Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.)
Abstract
1. Introduction
2. Results
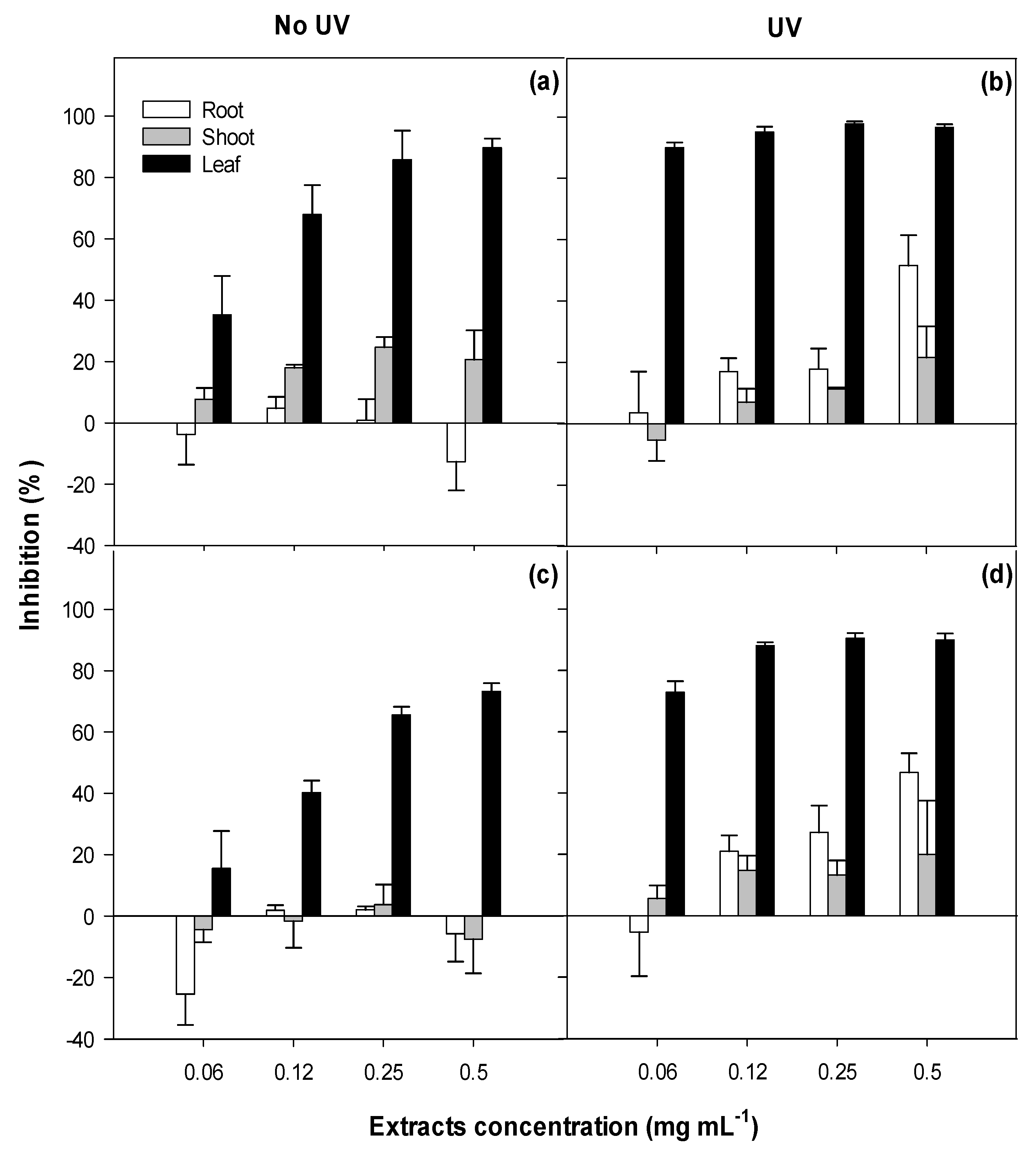
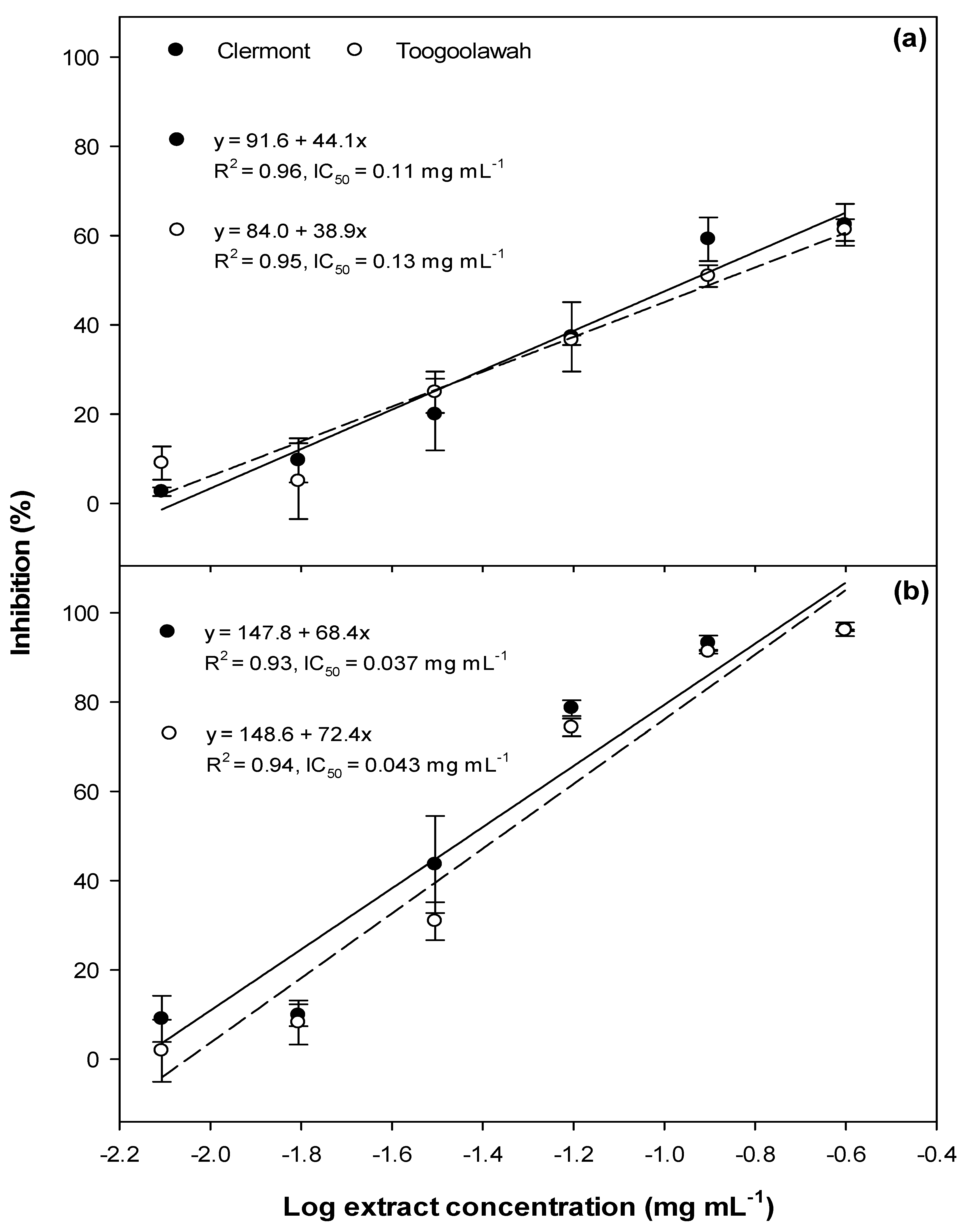
2.1. Phytotoxicity
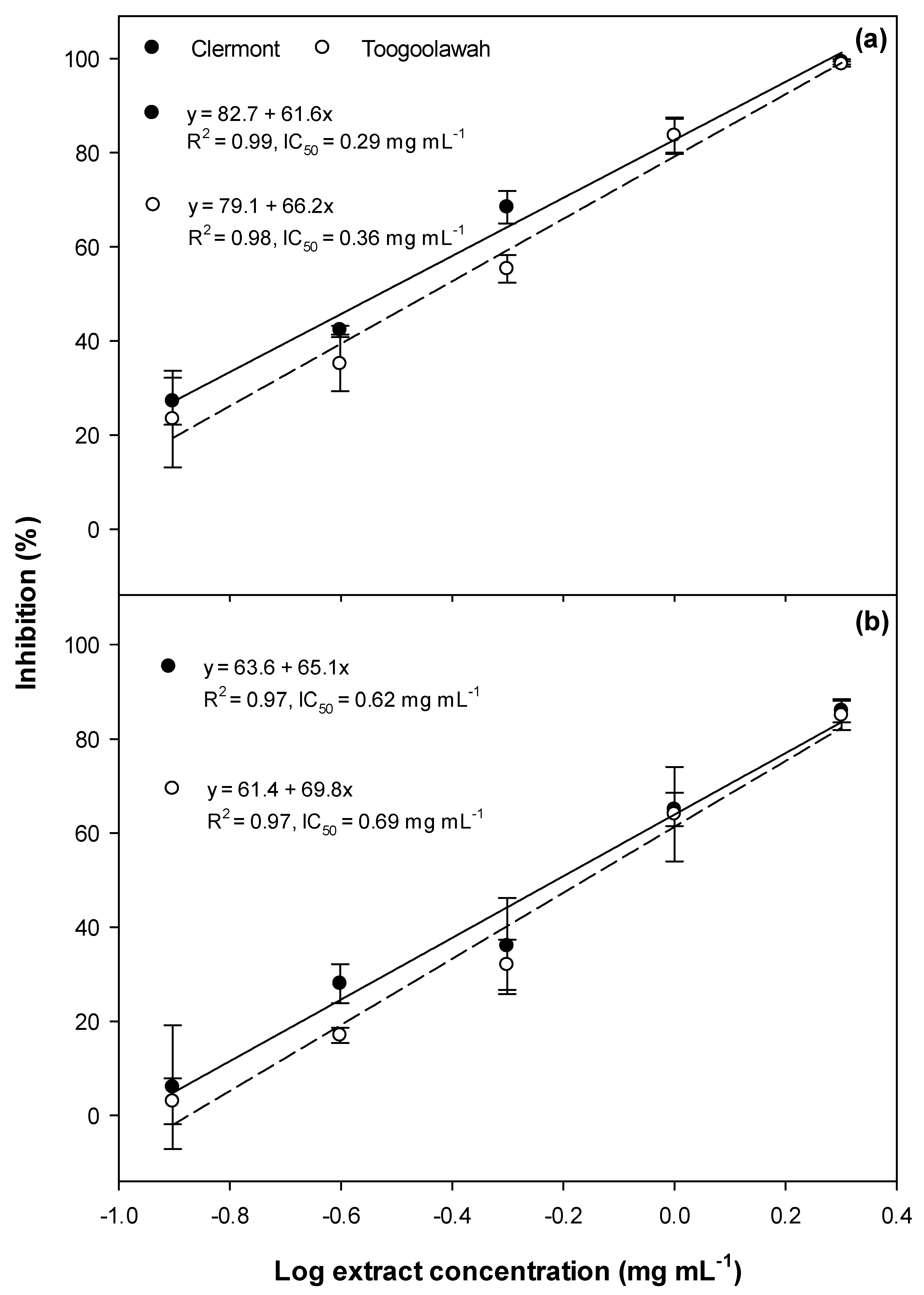
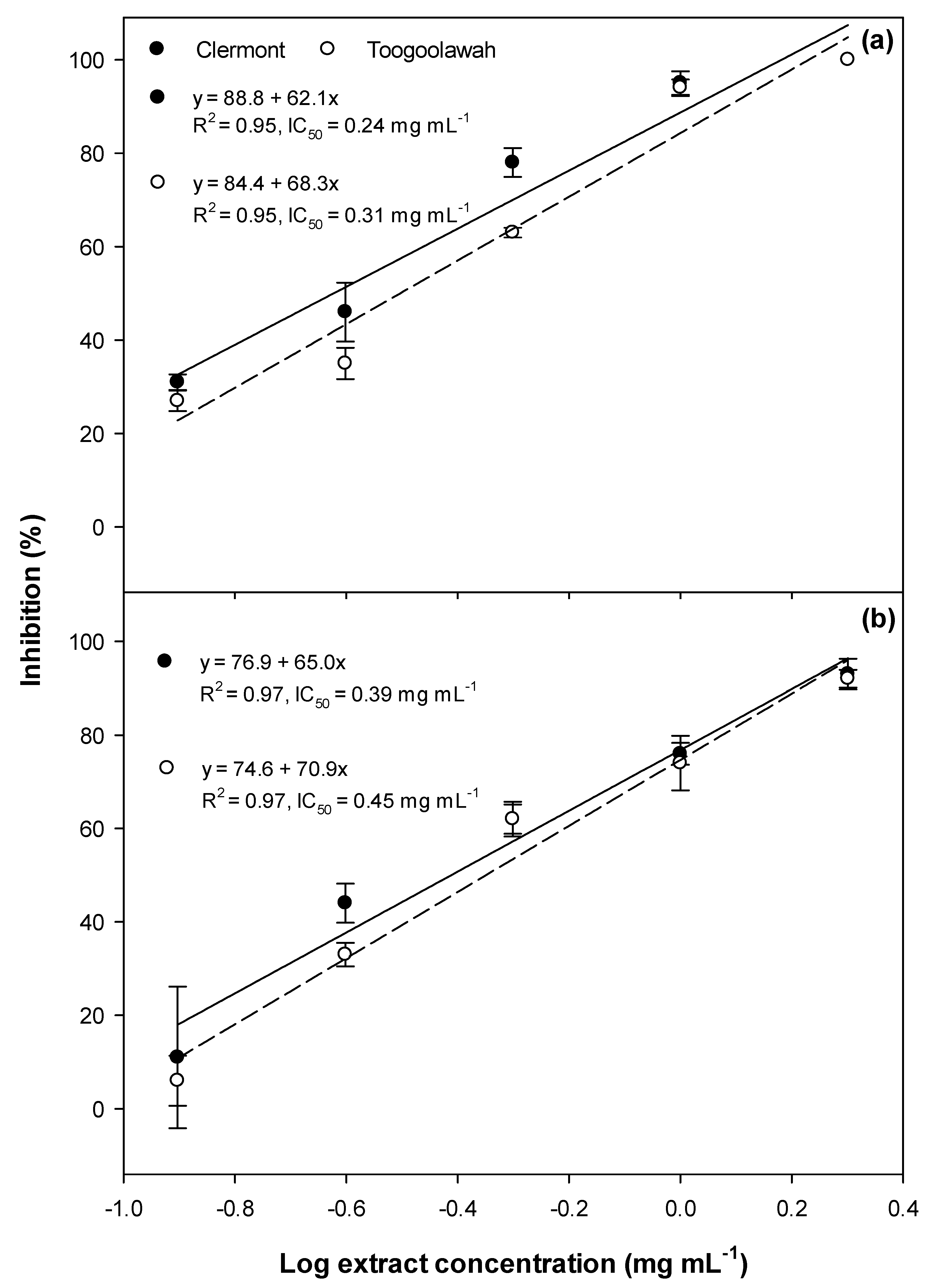
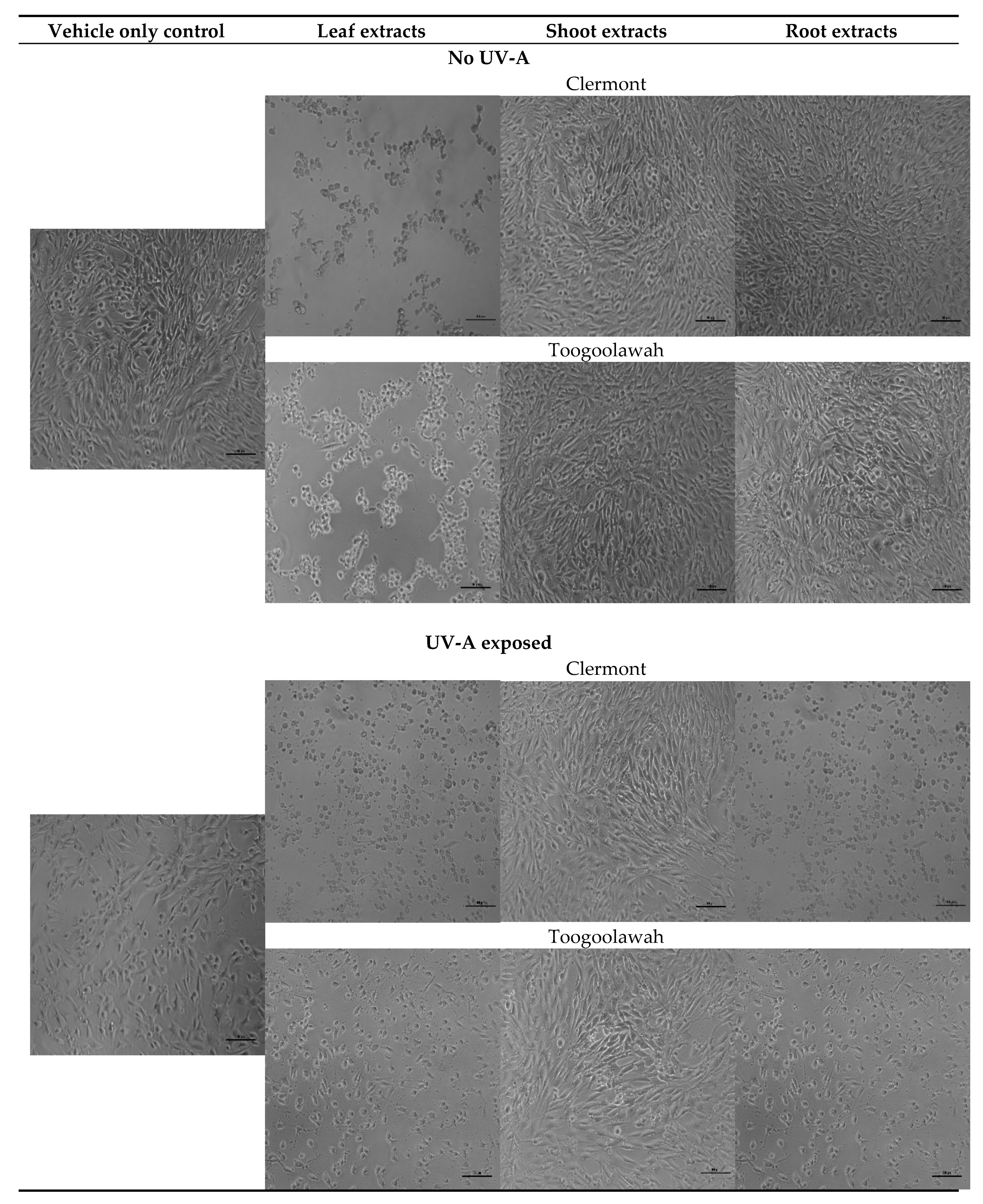
2.2. Cytotoxicity and Photocytotoxicity
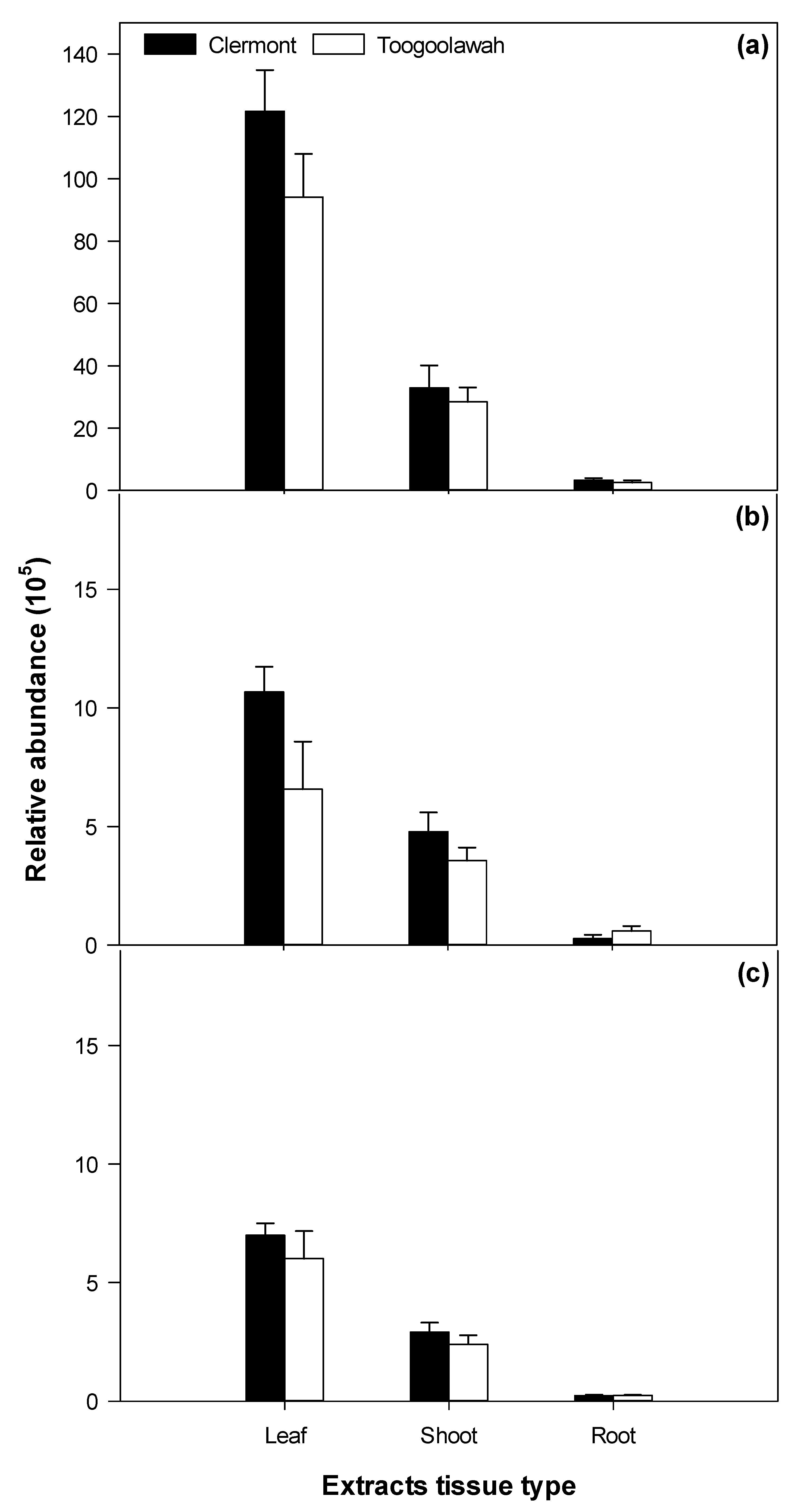
2.3. Quantitation of Major Known Secondary Metabolites
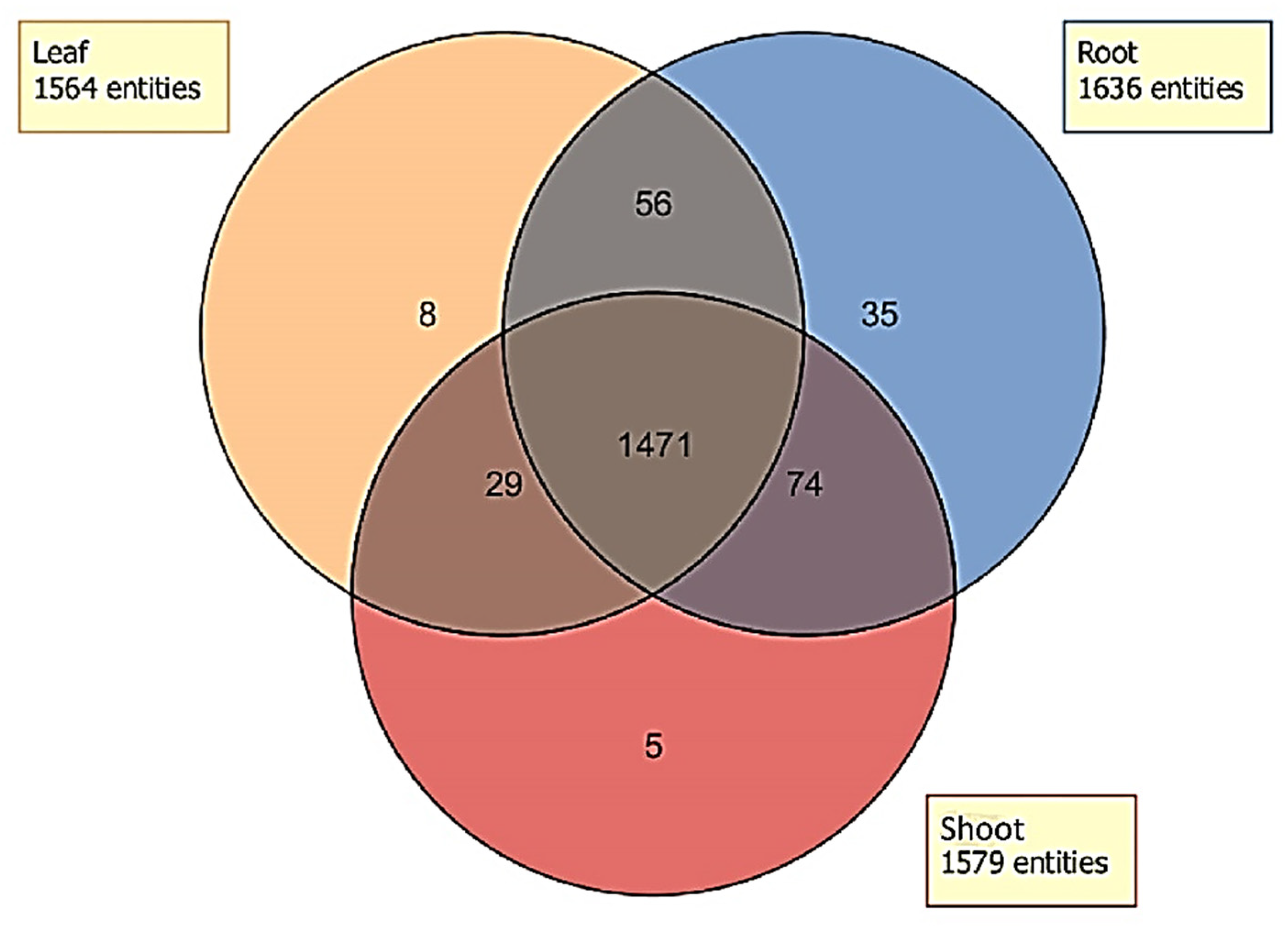
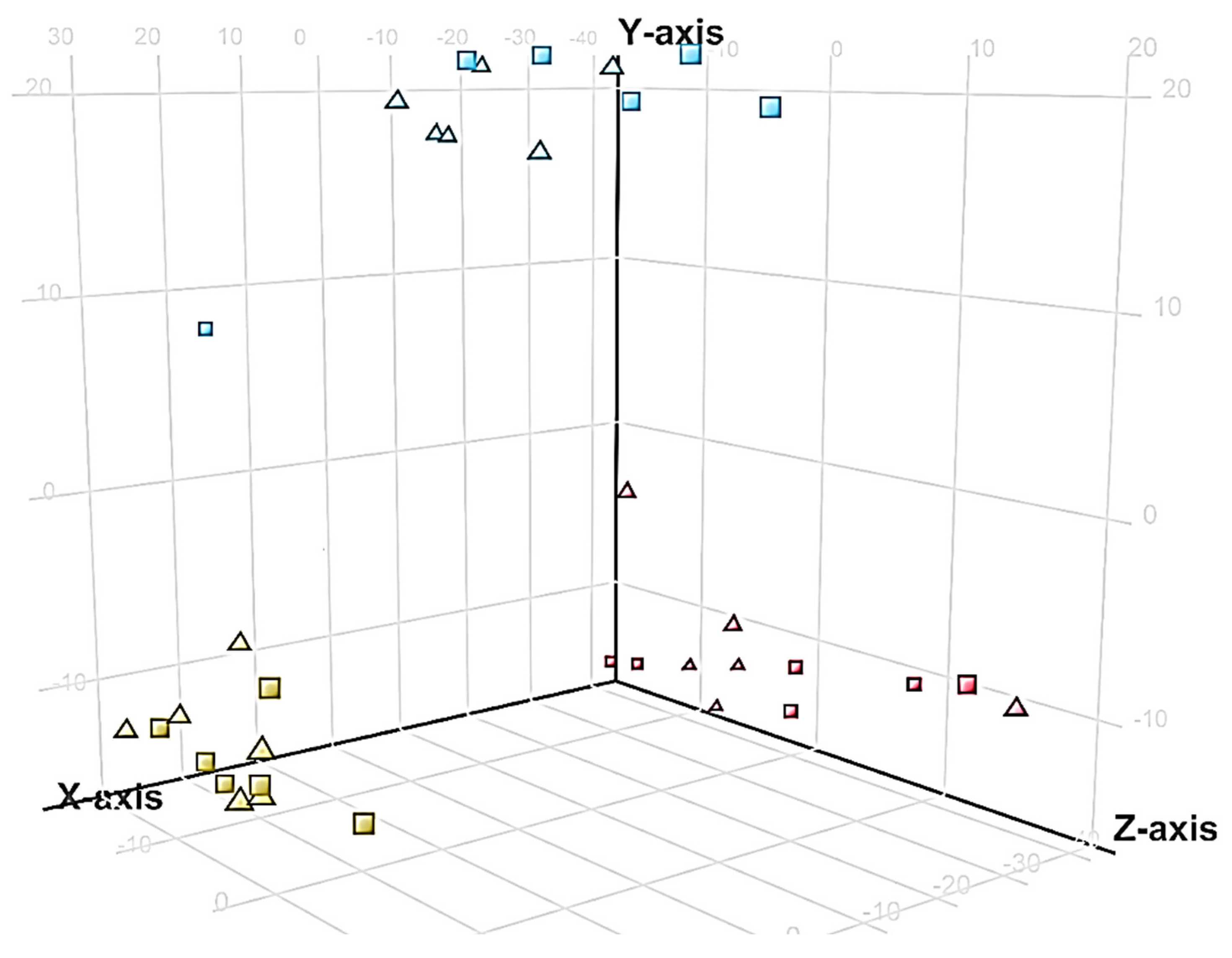
2.4. Distribution of Unidentified Molecular Entities
2.5. Associations between Compound Abundance and Toxicity
3. Discussion
3.1. Phytotoxicity/Allelopathy
3.2. Cytotoxicity and Photocytotoxicity
3.3. Biotype Differences
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Evaluation of Phytotoxicity
4.4. Evaluation of Cytotoxicity and Photocytotoxicity
4.5. Metabolic Profiling
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adkins, S.W.; McClay, A.; Bajwa, A.A.; Shabbir, A.; Dhileepan, K. Biology and ecology. In Parthenium Weed: Biology, Ecology and Management; CABI: Oxfordshire, UK, 2018; pp. 7–39. [Google Scholar]
- Adkins, S.; Shabbir, A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag. Sci. 2014, 70, 1023–1029. [Google Scholar]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Chippendale, J.; Panetta, F. The cost of parthenium weed to the Queensland cattle industry. Plant Prot. Q. 1994, 9, 73–76. [Google Scholar]
- Singh, H.; Batish, D.; Pandher, J.; Kohli, R. Assessment of allelopathic properties of Parthenium hysterophorus residues. Agric. Ecosyst. Environ. 2003, 95, 537–541. [Google Scholar] [CrossRef]
- Shi, B.; Adkins, S. Relative phytotoxicity of parthenium weed (Parthenium hysterophorus L.) residues on the seedling growth of a range of Australian native and introduced species. Crop Pasture Sci. 2018, 69, 837–845. [Google Scholar]
- Mersie, W.; Singh, M. Allelopathic effect of parthenium (Parthenium hysterophorus L.) extract and residue on some agronomic crops and weeds. J. Chem. Ecol. 1987, 13, 1739–1747. [Google Scholar]
- Kanchan, S.; Chandra, J. Pollen allelopathy—A new phenomenon. New Phytol. 1980, 84, 739–746. [Google Scholar] [CrossRef]
- Belgeri, A.; Adkins, S. Allelopathic potential of invasive parthenium weed (Parthenium hysterophorus L.) seedlings on grassland species in Australia. Allelopath. J. 2015, 36, 1–14. [Google Scholar]
- Batish, D.R.; Singh, H.P.; Pandher, J.K.; Arora, V.; Kohli, R.K. Phytotoxic effect of Parthenium residues on the selected soil properties and growth of chickpea and radish. Weed Biol. Manag. 2002, 2, 73–78. [Google Scholar] [CrossRef]
- Pandey, D. Inhibition of salvinia (Salvinia molesta Mitchell) by parthenium (Parthenium hysterophorus L.). II. Relative effect of flower, leaf, stem, and root residue on salvinia and paddy. J. Chem. Ecol. 1994, 20, 3123–3131. [Google Scholar]
- Ahmad, J.; Bagheri, R.; Bashir, H.; Baig, M.A.; Al-Huqail, A.; Ibrahim, M.M.; Qureshi, M.I. Organ-specific phytochemical profiling and antioxidant analysis of Parthenium hysterophorus L. Biomed. Res. Int. 2018, 2018, 9535232. [Google Scholar] [CrossRef]
- Belz, R.G.; Reinhardt, C.F.; Foxcroft, L.C.; Hurle, K. Residue allelopathy in Parthenium hysterophorus L.—Does parthenin play a leading role? Crop Prot. 2007, 26, 237–245. [Google Scholar] [CrossRef]
- Belz, R.G.; van der Laan, M.; Reinhardt, C.F.; Hurle, K. Soil degradation of parthenin—Does it contradict the role of allelopathy in the invasive weed Parthenium hysterophorus L.? J. Chem. Ecol. 2009, 35, 1137–1150. [Google Scholar] [CrossRef]
- Rodriguez, E.; Dillon, M.; Mabry, T.; Mitchell, J.; Towers, G. Dermatologically active sesquiterpene lactones in trichomes of Parthenium hysterophorus L. (Compositae). Experientia 1976, 32, 236–238. [Google Scholar]
- Picman, A.; Towers, G.; Subba Rao, P. Coronopilin--another major sesquiterpene lactone in Parthenium hysterophorus. Phytochemistry 1980, 10, 145–153. [Google Scholar] [CrossRef]
- De la Fuente, J.R.; Uriburu, M.; Burton, G.; Sosa, V.E. Sesquiterpene lactone variability in Parthenium hysterophorus L. Phytochemistry 2000, 55, 769–772. [Google Scholar] [CrossRef]
- Das, B.; Das, R. Chemical investigation in Parthenium hysterophorus L.—An allelopathic plant. Allelopath. J. 1995, 2, 99–104. [Google Scholar]
- Shen, M.; Rodriguez, E.; Kerr, K.; Mabry, T. Flavonoids of four species of Parthenium (compositae). Phytochemistry 1976, 15, 1045–1047. [Google Scholar] [CrossRef]
- Allan, S.; Shi, B.; Adkins, S.W. Impact of Parthenium Weed on Human and Animal Health. In Parthenium Weed: Biology, Ecology and Management; CABI: Oxfordshire, UK, 2018; Volume 7, pp. 105–128. [Google Scholar]
- Tudor, G.; Ford, A.L.; Armstrong, T.; Bromage, E. Taints in meat from sheep grazing Parthenium hysterophorus. Aust. J. Exp. Agric. 1982, 22, 43–46. [Google Scholar] [CrossRef]
- Narasimhan, T.R.; Ananth, M.; Swamy, M.N.; Babu, M.R.; Mangala, A.; Subba Rao, P. Toxicity of Parthenium hysterophorus L. to cattle and buffaloes. Experientia 1977, 33, 1358–1359. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, A.A.; Chauhan, B.S.; Adkins, S. Morphological, physiological and biochemical responses of two Australian biotypes of Parthenium hysterophorus to different soil moisture regimes. Environ. Sci. Pollut. Res. 2017, 24, 16186–16194. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Chauhan, B.S.; Adkins, S.W. Germination ecology of two Australian biotypes of ragweed parthenium (Parthenium hysterophorus) relates to their invasiveness. Weed Sci. 2018, 66, 62–70. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Zhu, X.; Chauhan, B.S.; Adkins, S.W.; Weston, L.A. Screening of gene regions for genetic diversity in global parthenium weed (Parthenium hysterophorus L.) populations. In Proceedings of the 21st Australasian Weeds Conference “Weed Biosecurity-Protecting our Future”, Sydney, NSW, Australia, 9–13 September 2018; pp. 318–321. [Google Scholar]
- Weston, L.A.; Skoneczny, D.; Weston, P.A.; Weidenhamer, J.D. Metabolic profiling: An overview—New approaches for the detection and functional analysis of biologically active secondary plant products. J. Allelochem. Interact. 2015, 1, 15–27. [Google Scholar]
- Zhu, X.; Skoneczny, D.; Weidenhamer, J.D.; Mwendwa, J.M.; Weston, P.A.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Identification and localization of bioactive naphthoquinones in the roots and rhizosphere of Paterson’s curse (Echium plantagineum), a noxious invader. J. Exp. Bot. 2016, 67, 3777–3788. [Google Scholar] [CrossRef]
- Skoneczny, D.; Weston, P.A.; Zhu, X.; Gurr, G.M.; Callaway, R.M.; Barrow, R.A.; Weston, L.A. Metabolic profiling and identification of shikonins in root periderm of two invasive Echium spp. weeds in Australia. Molecules 2017, 22, 330–348. [Google Scholar] [CrossRef]
- Quinn, J.C.; Kessell, A.; Weston, L.A. Secondary plant products causing photosensitization in grazing herbivores: Their structure, activity and regulation. Int. J. Mol. Sci. 2014, 15, 1441–1465. [Google Scholar] [CrossRef]
- Reinhardt, C.; Kraus, S.; Walker, F.; Foxcroft, L.; Robbertse, P.; Hurle, K. The allelochemical parthenin is sequestered at high level in capitate-sessile trichomes on leaf surfaces of Parthenium hysterophorus. J. Plant Dis. Prot. 2004, 19, 253–261. [Google Scholar]
- Aslam, F.; Khaliq, A.; Matloob, A.; Abbas, R.; Hussain, S.; Rasul, F. Differential allelopathic activity of Parthenium hysterophorus L. against canary grass and wild oat. J. Anim. Plant Sci. 2014, 24, 234–244. [Google Scholar]
- Weston, L.A. Are laboratory bioassays for allelopathy suitable for prediction of field responses? J. Chem. Ecol. 2000, 26, 2111–2118. [Google Scholar] [CrossRef]
- Reinhardt, C.; Van der Laan, M.; Belz, R.; Hurle, K.; Foxcroft, L. Production dynamics of the allelochemical parthenin in leaves of Parthenium hysterophorus L. J. Plant Dis. Prot. 2006, 20, 427–433. [Google Scholar]
- Saad, M.M.; Abdelgaleil, S.A.; Suganuma, T. Herbicidal potential of pseudoguaninolide sesquiterpenes on wild oat, Avena fatua L. Biochem. Syst. Ecol. 2012, 44, 333–337. [Google Scholar] [CrossRef]
- Padilla-Gonzalez, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene lactones: More than protective plant compounds with high toxicity. Crit. Rev. Plant Sci. 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Macías, F.A.; Fernández, A.; Varela, R.M.; Molinillo, J.M.; Torres, A.; Alves, P.L. Sesquiterpene lactones as allelochemicals. J. Nat. Prod. 2006, 69, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Hernández, A.; Allen, S.N.; Moraes, R.M.; Vroman, J.A.; Avery, M.A.; Duke, S.O. Comparative phytotoxicity of artemisinin and several sesquiterpene analogues. Phytochemistry 1999, 50, 607–614. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.; Kohli, R.; Saxena, D.; Kaur, S. Allelopathic effects of parthenin against two weedy species, Avena fatua and Bidens pilosa. Environ. Exp. Bot. 2002, 47, 149–155. [Google Scholar] [CrossRef]
- Nimbal, C.I.; Pedersen, J.F.; Yerkes, C.N.; Weston, L.A.; Weller, S.C. Phytotoxicity and distribution of sorgoleone in grain sorghum germplasm. J. Agric. Food Chem. 1996, 44, 1343–1347. [Google Scholar] [CrossRef]
- Bertin, C.; Harmon, R.; Akaogi, M.; Weidenhamer, J.D.; Weston, L.A. Assessment of the phytotoxic potential of m-tyrosine in laboratory soil bioassays. J. Chem. Ecol. 2009, 35, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Raghava, N.; Raghava, R.P.; Singh, L.; Srivastava, J. Role of allelopathy in sustainable agriculture-with special reference to parthenium. Plant Stress Toler. Physiol. Mol. Strateg. 2016, 16, 391–467. [Google Scholar]
- Liu, J.; Li, D.; Wang, D.; Liu, Y.; Song, H. Allelopathic effects, physiological responses and phenolic compounds in litter extracts of Juniperus rigidasieb. Et zucc. Chem. Biodivers. 2017, 14, e1700088. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Inoue, M.; Nishimura, H.; Mizutani, J.; Tsuzuki, E. Interactions oftrans-cinnamic acid, its related phenolic allelochemicals, and abscisic acid in seedling growth and seed germination of lettuce. J. Chem. Ecol. 1993, 19, 1775–1787. [Google Scholar] [CrossRef]
- Khaliq, A.; Aslam, F.; Matloob, A.; Hussain, S.; Tanveer, A.; Alsaadawi, I.; Geng, M. Residual phytotoxicity of parthenium: Impact on some winter crops, weeds and soil properties. Ecotoxicol. Environ. Saf. 2015, 122, 352–359. [Google Scholar] [CrossRef]
- Datta, S.; Saxena, D.B. Pesticidal properties of parthenin (from Parthenium hysterophorus) and related compounds. Pest Manag. Sci. 2001, 57, 95–101. [Google Scholar] [CrossRef]
- Subba Rao, P.; Mangala, A.; Rao, B.S.; Prakash, K. Clinical and immunological studies on persons exposed to Parthenium hysterophorus L. Experientia 1977, 33, 1387–1388. [Google Scholar]
- Narasimhan, T.; Harindranath, N.; Premlata, S.; Murthy, B.K.; Subba Rao, P. Toxicity of the sesquiterpene lactone parthenin to cultured bovine kidney cells. Planta Med. 1985, 51, 194–197. [Google Scholar] [CrossRef]
- Ramesh, C.; Ravindranath, N.; Das, B.; Prabhakar, A.; Bharatam, J.; Ravikumar, K.; Kashinatham, A.; McMorris, T. Pseudoguaianolides from the flowers of Parthenium hysterophorus. Phytochemistry 2003, 64, 841–844. [Google Scholar] [CrossRef]
- Das, B.; Reddy, V.S.; Krishnaiah, M.; Sharma, A.; Kumar, K.R.; Rao, J.V.; Sridhar, V. Acetylated pseudoguaianolides from Parthenium hysterophorus and their cytotoxic activity. Phytochemistry 2007, 68, 2029–2034. [Google Scholar] [CrossRef]
- Shi, B. Invasive Potential of the Weed Parthenium hysterophorus: The Role of Allelopathy. Ph.D. Thesis, School of Agriculture and Food Sciences, The University of Queensland, Brisbane, Australia, 2016. [Google Scholar] [CrossRef]
- Picman, A.K.; Towers, G. Sesquiterpene lactones in various populations of Parthenium hysterophorus. Biochem. Syst. Ecol. 1982, 10, 145–153. [Google Scholar] [CrossRef]
- Latif, S.; Gurusinghe, S.; Weston, P.A.; Quinn, J.C.; Piltz, J.W.; Weston, L.A. Metabolomic approaches for the identification of flavonoids associated with weed suppression in selected Hardseeded annual pasture legumes. Plant Soil 2019, 447, 199–218. [Google Scholar] [CrossRef]




| Correlation with Phytotoxicity Measures | |||||
|---|---|---|---|---|---|
| Compound | Formula | Accurate Mass | Germination | Radicle Length | Hypocotyl Length |
| Parthenin | C15H18O4 | 262.1140 | −0.57 | −0.41 | −0.43 |
| Ambrosin | C15H18O3 | 246.1256 | −0.72 * | −0.54 | −0.46 |
| Coronopilin | C15H20O4 | 264.1362 | −0.67 | −0.47 | −0.49 |
| Damsin | C15H20O3 | 248.1412 | −0.62 | −0.55 | −0.37 |
| Chlorogenic acid | C16H18O9 | 354.0951 | −0.60 | −0.63 * | −0.61 |
| Significant Compounds | Coefficient of Determination (R2) | Accurate Mass | Retention Time (min) | Molecular Formula |
|---|---|---|---|---|
| Germination inhibition | ||||
| Compound 187 | 0.75 | 430.2276 | 8.8 | C15H34N4O10 |
| Compound 187 + 124 | 0.92 | 341.1825 | 8.1 | C15H25N4O5 |
| Radicle elongation inhibition | ||||
| Compound 541 | 0.74 | 290.1880 | 12.4 | C18H26O3 |
| Compound 541 + 46 | 0.93 | 297.1576 | 5.2 | C15H23NO5 |
| Hypocotyl elongation inhibition | ||||
| Compound 612 | 0.61 | 352.2614 | 13.9 | C21H36O4 |
| Compound 612 + 1048 | 0.84 | 914.6030 | 21.1 | C56H75N6O5 |
| Compound 612 + 1048 + 840 | 0.92 | 582.4118 | 19.0 | C30H50N10O2 |
| Cytotoxicity | ||||
| Compound 19 | 0.74 | 218.0188 | 0.86 | C7H2N6O3 |
| Compound 19 + 32 | 0.89 | 281.1825 | 1.1 | C10H25N4O5 |
| Photocytotoxicity | ||||
| Compound 1242 | 0.84 | 580.3956 | 20.3 | C30H48N10O2 |
| Compound 1242 + 1075 | 0.99 | 402.2272 | 17.9 | C21H30N4O4 |
| Compound | Effect | Reference |
|---|---|---|
| Sesquiterpene lactones | ||
| Parthenin | Phytotoxic | [8,10,11,13] |
| Cytotoxic, allergenic | [16,23,45,47] | |
| Ambrosin | Allergenic/dermatitis | [16,17,18,19] |
| Coronopilin | Phytotoxic | |
| Damsin | Allelopathic | |
| Hysterin | Allelopathic | |
| Hymenin | - | |
| Phenolics | ||
| Caffeic acid | Phytotoxic | [8,19] |
| p-coumaric acid | ||
| Anisic acid | ||
| Vanillic acid | ||
| Ferulic acid | ||
| Fumaric acid | ||
| Aerulic acid | ||
| p-hydroxybenzoic acid | ||
| Chlorogenic acid | ||
| Scopoletin | Cytotoxic | [49] |
| Flavonoids | ||
| Kaempferol | Not known | [20] |
| Quercetin 3-o-glycosides | ||
| Quecetagetin 3,7-dimethyl ether | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajwa, A.A.; Weston, P.A.; Gurusinghe, S.; Latif, S.; Adkins, S.W.; Weston, L.A. Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.). Toxins 2020, 12, 447. https://doi.org/10.3390/toxins12070447
Bajwa AA, Weston PA, Gurusinghe S, Latif S, Adkins SW, Weston LA. Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.). Toxins. 2020; 12(7):447. https://doi.org/10.3390/toxins12070447
Chicago/Turabian StyleBajwa, Ali Ahsan, Paul A. Weston, Saliya Gurusinghe, Sajid Latif, Steve W. Adkins, and Leslie A. Weston. 2020. "Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.)" Toxins 12, no. 7: 447. https://doi.org/10.3390/toxins12070447
APA StyleBajwa, A. A., Weston, P. A., Gurusinghe, S., Latif, S., Adkins, S. W., & Weston, L. A. (2020). Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.). Toxins, 12(7), 447. https://doi.org/10.3390/toxins12070447









