OnabotulinumtoxinA Displays Greater Biological Activity Compared to IncobotulinumtoxinA, Demonstrating Non-Interchangeability in Both In Vitro and In Vivo Assays
Abstract
:1. Introduction
2. Results
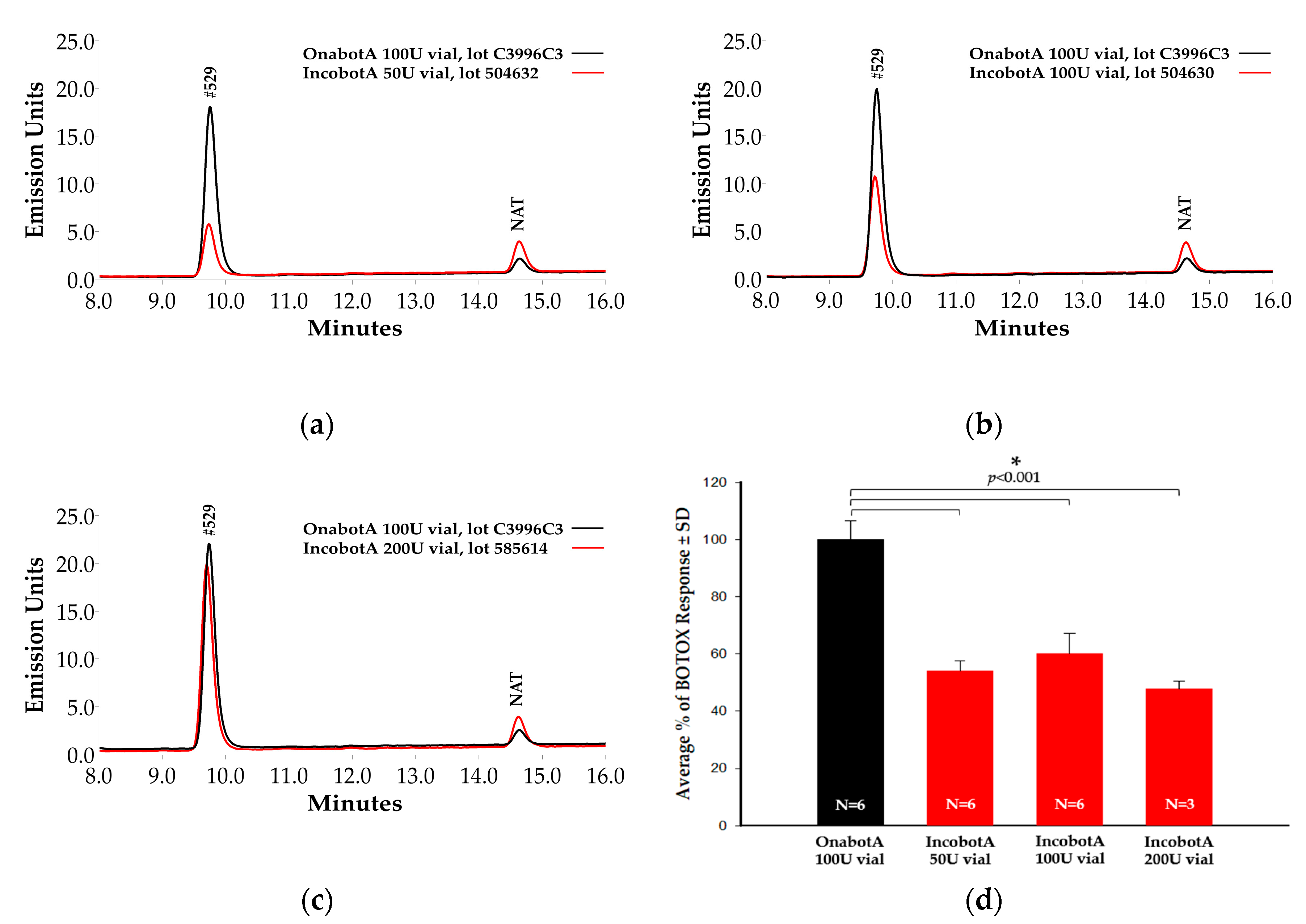
2.1. Light-Chain Activity High-Performance Liquid Chromatography (LCA-HPLC) Assay
2.2. Cell-Based Potency Assay (CBPA)
2.3. Compound Muscle Action Potential (CMAP) Electrophysiology Assay
2.4. Digit Abduction Score (DAS) Assay
3. Discussion
4. Materials and Methods
4.1. BoNT/A Products
4.2. Light-Chain Activity High-Performance Liquid Chromatography (LCA-HPLC) Assay
4.3. Cell-Based Potency Assay (CBPA)
4.4. Compound Muscle Action Potential (CMAP) Electrophysiology Assay
4.5. Digit Abduction Score (DAS) Assay
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dressler, D.; Saberi, F.A. Botulinum Toxin: Mechanisms of Action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fujinaga, Y.; Watanabe, T.; Ohyama, T.; Takeshi, K.; Moriishi, K.; Nakajima, H.; Oguma, K. Molecular composition of Clostridium botulinum type A progenitor toxins. Infect. Immun. 1996, 64, 1589–1594. [Google Scholar] [CrossRef] [Green Version]
- Simpson, L. The life history of a botulinum toxin molecule. Toxicon 2013, 68, 40–59. [Google Scholar] [CrossRef]
- Gart, M.S.; Gutowski, K.A. Overview of Botulinum Toxins for Aesthetic Uses. Clin. Plast. Surg. 2016, 43, 459–471. [Google Scholar] [CrossRef]
- Schantz, E.J.; Johnson, E.A. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol. Rev. 1992, 56, 80–99. [Google Scholar] [CrossRef]
- Beylot, C. Different botulinum toxins and their specifications. Ann. Dermatol. Venereol. 2009, 136, S77–S85. [Google Scholar] [CrossRef]
- Hambleton, P. Clostridium botulinum toxins: A general review of involvement in disease, structure, mode of action and preparation for clinical use. J. Neurol. 1992, 239, 16–20. [Google Scholar] [CrossRef]
- Federal Drug Administration (FDA). Center for drug evaluation and research, application number: 125274. Chemistry Review for Reloxin (Botulinum Toxin Type A); Ipsen Biopharm Limited: UK. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/125274s000_ChemR.pdf (accessed on 21 September 2009).
- Frevert, J.; Dressler, D. Complexing proteins in botulinum toxin type A drugs: A help or a hindrance? Boil. Targets Ther. 2010, 4, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Won, C.H.; Kim, H.K.; Kim, B.J.; Kang, H.; Hong, J.P.; Lee, S.-Y.; Kim, C.-S. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: A multicenter, randomized, double-blind, active-controlled study. Int. J. Dermatol. 2014, 54, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Brin, M.F.; James, C.; Maltman, J. Botulinum toxin type A products are not interchangeable: A review of the evidence. Boil. Targets Ther. 2014, 8, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, J.; Gourdeau, A. A direct comparison of onabotulinumtoxinA (botox) and incobotulinumtoxinA (xeomin) in the treatment of benign essential blepharospasm: A split-face technique. J. Neuroophthalmol. 2014, 34, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Tacik, P.; Saberi, F.A. Botulinum toxin therapy of cervical dystonia: Duration of therapeutic effects. J. Neural Transm. 2014, 122, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Rappl, T.; Parvizi, D.; Friedl, H.; Wiedner, M.; May, S.; Kranzelbinder, B.; Wurzer, P.; Hellbom, B. Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: A randomized, double-blind study. Clin. Cosmet. Investig. Dermatol. 2013, 6, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattler, G.; Callander, M.J.; Grablowitz, D.; Walker, T.; Bee, E.K.; Rzany, B.; Flynn, T.C.; Carruthers, A. Noninferiority of IncobotulinumtoxinA, Free from Complexing Proteins, Compared with Another Botulinum Toxin Type A in the Treatment of Glabellar Frown Lines. Dermatol. Surg. 2010, 36, 2146–2154. [Google Scholar] [CrossRef]
- Kane, M.A.C.; Gold, M.H.; Coleman, W.P.; Jones, D.H.; Tanghetti, E.A.; Alster, T.S.; Rohrer, T.E.; Burgess, C.M.; Shamban, A.T.; Finn, E. A Randomized, Double-Blind Trial to Investigate the Equivalence of IncobotulinumtoxinA and OnabotulinumtoxinA for Glabellar Frown Lines. Dermatol. Surg. 2015, 41, 1310–1319. [Google Scholar] [CrossRef]
- Jost, W.H.; Benecke, R.; Hauschke, D.; Jankovic, J.; Kaňovský, P.; Roggenkämper, P.; Simpson, D.M.; Comella, C.L. Clinical and pharmacological properties of incobotulinumtoxinA and its use in neurological disorders. Drug Des. Dev. Ther. 2015, 9, 1913–1926. [Google Scholar] [CrossRef] [Green Version]
- Frevert, J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D 2015, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kollewe, K.; Mohammadi, B.; Kohler, S.; Pickenbrock, H.; Dengler, R.; Dressler, D. Blepharospasm: Long-term treatment with either botox®, xeomin® or dysport®. J. Neural Transm. 2015, 122, 427–431. [Google Scholar] [CrossRef]
- Merz Pharma UK Ltd. Xeomin 200 Units Summary of Product Characteristics (SMPC). 2017. Available online: https://www.medicines.org.uk/emc/product/2162/smpc/print (accessed on 14 January 2019).
- Merz Pharma UK Ltd. Bocouture 50 Units Summary of Product Characteristics (SMPC). Available online: https://www.medicines.org.uk/emc/product/600/smpc (accessed on 9 May 2019).
- Moers-Carpi, M.M.; Dirschka, T.; Feller-Heppt, G.; Hilton, S.; Hoffmann, K.; Philipp-Dormston, W.G.; Rütter, A.; Tan, K.; Chapman, M.A.; Fulford-Smith, A. A randomised, double-blind comparison of 20 units of onabotulinumtoxinA with 30 units of incobotulinumtoxinA for glabellar lines. J. Cosmet. Laser Ther. 2012, 14, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Yeilding, R.H.; Fezza, J.P. A Prospective, Split-Face, Randomized, Double-Blind Study Comparing OnabotulinumtoxinA to IncobotulinumtoxinA for Upper Face Wrinkles. Plast. Reconstr. Surg. 2015, 135, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.W.; Carruthers, A.; Fagien, S.; Lowe, N.J. Comparisons among Botulinum Toxins: An Evidence-Based Review. Plast. Reconstr. Surg. 2008, 121, 413e–422e. [Google Scholar] [CrossRef]
- Brown, M.; Nicholson, G.; Ardila, M.C.; Satorius, A.; Broide, R.S.; Clarke, K.; Hunt, T.; Francis, J. Comparative evaluation of the potency and antigenicity of two distinct BoNT/A-derived formulations. J. Neural Transm. 2012, 120, 291–298. [Google Scholar] [CrossRef]
- Hunt, T.; Rupp, D.; Shimizu, G.; Tam, K.; Weidler, J.; Xie, J. Characterization of SNARE Cleavage Products Generated by Formulated Botulinum Neurotoxin Type-A Drug Products. Toxins 2010, 2, 2198–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, T.; Clarke, K. Potency Evaluation of a Formulated Drug Product Containing 150-kd Botulinum Neurotoxin Type A. Clin. Neuropharmacol. 2008, 32, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Frevert, J. Content of botulinum neurotoxin in botox®/vistabel®, dysport®/azzalure®, and xeomin®/bocouture®. Drugs R D 2010, 10, 67–73. [Google Scholar] [CrossRef]
- Dressler, D.; Mander, G.; Fink, K. Measuring the potency labelling of onabotulinumtoxinA (Botox®) and incobotulinumtoxinA (Xeomin®) in an LD50 assay. J. Neural Transm. 2011, 119, 13–15. [Google Scholar] [CrossRef]
- Kutschenko, A.; Manig, A.; Reinert, M.-C.; Mönnich, A.; Liebetanz, D. In-Vivo comparison of the neurotoxic potencies of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA. Neurosci. Lett. 2016, 627, 216–221. [Google Scholar] [CrossRef]
- Fernandez-Salas, E.; Wang, J.; Molina, Y.; Nelson, J.B.; Jacky, B.P.S.; Aoki, K.R. Botulinum Neurotoxin Serotype a Specific Cell-Based Potency Assay to Replace the Mouse Bioassay. PLoS ONE 2012, 7, e49516. [Google Scholar] [CrossRef] [Green Version]
- Torii, Y.; Goto, Y.; Takahashi, M.; Ishida, S.; Harakawa, T.; Sakamoto, T.; Kaji, R.; Kozaki, S.; Ginnaga, A. Quantitative determination of biological activity of botulinum toxins utilizing compound muscle action potentials (CMAP), and comparison of neuromuscular transmission blockage and muscle flaccidity among toxins. Toxicon 2010, 55, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.R. Preclinical update on BOTOX® (botulinum toxin type A)-purified neurotoxin complex relative to other botulinurn neurotoxin preparations. Eur. J. Neurol. 1999, 6, s3–s10. [Google Scholar] [CrossRef]
- Broide, R.S.; Rubino, J.; Nicholson, G.S.; Ardila, M.C.; Brown, M.S.; Aoki, K.R.; Francis, J. The rat Digit Abduction Score (DAS) assay: A physiological model for assessing botulinum neurotoxin-induced skeletal muscle paralysis. Toxicon 2013, 71, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Prager, W.; Teixeira, D.N.; Leventhal, P.S. IncobotulinumtoxinA for Aesthetic Indications. Dermatol. Surg. 2017, 43, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. Conversion ratio between botox®, dysport®, and xeomin® in clinical practice. Toxins 2016, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.J.; Chang, B.; Taglienti, A.J.; Chin, B.C.; Chang, C.S.; Folsom, N.; Percec, I. A quantitative analysis of onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA: A randomized, double-blind, prospective clinical trial of comparative dynamic strain reduction. Plast. Reconstr. Surg. 2016, 137, 1424–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa, A.; Pereira, E.S.P.; Pereira, M.D.O.; Dos Santos, F.B.C.; Fávaro, R.; De Matos, L.S.; Tannous, T.S.; Duarte, C.O.P.; Pereira, C.S. Six-Month Comparative Analysis Monitoring the Progression of the Largest Diameter of the Sweating Inhibition Halo of Different Botulinum Toxins Type-A. Aesthetic Surg. J. 2018, 39, 993–1004. [Google Scholar] [CrossRef]
- Banegas, R.A.; Farache, F.; Rancati, A.; Chain, M.; Gallagher, C.J.; Chapman, M.A.; Caulkins, C.A. The south american glabellar experience study (sage): A multicenter retrospective analysis of real-world treatment patterns following the introduction of incobotulinumtoxinA in argentina. Aesthetic Surg. J. 2013, 33, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Carruthers, A.; Carruthers, J.; Fagien, S.; Lei, X.; Kolodziejczyk, J.; Brin, M.F. Repeated OnabotulinumtoxinA Treatment of Glabellar Lines at Rest Over Three Treatment Cycles. Dermatol. Surg. 2016, 42, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Jost, W.H.; Blümel, J.; Grafe, S.; Blümel, J.; Jost, P.W.H. Botulinum Neurotoxin Type A Free of Complexing Proteins (XEOMIN®) in Focal Dystonia. Drugs 2007, 67, 669–683. [Google Scholar] [CrossRef]
- Benecke, R.; Jost, W.H.; Kaňovský, P.; Růžička, E.; Comes, G.; Gräfe, S. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology 2005, 64, 1949–1951. [Google Scholar] [CrossRef] [PubMed]
- Merz Pharma UK Ltd. Xeomin® (incobotulinumtoxinA). Available online: https://www.xeominaesthetic.com/wp-content/uploads/2019/05/XEOMIN-Full-Prescribing-Information-including-MedGuide.pdf (accessed on 14 January 2020).
- Allergan plc. Botox® (onabotulinumtoxinA). Available online: https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20190620-BOTOX-100-and-200-Units-v3-0USPI1145-v2-0MG1145.pdf (accessed on 17 December 2019).
- Ipsen Biopharmaceuticals Inc. Dysport® (abobotulinumtoxinA). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125274s107lbl.pdf (accessed on 14 January 2020).
- Sesardic, D.; Leung, T.; Das, R.G. Role for standards in assays of botulinum toxins: International collaborative study of three preparations of botulinum type A toxin. Boilogicals 2003, 31, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Elmas, I.; Baslo, M.B.; Ertas, M.; Kaya, M. Analysis of gastrocnemius compound muscle action potential in rat after death: Significance for the estimation of early postmortem interval. Forensic Sci. Int. 2001, 116, 125–132. [Google Scholar] [CrossRef]
- Sakamoto, T.; Torii, Y.; Takahashi, M.; Ishida, S.; Goto, Y.; Nakano, H.; Harakawa, T.; Ginnaga, A.; Kozaki, S.; Kaji, R. Quantitative determination of the biological activity of botulinum toxin type A by measuring the compound muscle action potential (CMAP) in rats. Toxicon 2009, 54, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Kang, W.-H.; Rhee, C.-H.; Yang, G.-H.; Cruz, D.J.M. Comparative Pharmacodynamics Study of 3 Different Botulinum Toxin Type A Preparations in Mice. Dermatol. Surg. 2020. [Google Scholar] [CrossRef]
- Pellett, S.; Tepp, W.H.; Whitemarsh, R.C.; Bradshaw, M.; Johnson, E.A. In Vivo onset and duration of action varies for botulinum neurotoxin A subtypes 1-5. Toxicon 2015, 107, 37–42. [Google Scholar] [CrossRef] [Green Version]



| Product | CBPA EC50 (U/mL) (Mean ± SEM) | CBPA Fold-Difference 3 (Mean ± SD) | CMAP ID50 (U/rat) (Mean ± SEM) | CMAP Fold-Difference 4 (Mean ± SD) | DAS ED50 (U/kg) (Mean ± SEM) | DAS Fold-Difference 4 (Mean ± SD) |
|---|---|---|---|---|---|---|
| OnabotA (100 U vial) 1 | 5.3 ± 1.1 | - | 0.043 ± 0.002 | - | 8.4 ± 0.9 | - |
| IncobotA (50 U vial) 1 | 6.6 ± 1.3 * | 1.3 ± 0.1 | 0.075 ± 0.008 * | 1.7 ± 0.2 | 13.1 ± 1.2 * | 1.6 ± 0.4 |
| OnabotA (100 U vial) 1 | 5.9 ± 0.6 | - | 0.063 ± 0.007 | - | 8.6 ± 0.4 | - |
| IncobotA (100 U vial) 1 | 7.4 ± 0.9 * | 1.3 ± 0.1 | 0.087 ± 0.012 | 1.4 ± 0.1 | 13.0 ± 0.8 * | 1.5 ± 0.1 |
| OnabotA (100 U vial) 1 | 6.9 ± 0.7 | - | 0.053 ± 0.005 | - | 8.9 ± 0.3 | - |
| IncobotA (200 U vial) 1 | 9.0 ± 1.3 | 1.3 ± 0.1 | 0.073 ± 0.005 * | 1.4 ± 0.1 | 15.4 ± 0.5 * | 1.7 ± 0.2 |
| Pooled onabotA (100 U vials) 2 | 5.9 ± 0.5 | - | 0.053 ± 0.004 | - | 8.7 ± 0.3 | - |
| Pooled incobotA (50 U, 100 U, and 200 U vials) 2 | 7.6 ± 0.7 | 1.3 | 0.078 ± 0.005 | 1.5 ± 0.3 | 14.2 ± 0.5 | 1.7 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupp, D.; Nicholson, G.; Canty, D.; Wang, J.; Rhéaume, C.; Le, L.; Steward, L.E.; Washburn, M.; Jacky, B.P.; Broide, R.S.; et al. OnabotulinumtoxinA Displays Greater Biological Activity Compared to IncobotulinumtoxinA, Demonstrating Non-Interchangeability in Both In Vitro and In Vivo Assays. Toxins 2020, 12, 393. https://doi.org/10.3390/toxins12060393
Rupp D, Nicholson G, Canty D, Wang J, Rhéaume C, Le L, Steward LE, Washburn M, Jacky BP, Broide RS, et al. OnabotulinumtoxinA Displays Greater Biological Activity Compared to IncobotulinumtoxinA, Demonstrating Non-Interchangeability in Both In Vitro and In Vivo Assays. Toxins. 2020; 12(6):393. https://doi.org/10.3390/toxins12060393
Chicago/Turabian StyleRupp, David, Greg Nicholson, David Canty, Joanne Wang, Catherine Rhéaume, Linh Le, Lance E. Steward, Mark Washburn, Birgitte P. Jacky, Ron S. Broide, and et al. 2020. "OnabotulinumtoxinA Displays Greater Biological Activity Compared to IncobotulinumtoxinA, Demonstrating Non-Interchangeability in Both In Vitro and In Vivo Assays" Toxins 12, no. 6: 393. https://doi.org/10.3390/toxins12060393
APA StyleRupp, D., Nicholson, G., Canty, D., Wang, J., Rhéaume, C., Le, L., Steward, L. E., Washburn, M., Jacky, B. P., Broide, R. S., Philipp-Dormston, W. G., Brin, M. F., & Brideau-Andersen, A. (2020). OnabotulinumtoxinA Displays Greater Biological Activity Compared to IncobotulinumtoxinA, Demonstrating Non-Interchangeability in Both In Vitro and In Vivo Assays. Toxins, 12(6), 393. https://doi.org/10.3390/toxins12060393





