Evaluation of the Efficacy of Mycotoxin Modifiers and Mycotoxin Binders by Using an In Vitro Rumen Model as a First Screening Tool
Abstract
1. Introduction
2. Results and Discussion
- Each mycotoxin binder was mixed into feed. The efficacy of the binders was tested at normal rumen pH (6.8) [18].
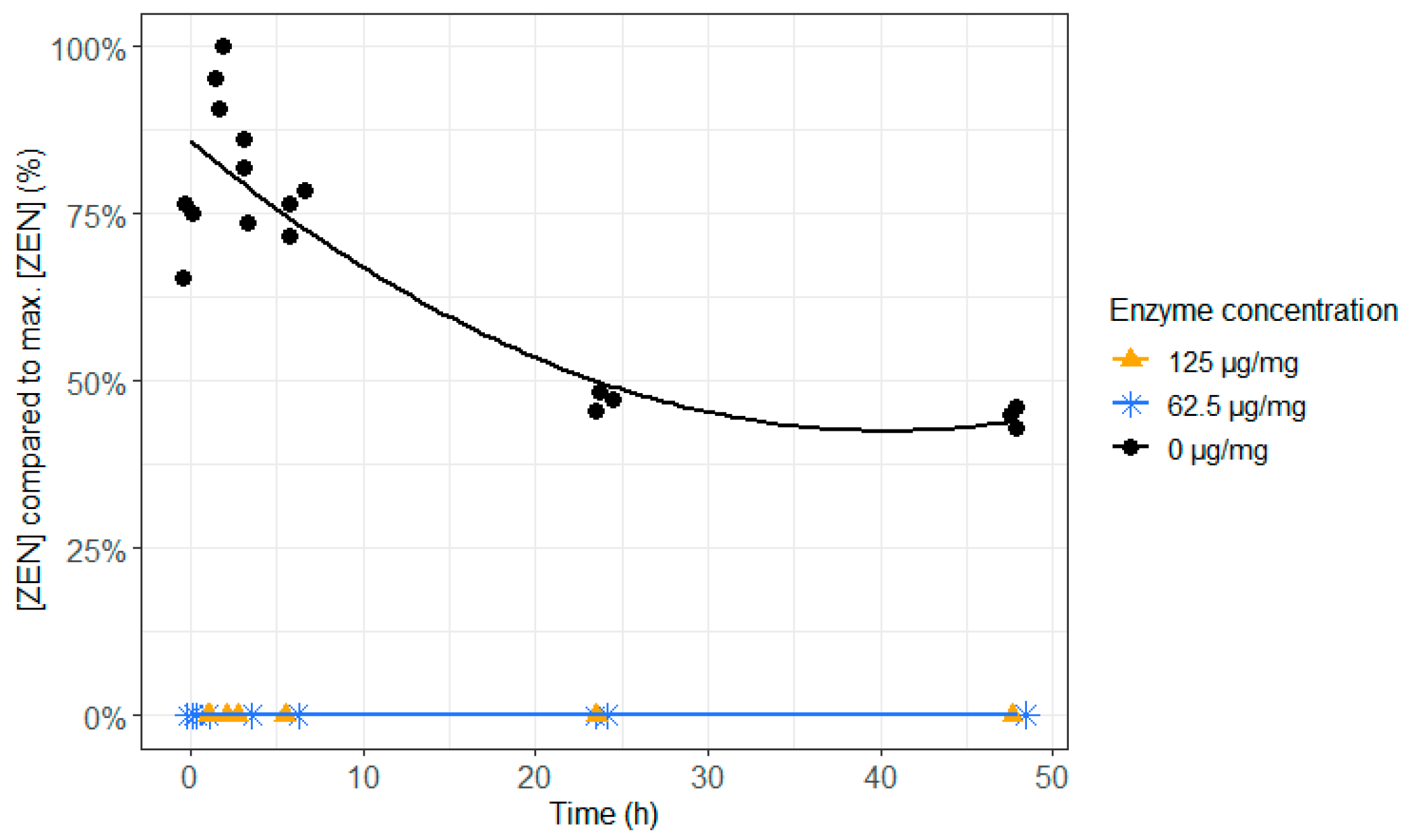
- The enzyme ZenA was added to feed. The efficacy of this mycotoxin biotransforming agent was initially tested at normal rumen pH (6.8).
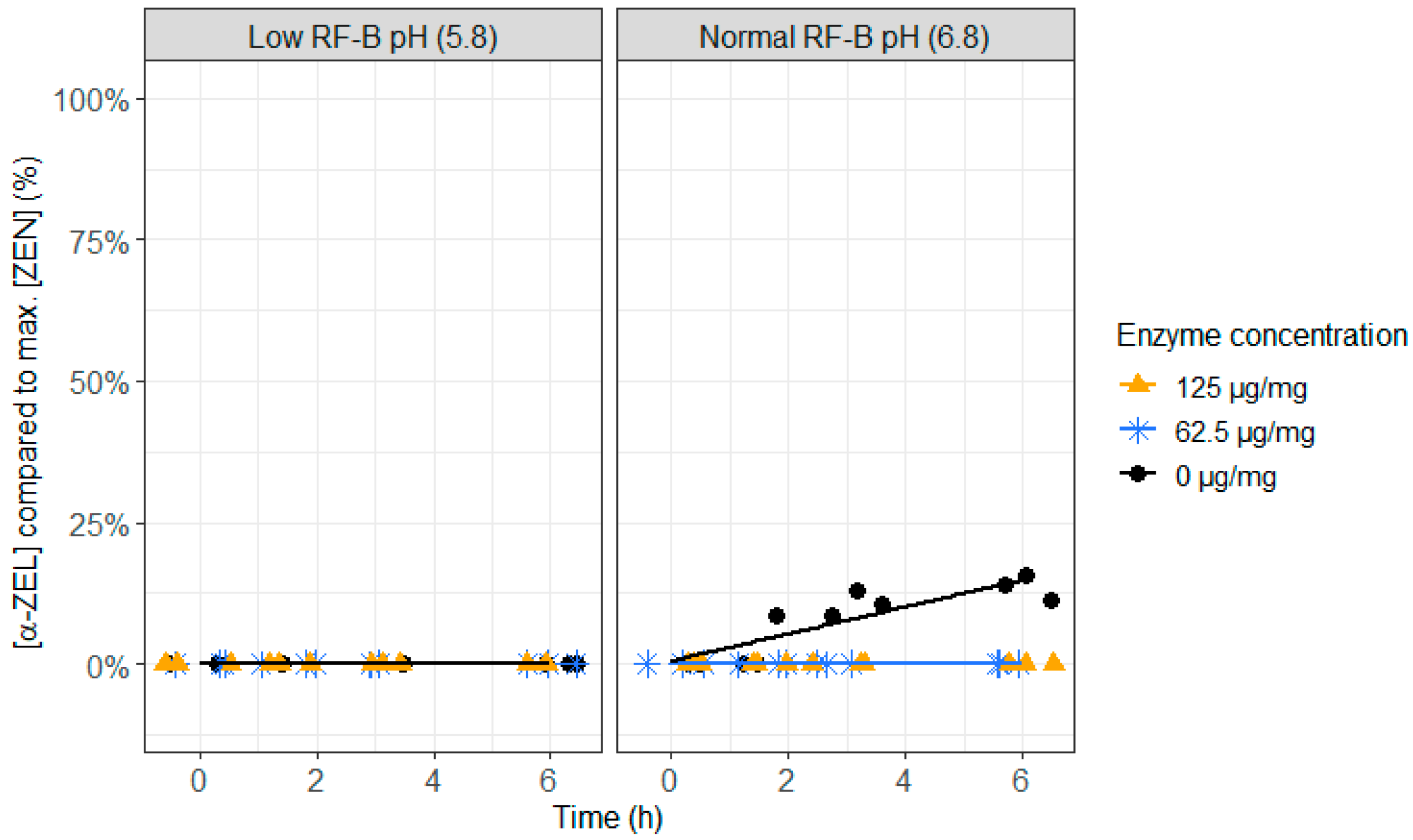
- An additional experiment with ZenA was performed to test the activity of the enzyme at different pH levels. Hence, the enzyme was added to the rumen fluid–buffer mixture at two different pH levels just before incubation: low pH (5.8 [18]) and normal pH (6.8).
- The anaerobic bacterial strain BBSH 797 was added to the rumen fluid–buffer just before incubation as to avoid contact with oxygen. The efficacy of this mycotoxin biotransforming agent was tested at two different pH levels (5.8 and 6.8).
2.1. Binders in Feed, Normal Rumen pH (6.8)
2.2. ZenA in Feed, Normal Rumen pH (6.8)
2.3. ZenA in Rumen Fluid, Normal (6.8) and Low (5.8) Rumen pH
2.4. BBSH 797 in Rumen Fluid, Normal (6.8) and Low (5.8) Rumen pH
3. Conclusions
4. Materials and Methods
4.1. Rumen Fluid, Maize Silage, Mycotoxins, Chemicals, Reagents, and Mycotoxin Detoxifiers
4.2. Standard Solutions and Rumen Fluid–Buffer Mixtures
4.3. In Vitro Rumen Simulation Experiments
4.3.1. Binder in Feed, Normal Rumen pH
4.3.2. ZenA in Feed, Normal Rumen pH
4.3.3. ZenA in Rumen Fluid, Normal and Low pH
4.3.4. BBSH 797 in Rumen Fluid, Normal and Low pH
4.4. Calibrator and Quality Control (QC) Samples Used for Mycotoxin Analysis
4.5. Rumen Fluid Sample Extraction and LC-MS/MS Mycotoxin Analysis
4.6. Data Modeling and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-ZAL | α-zearalanol |
| α-ZEL | α-zearalenol |
| β-ZAL | β-zearalanol |
| β-ZEL | β-zearalenol |
| DHZEN | decarboxylated hydrolyzed zearalenone |
| DOM-1 | deepoxy-deoxynivalenol |
| DON | deoxynivalenol |
| ENN B | enniatin B |
| HZEN | hydrolyzed zearalenone |
| MPA | mycophenolic acid |
| NIV | nivalenol |
| ROQ-C | roquefortine C |
| VFA | volatile fatty acids |
| ZAN | zearalanone |
| ZEN | zearalenone |
References
- Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Te Giffel, M.C. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam. Part B 2008, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Valgaeren, B.; Théron, L.; Croubels, S.; Devreese, M.; De Baere, S.; Van Pamel, E.; Daeseleire, E.; De Boevre, M.; De Saeger, S.; Vidal, A.; et al. The role of roughage provision on the absorption and disposition of the mycotoxin deoxynivalenol and its acetylated derivatives in calves: From field observations to toxicokinetics. Arch. Toxicol. 2018, 93, 293–310. [Google Scholar] [CrossRef]
- Tangni, E.K.; Pussemier, L.; Bastiaanse, H.; Haesaert, G.; Foucart, G.; Van Hove, F. Presence of mycophenolic acid, roquefortine C, citrinin and ochratoxin A in maize and grass silages supplied to dairy cattle in Belgium. J. Anim. Sci. Adv. 2013, 3, 598–612. [Google Scholar]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; te Giffel, M.C. Occurrence of mycotoxins in feedstuffs of dairy cows and estimation of total dietary intakes. J. Dairy Sci. 2008, 91, 4261–4271. [Google Scholar] [CrossRef]
- Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in Flanders’ fields: Occurrence and correlations with Fusarium species in whole-plant harvested maize. Microorganisms 2019, 7, 571. [Google Scholar] [CrossRef]
- Debevere, S.; Cools, A.; Baere, S.D.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. In vitro rumen simulations show a reduced disappearance of deoxynivalenol, nivalenol and enniatin B at conditions of rumen acidosis and lower microbial activity. Toxins 2020, 12, 101. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Bertuzzi, T.; Moschini, M.; Masoero, F. Study of the effects of PR toxin, mycophenolic acid and roquefortine C on in vitro gas production parameters and their stability in the rumen environment. J. Agric. Sci. 2015, 153, 163–176. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarch, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2011, 28, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, A.; Stroka, J. Substances for reduction of the contamination of feed by mycotoxins: A review. World Mycotoxin J. 2011, 4, 225–256. [Google Scholar] [CrossRef]
- AFSSA; CODA-CERVA; INRA Clermont-Ferrand; INRA Toulouse; IRTA; ISPA. Review of mycotoxin-detoxifying agents used as feed additives: Mode of action, efficacy and feed/food safety. EFSA Support. Publ. 2009, 6, E22. [Google Scholar]
- European Food Safety Authority. Statement on the establishment of guidelines for the assessment of additives from the functional group substances for reduction of the contamination of feed by mycotoxins. EFSA J. 2010, 8, 1693. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- AlZahal, O.; Kebreab, E.; France, J.; McBride, B.W. A mathematical approach to prediction biological values from ruminal pH measurements. J. Dairy Sci. 2007, 90, 3777–3785. [Google Scholar] [CrossRef]
- Gallo, A.; Masoero, F. In vitro models to evaluate the capacity of different sequestering agents to adsorb aflatoxins. Ital. J. Anim. Sci. 2010, 9, 109–116. [Google Scholar] [CrossRef]
- Niderkorn, V.; Morgavi, D.P.; Pujos, E.; Tissandier, A.; Boudra, H. Screening of fermentative bacteria for their ability to bind and biotransform deoxynivalenol, zearalenone and fumonisins in an in vitro model simulating corn silage. Food Addit. Contam. 2007, 24, 406–415. [Google Scholar] [CrossRef]
- Peltonen, K.D.; El-Nezami, H.S.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 by probiotic bacteria. J. Sci. Food Agric. 2000, 80, 1942–1945. [Google Scholar] [CrossRef]
- Oatley, J.T.; Rarick, M.D.; Ji, G.E.; Linz, J.E. Binding of aflatoxin B1 to Bifidobacteria in vitro. J. Food Prot. 2000, 63, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Karazhyan, R.; Shaker Sheyda, I.; Mehraban Sang Atash, M.; Tajalli, F.; Mojtahedi, M.; Sadegh, M. Effect of Saccharomyces cerevisiae yeast on ruminal detoxification of aflatoxin B1. J. Vet. Res. 2017, 72, 81–86. [Google Scholar]
- El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 1998, 61, 466–468. [Google Scholar] [CrossRef] [PubMed]
- El-Nezami, H.; Polychronaki, N.; Salminen, S.; Mykkänen, H. Binding rather than metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative α-zearalenol. Appl. Environ. Microbiol. 2002, 68, 3545–3549. [Google Scholar] [CrossRef]
- El-Nezami, H.; Polychronaki, N.; Lee, Y.K.; Haskard, C.; Juvonen, R.; Salminen, S.; Mykkänen, H. Chemical moieties and interactions involved in the binding of zearalenone to the surface of Lactobacillus rhamnosus strains GG. J. Agric. Food Chem. 2004, 52, 4577–4581. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Liu, S.; Zhao, X.J.; Wang, N.; Jiang, X.; Xin, H.S.; Zhang, Y.G. Lactobacillus rhamnosus GG modulates gastrointestinal absorption, excretion patterns, and toxicity in Holstein calves fed a single dose of aflatoxin B1. J. Dairy Sci. 2018, 102, 1330–1340. [Google Scholar] [CrossRef]
- Debevere, S.; De Baere, S.; Haesaert, G.; Rychlik, M.; Fievez, V.; Croubels, S. In vitro rumen simulation shows detoxification of the mycotoxin deoxynivalenol and activation of the mycotoxin zearalenone, but also adhesion of mycotoxins to maize silage. In Proceedings of the 43rd Animal Nutrition Research Forum, Wageningen, The Netherlands, 11 April 2018; pp. 41–42. [Google Scholar]
- Debevere, S.; De Baere, S.; Haesaert, G.; Rychlik, M.; Fievez, V.; Croubels, S. Development of an UPLC-MS/MS method for the analysis of mycotoxins in rumen fluid with and without maize silage emphasizes the importance of using matrix-matched calibration. Toxins 2019, 11, 519. [Google Scholar] [CrossRef]
- Jouany, J.-P.; Yiannikouris, A.; Bertin, G. The chemical bonds between mycotoxins and cell wall components of Saccharomyces cerevisiae have been identified. Arch. Zootech. 2005, 8, 26–50. [Google Scholar]
- Joannis-Cassan, C.; Tozlovanu, M.; Hadjeba-Medjdoub, K.; Ballet, N.; Pfohl-Leszkowicz, A. Binding of zearalenone, aflatoxin B1, and ochratoxin a by yeast-based products: A method for quantification of adsorption performance. J. Food Prot. 2011, 74, 1175–1185. [Google Scholar] [CrossRef]
- Lauwers, M.; Croubels, S.; Letor, B.; Gougoulias, C.; Devreese, M. Biomarkers for exposure as a tool for efficacy testing of a mycotoxin detoxifier in broiler chickens and pigs. Toxins 2019, 11, 187. [Google Scholar] [CrossRef]
- Ramos, A.J.; Hernández, E.; Plá-Delfina, J.M.; Merino, M. Intestinal absorption of zearalenone and in vitro study of non-nutritive sorbent materials. Int. J. Pharm. 1996, 128, 129–137. [Google Scholar] [CrossRef]
- Schoeters, E.; Li, Z.; Dyck, S.M.O.V.; Lao, Y. Mycotoxin binder. U.S. Patent No. 8,507,019 B2, 13 August 2013. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on the safety and efficacy of a preparation of bentonite-and sepiolite (Toxfin ® Dry) as feed additive for all species. EFSA J. 2013, 11, 3179. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated carbon and other adsorbent materials. Food Chem. Toxicol. 2004, 42, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Schatzmayr, G.; Zehner, F.; Täubel, M.; Schatzmayr, D.; Klimitsch, A.; Loibner, A.P.; Binder, E.M. Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 2006, 50, 543–551. [Google Scholar] [CrossRef]
- Osselaere, A.; Santos, R.; Hautekiet, V.; De Backer, P.; Chiers, K.; Ducatelle, R.; Croubels, S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- De Baere, S.; De Mil, T.; Antonissen, G.; Devreese, M.; Croubels, S. In vitro model to assess the adsorption of oral veterinary drugs to mycotoxin binders in a feed- and aflatoxin B1-containing buffered matrix. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2018, 35, 1728–1738. [Google Scholar] [CrossRef]
- European Food Safety Authority. Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar]
- Vekiru, E.; Hametner, C.; Mitterbauer, R.; Rechthaler, J.; Adam, G.; Schatzmayr, G.; Krska, R.; Schuhmacher, R. Cleavage of zearalenone by trichosporon mycotoxinivorans to a novel nonestrogenic metabolite. Appl. Environ. Microbiol. 2010, 76, 2353–2359. [Google Scholar] [CrossRef]
- Vekiru, E.; Fruhauf, S.; Hametner, C.; Schatzmayr, G.; Krska, R.; Moll, W.D.; Schuhmacher, R. Isolation and characterisation of enzymatic zearalenone hydrolysis reaction products. World Mycotoxin J. 2016, 9, 353–363. [Google Scholar] [CrossRef]
- Fruhauf, S.; Novak, B.; Nagl, V.; Hackl, M.; Hartinger, D.; Rainer, V.; Labudov, S.; Adam, G.; Aleschko, M.; Moll, W.; et al. Biotransformation of the mycotoxin zearalenone to its metabolites hydrolyzed aearalenone (HZEN) and decarboxylated hydrolyzed zearalenone (DHZEN) diminishes its estrogenicity in vitro and in vivo. Toxins 2019, 11, 481. [Google Scholar] [CrossRef]
- Fuchs, E.; Binder, E.M.; Heidler, D.; Krska, R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 2002, 19, 379–386. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the safety of the product “Biomin BBSH 797” for piglets, pigs for fattening and chickens for fattening. EFSA J. 2005, 3, 169. [Google Scholar] [CrossRef]
- European Commission. Commission implementing regulation (EU) No 1016/2013 of 23 October 2013 concerning the authorisation of a preparation of a micro-organism strain DSM 11798 of the Coriobacteriaceae family as a feed additive for pigs. Off. J. Eur. Union 2013, L282, 36–38. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2017/930 of 31 May 2017 concerning the authorisation of a preparation of a microorganism strain DSM 11798 of the Coriobacteriaceae family as a feed additive for all avian species and amending Implementing Regulation. Off. J. Eur. Union 2017, L141, 6–9. [Google Scholar]
- Jenkins, T. Mycotoxin Impacts on Ruminants. Available online: https://www2.biomin.net/us/blog-posts/mycotoxin-impacts-on-ruminants/ (accessed on 25 February 2020).
- Julien, C.; Troegeler-Meynadier, A.; Marden, J.P.; Enjalbert, F.; Bayourthe, C. In vivo and in vitro measurements of ruminal redox potential: A comparative study. In Proceedings of the ADSA/ASAS Joint Annual Meeting, Montréal, QC, Canada, 12 July 2009. [Google Scholar]
- Hu, L.; Rychlik, M. Biosynthesis of 15N3-labeled enniatins and beauvericin and their application to stable isotope dilution assays. J. Agric. Food Chem. 2012, 60, 7129–7136. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Recommendation No 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Wambacq, E.; Vanhoutte, I.; Audenaert, K.; De Gelder, L.; Haesaert, G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J. Sci. Food Agric. 2016, 96, 2284–2302. [Google Scholar] [CrossRef]
- European Commission. Commission Decision 2002/657/EC implementing Council Directive 96/23/EC concerning the performances of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–36. [Google Scholar]
- Knecht, J.; Stork, G. Prozentuales und logarithmisches verfahren zur berechnung von eichkurven. Z. Anal. Chem. 1974, 270, 97–98. [Google Scholar] [CrossRef]
- Heitzman, R.J. Veterinary Drug Residues. Residues in Food Producing Animals and Their Products: Reference Materials and Methods, 2nd ed.; Heitzman, R.J., Ed.; CEC: Luxembourg, 1992; ISBN 92-826-4095-7. [Google Scholar]
- Committee for Medicinal Products for Veterinary Use (CVMP). VICH Topic GL49: Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies; Committee for Medicinal Products for Veterinary Use (CVMP): London, UK, 2015. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference, Version 1.43.6. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 17 June 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]








| Mycotoxin | Binder 1 | Binder 2 | Binder 3 |
|---|---|---|---|
| ENN B | + | + | + |
| ROQ-C | + | No | No |
| MPA | No | No | No |
| DON | No | No | No |
| DOM-1 | No | No | No |
| NIV | No | No | No |
| ZEN | No | No | No |
| Diet Components (kg) | Study 1 (Lactating Cow n°) | Study 2 (Lactating Cow n°) | Study 3 & 4 (Dry Cow n°) | |||||
|---|---|---|---|---|---|---|---|---|
| n° 1 | n° 2 | n° 3 | n° 1 | n° 2 | n° 3 | n° 1 | n° 2 | |
| Maize silage | 22.62 | 22.62 | 21.3 | 22.62 | 21.3 | 21.3 | 17.3 | 17.3 |
| Grass silage | 18.06 | 18.06 | 14.5 | 18.06 | 14.5 | 14.5 | 20.1 | 20.1 |
| Maize meal | 0.85 | 0.85 | 2.0 | 0.85 | 2.0 | 2.0 | 0.9 | 0.9 |
| Sugar beet pulp | / | / | 8.7 | / | 8.7 | 8.7 | 7.2 | 7.2 |
| Straw | / | / | 0.3 | / | 0.3 | 0.3 | 0.2 | 0.2 |
| Soybean meal | / | / | 1.4 | / | 1.4 | 1.4 | 1.7 | 1.7 |
| Crushed barley | / | / | 0.2 | / | 0.2 | 0.2 | 0.24 | 0.24 |
| Sodium bicarbonate | / | / | 0.2 | / | 0.2 | 0.2 | 0.2 | 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debevere, S.; Schatzmayr, D.; Reisinger, N.; Aleschko, M.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. Evaluation of the Efficacy of Mycotoxin Modifiers and Mycotoxin Binders by Using an In Vitro Rumen Model as a First Screening Tool. Toxins 2020, 12, 405. https://doi.org/10.3390/toxins12060405
Debevere S, Schatzmayr D, Reisinger N, Aleschko M, Haesaert G, Rychlik M, Croubels S, Fievez V. Evaluation of the Efficacy of Mycotoxin Modifiers and Mycotoxin Binders by Using an In Vitro Rumen Model as a First Screening Tool. Toxins. 2020; 12(6):405. https://doi.org/10.3390/toxins12060405
Chicago/Turabian StyleDebevere, Sandra, Dian Schatzmayr, Nicole Reisinger, Markus Aleschko, Geert Haesaert, Michael Rychlik, Siska Croubels, and Veerle Fievez. 2020. "Evaluation of the Efficacy of Mycotoxin Modifiers and Mycotoxin Binders by Using an In Vitro Rumen Model as a First Screening Tool" Toxins 12, no. 6: 405. https://doi.org/10.3390/toxins12060405
APA StyleDebevere, S., Schatzmayr, D., Reisinger, N., Aleschko, M., Haesaert, G., Rychlik, M., Croubels, S., & Fievez, V. (2020). Evaluation of the Efficacy of Mycotoxin Modifiers and Mycotoxin Binders by Using an In Vitro Rumen Model as a First Screening Tool. Toxins, 12(6), 405. https://doi.org/10.3390/toxins12060405






