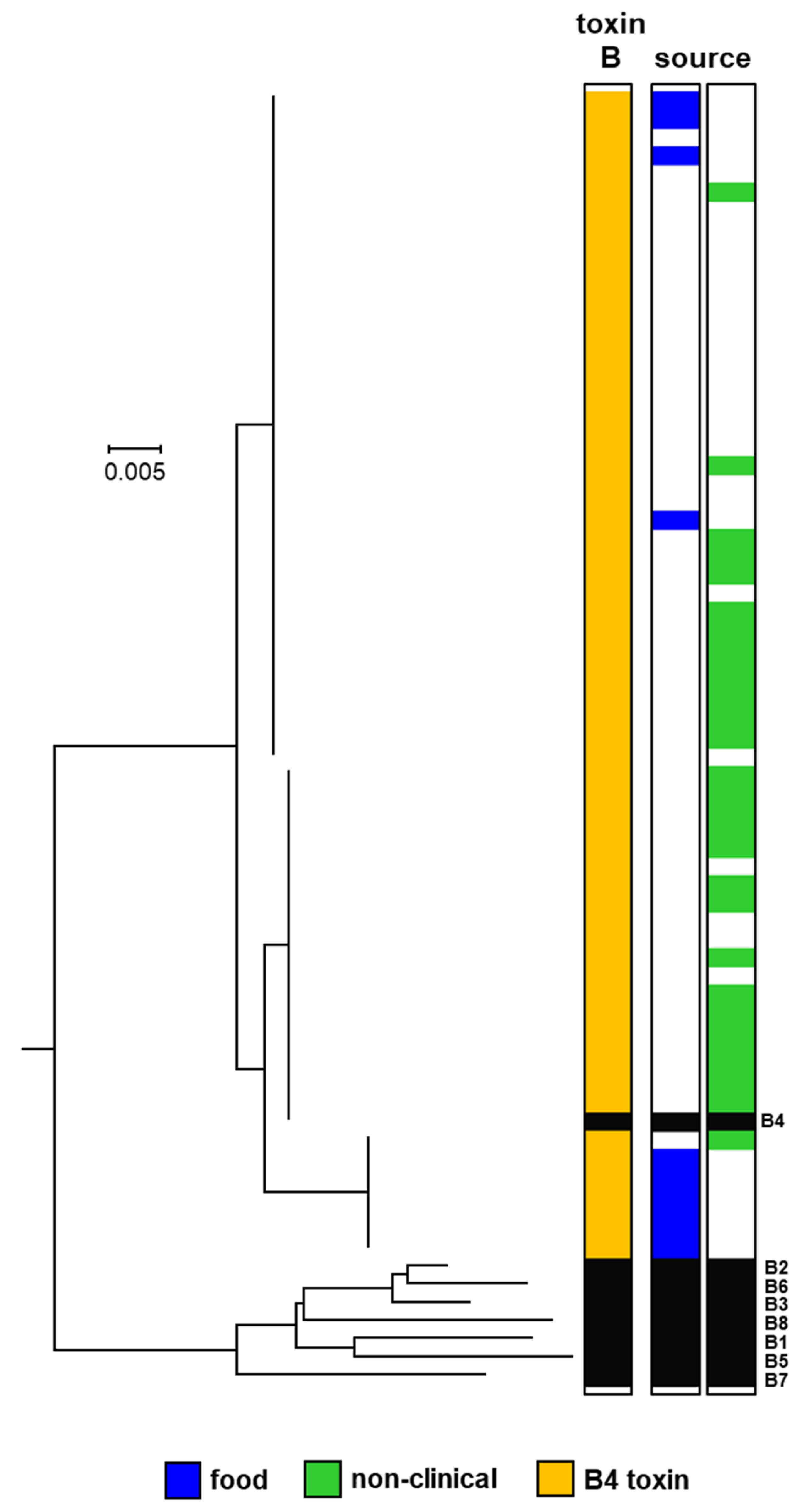
2.1. Botulinum Neurotoxins and Their Encoding Genes
2.2. Whole-Genome Analysis Based on Single-Nucleotide Polymorphisms (SNPs)
2.3. Pan-genome Comparison of the Two C. botulinum Group II Genomic Lineages
3. Conclusions
4. Materials and Methods
4.1. C. botulinum Group II Isolates
4.2. Genomic DNA Preparation
4.3. Whole-Genome Sequencing
4.4. Genome Assembly and Quality Control
4.5. Identification of Botulinum Neurotoxin Subtypes and Accessory Protein Configuration
4.6. Pangenome Analyses, Target Gene Identification and in Silico PCR
4.7. Core Genome SNPs Analysis and Phylogenetic Trees
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bruggemann, H.; Wollherr, A.; Mazuet, C.; Popoff, M. Clostridium botulinum. In Genomes of Foodborne and Waterborne Pathogens; Fratamico, P., Liu, Y., Kathariou, S., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 185–212. ISBN 978-1555814571. [Google Scholar]
- Carter, A.T.; Paul, C.J.; Mason, D.R.; Twine, S.M.; Alston, M.J.; Logan, S.M.; Austin, J.W.; Peck, M.W. Independent evolution of neurotoxin and flagellar genetic loci in proteolytic Clostridium botulinum. BMC Genom. 2009, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.T.; Peck, M.W. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Res. Microbiol. 2015, 166, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A. Food Microbiology: Fundamentals and Frontiers; Doyle, M.P., Buchanan, R.L., Eds.; ASM Press: Washington, DC, USA, 2013; pp. 441–464. [Google Scholar]
- Hill, K.K.; Smith, T.J.; Helma, C.H.; Ticknor, L.O.; Foley, B.T.; Svensson, R.T.; Brown, J.L.; Johnson, E.A.; Smith, L.A.; Okinaka, R.T.; et al. Genetic diversity among Botulinum Neurotoxin-producing clostridial strains. J. Bacteriol. 2007, 189, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W. Biology and genomic analysis of Clostridium botulinum. Adv. Microb. Physiol. 2009, 55, 183–320. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Williamson, C.H.D.; Hill, K.; Sahl, J.; Keim, P. Botulinum Neurotoxin-Producing Bacteria. Isn’t It Time that We Called a Species a Species? MBio 2018, 9, e01469-18. [Google Scholar] [CrossRef]
- Williamson, C.H.; Sahl, J.W.; Smith, T.J.; Xie, G.; Foley, B.T.; Smith, L.A.; Fernandez, R.A.; Lindström, M.; Korkeala, H.; Keim, P.; et al. Comparative genomic analyses reveal broad diversity in botulinum-toxin-producing Clostridia. BMC Genom. 2016, 17, 180. [Google Scholar] [CrossRef]
- Brunt, J.; Carter, A.T.; Stringer, S.C.; Peck, M.W. Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett. 2018, 592, 310–317. [Google Scholar] [CrossRef]
- Mansfield, M.J.; Doxey, A.C. Genomic insights into the evolution and ecology of botulinum neurotoxins. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef]
- Zhang, S.; Lebreton, F.; Mansfield, M.J.; Miyashita, S.I.; Zhang, J.; Schwartzman, J.A.; Tao, L.; Masuyer, G.; Martinez-Carranza, M.; Stenmark, P.; et al. Identification of a Botulinum Neurotoxin-like Toxin in a Commensal Strain of Enterococcus faecium. Cell Host Microbe 2018, 23, 169–176. [Google Scholar] [CrossRef]
- Hatheway, C.L. Toxigenic clostridia. Clin. Microbiol. Rev. 1990, 3, 66–98. [Google Scholar] [CrossRef]
- Poulain, B.; Popoff, M.R. Why Are Botulinum Neurotoxin-Producing Bacteria So Diverse and Botulinum Neurotoxins So Toxic? Toxins 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Rummel, A. The long journey of botulinum neurotoxins into the synapse. Toxicon 2015, 107, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and current perspectives on Clostridium botulinum diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef]
- Kaji, R. Clinical differences between A1 and A2 botulinum toxin subtypes. Toxicon 2015, 107, 85–88. [Google Scholar] [CrossRef]
- Moritz, M.S.; Tepp, W.H.; Bradshaw, M.; Johnson, E.A.; Pellett, S. Isolation and Characterization of the Novel Botulinum Neurotoxin A Subtype 6. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Moritz, M.S.; Tepp, W.H.; Inzalaco, H.N.T.; Johnson, E.A.; Pellett, S. Comparative functional analysis of mice after local injection with botulinum neurotoxin A1, A2, A6, and B1 by catwalk analysis. Toxicon 2019, 167, 20–28. [Google Scholar] [CrossRef]
- Pellett, S.; Tepp, W.H.; Whitemarsh, R.C.; Bradshaw, M.; Johnson, E.A. In vivo onset and duration of action varies for botulinum neurotoxin a subtypes 1-5. Toxicon 2015, 107, 37–42. [Google Scholar] [CrossRef]
- Whitemarsh, R.C.; Tepp, W.H.; Bradshaw, M.; Lin, G.; Pier, C.L.; Scherf, J.M.; Johnson, E.A.; Pellett, S. Characterization of botulinum neurotoxin A subtypes 1 through 5 by investigation of activities in mice, in neuronal cell cultures, and in vitro. Infect. Immun. 2013, 81, 3894–3902. [Google Scholar] [CrossRef]
- Carter, A.T.; Austin, J.W.; Weedmark, K.A.; Corbett, C.; Peck, M.W. Three classes of plasmid (47-63 kb) carry the type B neurotoxin gene cluster of group II Clostridium botulinum. Genome Biol. Evol. 2014, 6, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.T.; Austin, J.W.; Weedmark, K.A.; Peck, M.W. Evolution of Chromosomal Clostridium botulinum Type E Neurotoxin Gene Clusters: Evidence Provided by Their Rare Plasmid-Borne Counterparts. Genome Biol. Evol. 2016, 8, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.T.; Stringer, S.C.; Webb, M.D.; Peck, M.W. The type F6 neurotoxin gene cluster locus of group II Clostridium botulinum has evolved by successive disruption of two different ancestral precursors. Genome Biol. Evol. 2013, 5, 1032–1037. [Google Scholar] [CrossRef]
- Hill, K.K.; Smith, T.J. Genetic diversity within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. Curr. Top. Microbiol. Immunol. 2013, 364, 1–20. [Google Scholar] [CrossRef]
- Hill, K.K.; Xie, G.; Foley, B.T.; Smith, T.J. Genetic diversity within the botulinum neurotoxin-producing bacteria and their neurotoxins. Toxicon 2015, 107, 2–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Hintsa, H.; Chen, Y.; Korkeala, H.; Lindström, M. Plasmid-Borne Type E Neurotoxin Gene Clusters in Clostridium botulinum Strains. Appl. Environ. Microbiol. 2013, 79, 3856–3859. [Google Scholar] [CrossRef]
- Chen, Y.; Korkeala, H.; Aarnikunnas, J.; Lindström, M. Sequencing the botulinum neurotoxin gene and related genes in Clostridium botulinum type E strains reveals orfx3 and a novel type E neurotoxin subtype. J. Bacteriol. 2007, 189, 8643–8650. [Google Scholar] [CrossRef]
- Kubota, T.; Yonekura, N.; Hariya, Y.; Isogai, E.; Isogai, H.; Amano, K.; Fujii, N. Gene arrangement in the upstream region of Clostridium botulinum type E and Clostridium butyricum BL6340 progenitor toxin genes is different from that of other types. FEMS Microbiol. Lett. 1998, 158, 215–221. [Google Scholar] [CrossRef]
- Nowakowska, M.B.; Douillard, F.P.; Lindström, M. Looking for the X Factor in Bacterial Pathogenesis: Association of orfX-p47 Gene Clusters with Toxin Genes in Clostridial and Non-Clostridial Bacterial Species. Toxins 2019, 12, 19. [Google Scholar] [CrossRef]
- Barker, G.C.; Malakar, P.K.; Plowman, J.; Peck, M.W. Quantification of Nonproteolytic Clostridium botulinum Spore Loads in Food Materials. Appl. Environ. Microbiol. 2016, 82, 1675–1685. [Google Scholar] [CrossRef]
- Hielm, S.; Björkroth, J.; Hyytiä, E.; Korkeala, H. Prevalence of Clostridium botulinum in Finnish trout farms: Pulsed-field gel electrophoresis typing reveals extensive genetic diversity among type E isolates. Appl. Environ. Microbiol. 1998, 64, 4161–4167. [Google Scholar] [CrossRef] [PubMed]
- Hyytiä, E.; Hielm, S.; Korkeala, H. Prevalence of Clostridium botulinum type E in Finnish fish and fishery products. Epidemiol. Infect. 1998, 120, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W.; Plowman, J.; Aldus, C.F.; Wyatt, G.M.; Penaloza Izurieta, W.; Stringer, S.C.; Barker, G.C. Development and Application of a New Method for Specific and Sensitive Enumeration of Spores of Nonproteolytic Clostridium botulinum Types B, E, and F in Foods and Food Materials. Appl. Environ. Microbiol. 2010, 76, 6607. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.D.S.; Sugiyama, H. Botulism: The Organism, Its Toxins, the Disease, 2nd ed.; Charles C Thomas: Springfield, IL, USA, 1988; p. 171. [Google Scholar]
- Van Ermengem, E. Classics in infectious diseases. A new anaerobic bacillus and its relation to botulism. E. van Ermengem. Originally published as “Ueber einen neuen anaeroben Bacillus und seine Beziehungen zum Botulismus” in Zeitschrift fur Hygiene und Infektionskrankheiten 26: 1-56, 1897. Rev. Infect. Dis. 1979, 1, 701–719. [Google Scholar]
- Peck, M.W. Clostridium botulinum and the safety of minimally heated, chilled foods: An emerging issue? J. Appl. Microbiol. 2006, 101, 556–570. [Google Scholar] [CrossRef]
- Peck, M.W.; Goodburn, K.E.; Betts, R.P.; Stringer, S.C. Assessment of the potential for growth and neurotoxin formation by non-proteolytic Clostridium botulinum in short shelf-life commercial foods designed to be stored chilled. Trends Food Sci. Tech. 2008, 19, 207–216. [Google Scholar] [CrossRef]
- Peck, M.W.; Stringer, S.C.; Carter, A.T. Clostridium botulinum in the post-genomic era. Food Microbiol. 2011, 28, 183–191. [Google Scholar] [CrossRef]
- Peck, M.W.; Webb, M.D.; Goodburn, K.E. Assessment of the risk of botulism from chilled, vacuum/modified atmosphere packed fresh beef, lamb and pork held at 3 °C to 8 °C. Food Microbiol. 2020, in press. [Google Scholar]
- Macdonald, T.E.; Helma, C.H.; Shou, Y.; Valdez, Y.E.; Ticknor, L.O.; Foley, B.T.; Davis, S.W.; Hannett, G.E.; Kelly-Cirino, C.D.; Barash, J.R.; et al. Analysis of Clostridium botulinum serotype E strains by using multilocus sequence typing, amplified fragment length polymorphism, variable-number tandem-repeat analysis, and botulinum neurotoxin gene sequencing. Appl. Environ. Microbiol. 2011, 77, 8625–8634. [Google Scholar] [CrossRef]
- Mazuet, C.; Legeay, C.; Sautereau, J.; Ma, L.; Bouchier, C.; Bouvet, P.; Popoff, M.R. Diversity of Group I and II Clostridium botulinum Strains from France Including Recently Identified Subtypes. Genome Biol. Evol. 2016, 8, 1643–1660. [Google Scholar] [CrossRef]
- Peck, M.W.; van Vliet, A.H. Impact of Clostridium botulinum genomic diversity on food safety. Curr. Opin. Food Sci. 2016, 10, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Stringer, S.C.; Carter, A.T.; Webb, M.D.; Wachnicka, E.; Crossman, L.C.; Sebaihia, M.; Peck, M.W. Genomic and physiological variability within Group II (non-proteolytic) Clostridium botulinum. BMC Genom. 2013, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Weedmark, K.A.; Mabon, P.; Hayden, K.L.; Lambert, D.; Van Domselaar, G.; Austin, J.W.; Corbett, C.R. Clostridium botulinum Group II Isolate Phylogenomic Profiling Using Whole-Genome Sequence Data. Appl. Environ. Microbiol. 2015, 81, 5938–5948. [Google Scholar] [CrossRef]
- Williamson, C.H.D.; Vazquez, A.J.; Hill, K.; Smith, T.J.; Nottingham, R.; Stone, N.E.; Sobek, C.J.; Cocking, J.H.; Fernandez, R.A.; Caballero, P.A.; et al. Differentiating Botulinum Neurotoxin-Producing Clostridia with a Simple, Multiplex PCR Assay. Appl. Environ. Microbiol. 2017, 83, e00806–e00817. [Google Scholar] [CrossRef]
- Keto-Timonen, R.; Heikinheimo, A.; Eerola, E.; Korkeala, H. Identification of Clostridium species and DNA fingerprinting of Clostridium perfringens by amplified fragment length polymorphism analysis. J. Clin. Microbiol. 2006, 44, 4057–4065. [Google Scholar] [CrossRef]
- Keto-Timonen, R.; Nevas, M.; Korkeala, H. Efficient DNA fingerprinting of Clostridium botulinum types A, B, E, and F by amplified fragment length polymorphism analysis. Appl. Environ. Microbiol. 2005, 71, 1148–1154. [Google Scholar] [CrossRef]
- Raphael, B.H.; Lautenschlager, M.; Kalb, S.R.; de Jong, L.I.; Frace, M.; Luquez, C.; Barr, J.R.; Fernandez, R.A.; Maslanka, S.E. Analysis of a unique Clostridium botulinum strain from the Southern hemisphere producing a novel type E botulinum neurotoxin subtype. BMC Microbiol. 2012, 12, 245. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Public Health Risk Associated with Botulism as Foodborne Zoonoses. Toxins 2019, 12, 17. [Google Scholar] [CrossRef]
- Brunt, J.; Carter, A.T.; Pye, H.V.; Peck, M.W. The orphan germinant receptor protein GerXAO (but not GerX3b) is essential for L-alanine induced germination in Clostridium botulinum Group II. Sci. Rep. 2018, 8, 7060. [Google Scholar] [CrossRef]
- Brunt, J.; van Vliet, A.H.M.; van den Bos, F.; Carter, A.T.; Peck, M.W. Diversity of the Germination Apparatus in Clostridium botulinum Groups I, II, III, and IV. Front. Microbiol. 2016, 7, 1702. [Google Scholar] [CrossRef]
- Söderholm, H.; Jaakkola, K.; Somervuo, P.; Laine, P.; Auvinen, P.; Paulin, L.; Lindström, M.; Korkeala, H. Comparison of Clostridium botulinum genomes shows the absence of cold shock protein coding genes in type E neurotoxin producing strains. Botulinum J. 2013, 2. [Google Scholar] [CrossRef]
- Artin, I.; Carter, A.T.; Holst, E.; Lövenklev, M.; Mason, D.R.; Peck, M.W.; Rådström, P. Effects of Carbon Dioxide on Neurotoxin Gene Expression in Nonproteolytic Clostridium botulinum Type E. Appl. Environ. Microbiol. 2008, 74, 2391. [Google Scholar] [CrossRef] [PubMed]
- Mascher, G.; Mertaoja, A.; Korkeala, H.; Lindström, M. Neurotoxin synthesis is positively regulated by the sporulation transcription factor Spo0A in Clostridium botulinum type E. Environ. Microbiol. 2017, 19, 4287–4300. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Korkeala, H.; Linden, J.; Lindström, M. Quantitative real-time reverse transcription-PCR analysis reveals stable and prolonged neurotoxin cluster gene activity in a Clostridium botulinum type E strain at refrigeration temperature. Appl. Environ. Microbiol. 2008, 74, 6132–6137. [Google Scholar] [CrossRef][Green Version]
- McLauchlin, J.; Grant, K.A.; Little, C.L. Food-borne botulism in the United Kingdom. J. Public Health 2006, 28, 337–342. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Weedmark, K.A.; Lambert, D.L.; Mabon, P.; Hayden, K.L.; Urfano, C.J.; Leclair, D.; Van Domselaar, G.; Austin, J.W.; Corbett, C.R. Two novel toxin variants revealed by whole-genome sequencing of 175 Clostridium botulinum type E strains. Appl. Environ. Microbiol. 2014, 80, 6334–6345. [Google Scholar] [CrossRef]
- Midura, T.F.; Nygaard, G.S.; Wood, R.M.; Bodily, H.L. Clostridium botulinum type F: Isolation from venison jerky. Appl. Microbiol. 1972, 24, 165–167. [Google Scholar] [CrossRef][Green Version]
- Hill, K.K.; Xie, G.; Foley, B.T.; Smith, T.J.; Munk, A.C.; Bruce, D.; Smith, L.A.; Brettin, T.S.; Detter, J.C. Recombination and insertion events involving the botulinum neurotoxin complex genes in Clostridium botulinum types A, B, E and F and Clostridium butyricum type E strains. BMC Biol. 2009, 7, 66. [Google Scholar] [CrossRef]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef]
- Stover, B.C.; Muller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010, 11, 7. [Google Scholar] [CrossRef]
- Wachnicka, E.; Stringer, S.C.; Barker, G.C.; Peck, M.W. Systematic Assessment of Nonproteolytic Clostridium botulinum Spores for Heat Resistance. Appl. Environ. Microbiol. 2016, 82, 6019–6029. [Google Scholar] [CrossRef] [PubMed]
- Mazuet, C.; Sautereau, J.; Legeay, C.; Bouchier, C.; Bouvet, P.; Popoff, M.R. An atypical outbreak of food-borne botulism due to Clostridium botulinum types B and E from ham. J. Clin. Microbiol. 2015, 53, 722–726. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Sebaihia, M.; Peck, M.W.; Minton, N.P.; Thomson, N.R.; Holden, M.T.; Mitchell, W.J.; Carter, A.T.; Bentley, S.D.; Mason, D.R.; Crossman, L.; et al. Genome sequence of a proteolytic (Group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res. 2007, 17, 1082–1092. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Van Vliet, A.H.M. Use of pan-genome analysis for the identification of lineage-specific genes of Helicobacter pylori. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Brynildsrud, O.; Bohlin, J.; Scheffer, L.; Eldholm, V. Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol. 2016, 17, 238. [Google Scholar] [CrossRef] [PubMed]
- Kruczkiewicz, P.; Mutschalll, S.; Barker, D.; Thomas, J.; Van Domselaar, G.; Gannon, V.P.J.; Carrillo, C.D.; Taboada, E.N. MIST: A Tool for Rapid in silico Generation of Molecular Data from Bacterial Genome Sequences. In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms, Barcelona, Spain, 11–14 February 2013; pp. 316–323. [Google Scholar]
- Pornsukarom, S.; van Vliet, A.H.M.; Thakur, S. Whole genome sequencing analysis of multiple Salmonella serovars provides insights into phylogenetic relatedness, antimicrobial resistance, and virulence markers across humans, food animals and agriculture environmental sources. BMC Genom. 2018, 19, 801. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunt, J.; van Vliet, A.H.M.; Stringer, S.C.; Carter, A.T.; Lindström, M.; Peck, M.W. Pan-Genomic Analysis of Clostridium botulinum Group II (Non-Proteolytic C. botulinum) Associated with Foodborne Botulism and Isolated from the Environment. Toxins 2020, 12, 306. https://doi.org/10.3390/toxins12050306
Brunt J, van Vliet AHM, Stringer SC, Carter AT, Lindström M, Peck MW. Pan-Genomic Analysis of Clostridium botulinum Group II (Non-Proteolytic C. botulinum) Associated with Foodborne Botulism and Isolated from the Environment. Toxins. 2020; 12(5):306. https://doi.org/10.3390/toxins12050306
Chicago/Turabian StyleBrunt, Jason, Arnoud H. M. van Vliet, Sandra C. Stringer, Andrew T. Carter, Miia Lindström, and Michael W. Peck. 2020. "Pan-Genomic Analysis of Clostridium botulinum Group II (Non-Proteolytic C. botulinum) Associated with Foodborne Botulism and Isolated from the Environment" Toxins 12, no. 5: 306. https://doi.org/10.3390/toxins12050306
APA StyleBrunt, J., van Vliet, A. H. M., Stringer, S. C., Carter, A. T., Lindström, M., & Peck, M. W. (2020). Pan-Genomic Analysis of Clostridium botulinum Group II (Non-Proteolytic C. botulinum) Associated with Foodborne Botulism and Isolated from the Environment. Toxins, 12(5), 306. https://doi.org/10.3390/toxins12050306




