Multi Mycotoxin Determination in Dried Beef Using Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry (LC-MS/MS)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation
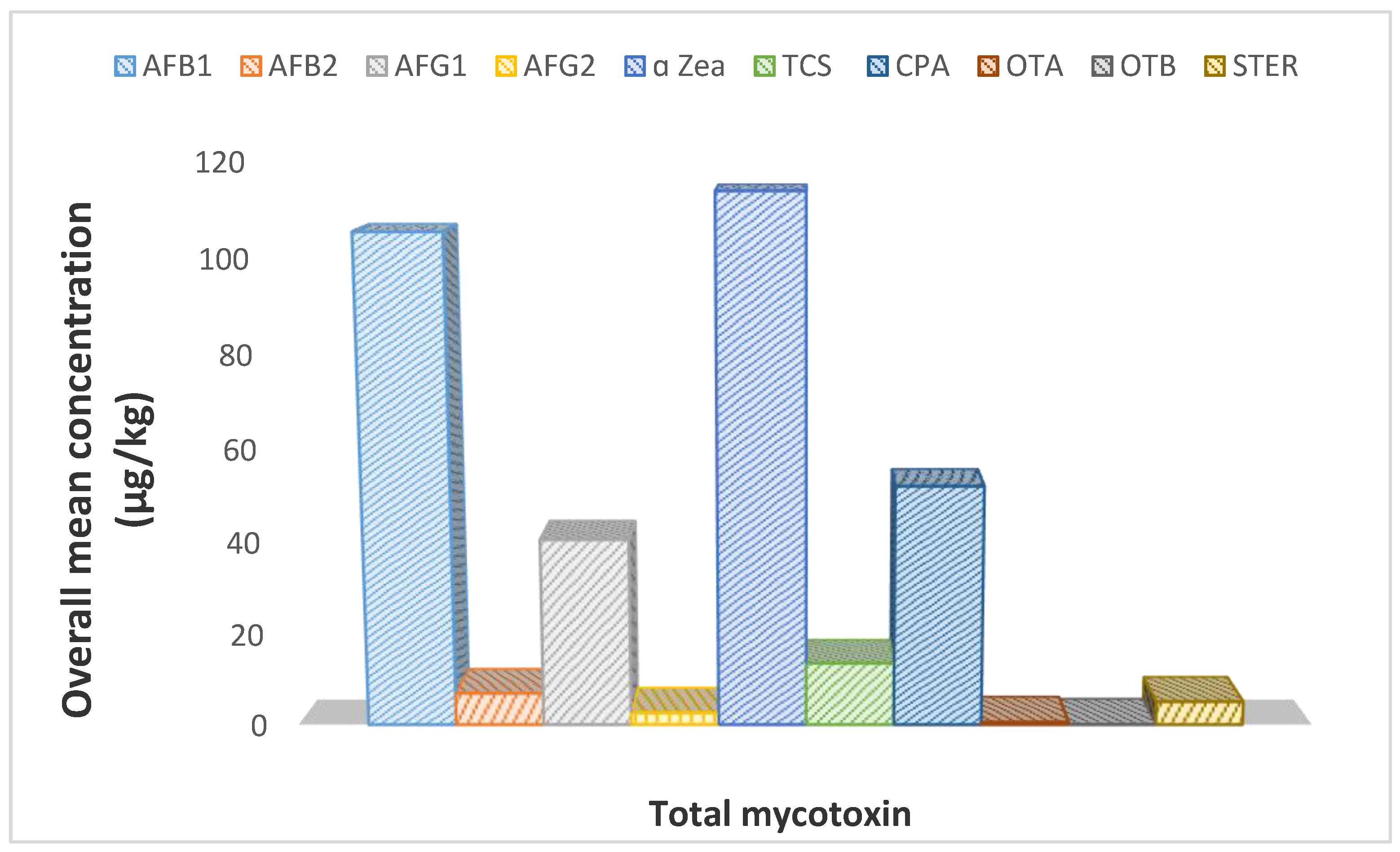
2.2. Occurrence of Mycotoxins in Dried Beef
2.3. Co-Occurence of Mycotoxins in Meats from the Different Sampling Locations
2.4. Trichothecenes Level in Dried Beef
2.5. Ochratoxin A Level in Dried Beef
2.6. Aflatoxin Total Level in Dried Beef
2.7. Other Mycotoxins in the Dried Beef
2.8. Potential Health Risks for Consumption of Contaminated Dried Beef
3. Conclusions
4. Materials and Methods
4.1. Reagents, Chemicals, and Extraction Kits
4.2. Standards for LC-MS
4.3. Sampling
4.4. Extraction Procedure for LC-MS
4.5. Instrumentation for LC-MS
4.6. Validation of the Method for LC-MS
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Binder, E.; Tan, L.; Chin, L.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- El Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleadin, J.; Staver, M.M.; Vahčić, N.; Kovačević, D.; Milone, S.; Saftić, L.; Scortichini, G. Survey of aflatoxin B1 and ochratoxin A occurrence in traditional meat products coming from Croatian households and markets. Food Control 2015, 52, 71–77. [Google Scholar] [CrossRef]
- Duarte, S.; Pena, A.; Lino, C. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010, 27, 187–198. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre-and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.C.; Pena, A.; Lino, C.M. Ochratoxin A in Portugal: A review to assess human exposure. Toxins 2010, 2, 1225–1249. [Google Scholar] [CrossRef] [PubMed]
- Perši, N.; Pleadin, J.; Kovačević, D.; Scortichini, G.; Milone, S. Ochratoxin A in raw materials and cooked meat products made from OTA-treated pigs. Meat Sci. 2014, 96, 203–210. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Nisar, S.; Asi, M.R.; Jinap, S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control 2014, 43, 98–103. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization and International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking (No. 83); IARC: Lyon, France, 2004. [Google Scholar]
- Royer, D.; Humpf, H.-U.; Guy, P. Quantitative analysis of Fusarium mycotoxins in maize using accelerated solvent extraction before liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Food Addit. Contam. 2004, 21, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Wagacha, J.; Muthomi, J. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bankole, S.; Adebanjo, A. Mycotoxins in food in West Africa: Current situation and possibilities of controlling it. Afr. J. Biotechnol. 2003, 2, 254–263. [Google Scholar]
- Thompson, C.; Henke, S.E. Effect of climate and type of storage container on aflatoxin production in corn and its associated risks to wildlife species. J. Wildl. Dis. 2000, 36, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markov, K.; Pleadin, J.; Bevardi, M.; Vahčić, N.; Sokolić-Mihalak, D.; Frece, J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control 2013, 34, 312–317. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Martín, A.; Nuñez, F.; Córdoba, J.J. Evaluation of hazard of aflatoxin B1, ochratoxin A and patulin production in dry-cured ham and early detection of producing moulds by qPCR. Food Control 2012, 27, 118–126. [Google Scholar] [CrossRef]
- Tabuc, C.; Bailly, J.; Bailly, S.; Querin, A.; Guerre, P. Toxigenic potential of fungal mycoflora isolated from dry cured meat products: Preliminary study. Rev. De Med. Vet. 2004, 155, 287–291. [Google Scholar]
- Dall’Asta, C.; Galaverna, G.; Bertuzzi, T.; Moseriti, A.; Pietri, A.; Dossena, A.; Marchelli, R. Occurrence of ochratoxin A in raw ham muscle, salami and dry-cured ham from pigs fed with contaminated diet. Food Chem. 2010, 120, 978–983. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Herzallah, S.M. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009, 114, 1141–1146. [Google Scholar] [CrossRef]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Quality and safety assessment of sun dried meat product (kundi) from Ibadan, Oyo state, Nigeria. Cogent Food Agric. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Okoro, C.D. Production, Properties and Shelf-Life of Intermediate Moisture Snail Meat. Ph.D. Thesis, University of Nigeria, Nsukka, Nigeria, 2015. [Google Scholar]
- Ekwomadu, T.I.; Dada, T.A.; Nleya, N.; Gopane, R.; Sulyok, M.; Mwanza, M. Variation of Fusarium free, masked, and emerging mycotoxin metabolites in maize from agriculture regions of South Africa. Toxins 2020, 12, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubert, J.; Soler, C.; Mañes, J. Application of an HPLC–MS/MS method for mycotoxin analysis in commercial baby foods. Food Chem. 2012, 133, 176–183. [Google Scholar] [CrossRef]
- Hanssen, H.-P. Mould control in the meat processing industry: Using a BioRid coating system. Fleischwirtschaft 1995, 75, 52–60. [Google Scholar]
- Pietri, A.; Bertuzzi, T.; Gualla, A.; Piva, G. Occurrence of ochratoxin A in raw ham muscles and in pork products from northern Italy. Ital. J. Food Sci. 2006, 18, 99–106. [Google Scholar]
- Völkel, I.; Schröer-Merker, E.; Czerny, C.P. The carry-over of mycotoxins in products of animal origin with special regard to its implications for the European food safety legislation. Food Nutr. Sci. 2011, 2, 852–867. [Google Scholar] [CrossRef] [Green Version]
- Sirot, V.; Fremy, J.-M.; Leblanc, J.-C. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Abd-Elghany, S.M.; Sallam, K.I. Rapid determination of total aflatoxins and ochratoxins A in meat products by immuno-affinity fluorimetry. Food Chem. 2015, 179, 253–256. [Google Scholar] [CrossRef]
- Malekinejad, H.; Akbari, P.; Allymehr, M.; Hobbenaghi, R.; Rezaie, A. Cyclopiazonic acid augments the hepatic and renal oxidative stress in broiler chicks. Hum. Exp. Toxicol. 2011, 30, 910–919. [Google Scholar] [CrossRef]
- D’Mello, J.; Macdonald, A.; Postel, D.; Hunter, E. 3-Acetyl deoxynivalenol production in a strain of Fusarium culmorum insensitive to the fungicide difenoconazole. Mycotoxin Res. 1997, 13, 73–80. [Google Scholar] [CrossRef]
- Miller, J.D. Epidemiology of Fusarium Ear Diseases of Cereals; Eagan Press: St. Paul, MN, USA, 1994; pp. 19–36. [Google Scholar]
- Steyn, P.S. Mycotoxins, general view, chemistry and structure. Toxicol. Lett. 1995, 82, 843–851. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Petkova-Bocharova, T.; Chernozemsky, I.; Castegnaro, M. Balkan endemic nephropathy and associated urinary tract tumours: A review on aetiological causes and the potential role of mycotoxins. Food Addit. Contam. 2002, 19, 282–302. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, J.; Xing, L.; Shen, H.; Yan, X.; Wang, J.; Zhang, X. Impairment of cell cycle progression by sterigmatocystin in human pulmonary cells in vitro. Food Chem. Toxicol. 2014, 66, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Aikawa, T.; Kera, K. A synergistic effect of nitrosodimethylamine on sterigmatocystin carcinogenesis in rats. Food Cosmet. Toxicol. 1978, 16, 591–596. [Google Scholar] [CrossRef]
- Asam, S.; Rychlik, M. Potential health hazards due to the occurrence of the mycotoxin tenuazonic acid in infant food. Eur. Food Res. Technol. 2013, 236, 491–497. [Google Scholar] [CrossRef]
- Adekoya, I.; Njobeh, P.; Obadina, A.; Chilaka, C.; Okoth, S.; De Boevre, M.; De Saeger, S. Awareness and prevalence of mycotoxin contamination in selected Nigerian fermented foods. Toxins 2017, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Sulyok, M.; Krska, R.; Schuhmacher, R. A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef]
- Han, Z.; Ren, Y.; Liu, X.; Luan, L.; Wu, Y. A reliable isotope dilution method for simultaneous determination of fumonisins B1, B2 and B3 in traditional Chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2010, 33, 2723–2733. [Google Scholar] [CrossRef]

| S/No | Mycotoxin | Ret. Time (min) | Precursor (mz) | Products (mz) | Q1 Pre Bias (V) | CE | Q3 Pre Bias (V) |
|---|---|---|---|---|---|---|---|
| 1 | 3ACDON | 6.589 | 338.9 | 231 | −24 | −15 | −22 |
| 213 | −12 | −17 | −20 | ||||
| 2 | HT-2 | 8.817 | 425 | 263 | −20 | −12 | −18 |
| 104.9 | −16 | −47 | −19 | ||||
| 3 | AFG2 | 7.507 | 331 | 245.1 | −12 | −32 | −24 |
| 313 | −12 | −24 | −20 | ||||
| 4 | AFG1 | 7.767 | 329 | 243 | −12 | −28 | −23 |
| 311.1 | −16 | −24 | −14 | ||||
| 5 | AFB1 | 8.25 | 313 | 241 | −22 | −41 | −23 |
| 285.1 | −22 | −24 | −29 | ||||
| 6 | B-ZEA | 8.949 | 323.1 | 277.2 | −16 | −16 | −18 |
| 305.2 | −16 | −11 | −20 | ||||
| 7 | a-ZEA | 9.415 | 323.1 | 277.2 | −17 | −17 | −18 |
| 305.2 | −24 | −9 | −20 | ||||
| 8 | T-2 Toxin | 9.674 | 467.2 | 245.1 | −13 | −11 | −16 |
| 305.2 | −22 | −11 | −20 | ||||
| 9 | ZEA | 10.063 | 319.1 | 185 | −12 | −27 | −30 |
| 187.1 | −15 | −21 | −19 | ||||
| 10 | AME | 10.125 | 273 | 128.1 | −10 | −49 | −21 |
| 115.05 | −18 | −54 | −19 | ||||
| 11 | OTB | 9.331 | 370.1 | 205 | −13 | −22 | −21 |
| 324.1 | −13 | −14 | −22 | ||||
| 12 | OTA | 10.132 | 403.8 | 239 | −15 | −27 | −24 |
| 221 | −12 | −38 | −21 | ||||
| 13 | AFB2 | 8.007 | 315 | 259.1 | −22 | −31 | −25 |
| 287 | −23 | −26 | −30 | ||||
| 14 | TCS | 5.02 | 198.15 | 125.05 | −10 | −18 | −21 |
| - | - | - | - | ||||
| 15 | Citrinin | 5.00 | 251 | 205.1 | −13 | −26 | −21 |
| 233 | −17 | −17 | −24 | ||||
| 16 | STECY | 5.01 | 324.90 | 310 | −22 | −24 | −30 |
| 281.1 | −22 | −40 | −27 | ||||
| 17 | CPA | 5.01 | 337.1 | 196.1 | −10 | −23 | −18 |
| 182.1 | −10 | −20 | −20 |
| Mycotoxin | Matrix Effect (%) | Linearity r2 | LOD µg/kg | LOQ µg/kg | Recovery % Spiked Level (µg/kg) | Intra-day(RSD) % Spiked Level (µg/kg) | Inter-day (RSD) % Spiked Level (µg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 25 | 50 | 100 | 1 | 3 | 5 | |||||
| AFB1 | 73 | 0.998 | 2 | 6.8 | 114 | 121 | 124 | 10.9 | 6.5 | 4.8 | 4.22 | 3.71 | 3.75 |
| AFB2 | 75 | 0.999 | 6 | 17 | 121 | 125 | 127 | 4.8 | 2.7 | 3.15 | 3.42 | 4.35 | 3.59 |
| AFG1 | 77 | 0.997 | 6 | 16 | 130 | 129 | 130 | 6.68 | 6.73 | 3.07 | 3.63 | 4.48 | 3.69 |
| AFG2 | 83 | 0.994 | 44 | 134 | 114 | 120 | 126 | 8.2 | 9.03 | 3.63 | 3.57 | 2.12 | 3.47 |
| STER | 47 | 0.999 | 8 | 23 | 73 | 71 | 71 | 3.71 | 6.89 | 13.1 | 13.2 | 15.1 | 14.7 |
| OTA | 60 | 0.997 | 0.13 | 0.4 | 71 | 70 | 70 | 12.4 | 5.01 | 3.29 | 5.18 | 5.38 | 6.81 |
| OTB | 48 | 0.999 | 0.41 | 1.2 | 36 | 36 | 41 | 5.43 | 5.47 | 7.33 | 9.51 | 5.81 | 6.46 |
| ZEA | 73 | 0.999 | 123 | 374 | 108 | 113 | 114 | 5.47 | 5.56 | 4.7 | 3.12 | 5.38 | 3.1 |
| T-2 | 52 | 0.992 | 84 | 254 | 59 | 64 | 58 | 4.64 | 6.31 | 3.85 | 6.19 | 6.09 | 6.14 |
| HT-2 | 35 | 0.999 | 3 | 10 | 81 | 79 | 78 | 16.4 | 7.62 | 4.59 | 4.86 | 4.62 | 4.58 |
| 3-ACDON | 39 | 0.997 | 25 | 75 | 48 | 52 | 57 | 2.33 | 10.1 | 6.92 | 6.94 | 6.18 | 6.28 |
| AME | 66 | 0.994 | 55 | 166 | 112 | 114 | 111 | 4.41 | 3.85 | 2.12 | 2.34 | 2.45 | 2.71 |
| α-ZEA | 72 | 0.998 | 127 | 385 | 117 | 116 | 114 | 3.49 | 5.54 | 2.57 | 3.29 | 3.55 | 3.3 |
| β-ZEA | 70 | 0.998 | 19 | 57 | 102 | 99 | 99 | 2.75 | 4.42 | 1.81 | 2.49 | 3.1 | 3.37 |
| Citrinin | 87 | 0.998 | 18 | 54 | 101 | 107 | 107 | 2.17 | 2.6 | 3.09 | 4.59 | 3.33 | 4.71 |
| CPA | 75 | 0.996 | 7 | 20 | 81 | 71 | 71 | 5.23 | 4.61 | 3.15 | 6.91 | 6.12 | 6.11 |
| TCS | 43 | 0.998 | 8 | 25 | 53 | 26 | 11 | 8.39 | 5.23 | 5.65 | 6.38 | 6.27 | 6.21 |
| Location | HT 2 | CPA | TCS | AFB1 | AFB2 | AFG1 | AFG2 | ZEA | α ZEA | β ZEA | CIT | T2 | OTA | OTB | STER | AME |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ikole | 0(15) | 14(15) | 4(15) | 8(15) | 9(15) | 7(15) | 4(15) | 0(15) | 6(15) | 0(15) | 0(15) | 0(15) | 1(15) | 3(15) | 6(15) | 0(15) |
| Ilawe | 0(6) | 5(6) | 1(6) | 1(6) | 0(6) | 0(6) | 0(6) | 0(6) | 2(6) | 0(6) | 0(6) | 0(6) | 0(6) | 0(6) | 0(6) | 0(6) |
| Aramoko | 0(12) | 12(12) | 1(12) | 6(12) | 2(12) | 7(12) | 0(12) | 0(12) | 5(12) | 0(12) | 0(12) | 0(12) | 1(12) | 1(12) | 1(12) | 0(12) |
| Ijero | 0(10) | 10(10) | 0(10) | 7(10) | 5(10) | 4(10) | 0(10) | 0(10) | 2(10) | 0(10) | 0(10) | 0(10) | 5(10) | 1(10) | 4(10) | 0(10) |
| Oye | 0(6) | 6(6) | 3(6) | 4(6) | 2(6) | 0(6) | 1(6) | 0(6) | 2(6) | 0(6) | 0(6) | 0(6) | 3(6) | 1(6) | 2(6) | 0(6) |
| Otun | 0(15) | 13(15) | 0(15) | 4(15) | 2(15) | 4(15) | 1(15) | 0(15) | 5(15) | 0(15) | 0(15) | 0(15) | 0(15) | 1(15) | 3(15) | 0(15) |
| Ado | 0(20) | 20(20) | 3(20) | 12(20) | 11(20) | 6(20) | 0(20) | 0(20) | 5(20) | 0(20) | 0(20) | 0(20) | 6(20) | 4(20) | 5(20) | 0(20) |
| Omuo | 0(14) | 13(14) | 2(14) | 7(14) | 5(14) | 4(14) | 1(14) | 0(14) | 5(14) | 0(14) | 0(14) | 0(14) | 1(14) | 2(14) | 2(14) | 0(14) |
| Igede | 0(6) | 6(6) | 1(6) | 4(6) | 3(6) | 3(6) | 2(6) | 0(6) | 2(6) | 0(6) | 0(6) | 0(6) | 0(6) | 1(6) | 1(6) | 0(6) |
| Ise/Emure | 0(4) | 3(4) | 0(4) | 1(4) | 2(4) | 1(4) | 0(4) | 0(4) | 2(4) | 0(4) | 0(4) | 0(4) | 0(4) | 0(4) | 0(4) | 0(4) |
| Total % | 0% | 102 94% | 15 14% | 54 50% | 41 38% | 36 33% | 9 8% | 0% | 36 33% | 0% | 0% | 0% | 17 16% | 14 13% | 24 22% | 0% |
| Location | Ikole | Ilawe | Aramoko | Ijero | Oye | Otun | Ado | Omuo | Igede | Ise/Emure |
|---|---|---|---|---|---|---|---|---|---|---|
| HT 2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| CPA | 118.87 ± 52.05 b | 6.42 ± 4.74 i | 13.36 ± 5.11 g | 25.81 ± 10.54 f | 163.93 ± 94.75 a | 10.36 ± 8.51 h | 92.62 ± 44.61 c | 56.43 ± 38.35 d | 30.03 ± 14.90 e | 2.11 ± 1.11 j |
| TCS | 35.88 ± 28.74 | 0.30 ± 0.30 | 55.83 ± 55.83 | 0 | 9.27 ± 4.47 | 0 | 18.79 ± 11.19 | 15.6 ± 14.93 | 0.16 ± 0.16 | 0 |
| AFB1 | 282.75 ± 136.66 | 5.85 ± 5.85 | 15.6 ± 9.62 | 66.57 ± 45.71 | 14.1 ± 8.87 | 59.81 ± 58.53 | 137.87 ± 60.05 | 70.69 ± 51.14 | 295.41 ± 272.73 | 3.91 ± 3.91 |
| AFB2 | 15.69 ± 7.32 ab | 0 | 0.65 ± 0.47 b | 1.72 ± 0.67 b | 1.69 ± 1.24 b | 2.5 ± 2.31 b | 7.42 ± 2.88 b | 3.6 ± 2.51 b | 33.13 ± 31.02 a | 2.85 ± 2.48 b |
| AFG1 | 88.66 ± 39.09 b | 0 | 4.94 ± 1.91 c | 13.95 ± 9.96 b | 0 | 6.82 ± 5.78 c | 15.45 ± 8.21 b | 15.56 ± 8.81 b | 257.35 ± 208.95 a | 2.24 ± 2.24 d |
| AFG2 | 5.53 ± 2.55 b | 0 | 0 | 0 | 0.64 ± 0.64 b | 0.45 ± 0.45 b | 0 | 0.38 ± 0.38 b | 19.08 ± 14.87 a | 0 |
| ZEA | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| α Zea | 137.2 ± 49.00 | 148.89 ± 124.93 | 167.34 ± 70.53 | 47.6 ± 39.14 | 81.64 ± 51.73 | 100.83 ± 44.62 | 103.35 ± 44.46 | 136.96 ± 52.71 | 80.33 ± 50.97 | 134.11 ± 77.59 |
| β Zea | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| CIT | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| T2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| OTA | 0.02 ± 0.02 b | 0 | 0.03 ± 0.03 d | 0.28 ± 0.10 c | 3.96 ± 3.60 a | 0 | 1.12 ± 0.64 b | 0.02 ± 0.02 d | 0 | 0 |
| OTB | 0.03 ± 0.02 | 0 | 0.01 ± 0.05 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.01 ± 0.00 | 0.22 ± 0.19 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0 |
| STER | 25.37 ± 13.77 | 0 | 2.64 ± 2.64 | 3.56 ± 1.78 | 3.06 ± 2.38 | 1.08 ± 0.59 | 4.36 ± 3.42 | 4.98 ± 4.62 | 5.89 ± 5.89 | 0 |
| AME | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 3-AC DON | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dada, T.A.; Ekwomadu, T.I.; Mwanza, M. Multi Mycotoxin Determination in Dried Beef Using Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry (LC-MS/MS). Toxins 2020, 12, 357. https://doi.org/10.3390/toxins12060357
Dada TA, Ekwomadu TI, Mwanza M. Multi Mycotoxin Determination in Dried Beef Using Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry (LC-MS/MS). Toxins. 2020; 12(6):357. https://doi.org/10.3390/toxins12060357
Chicago/Turabian StyleDada, Toluwase Adeseye, Theodora Ijeoma Ekwomadu, and Mulunda Mwanza. 2020. "Multi Mycotoxin Determination in Dried Beef Using Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry (LC-MS/MS)" Toxins 12, no. 6: 357. https://doi.org/10.3390/toxins12060357





