Neotropical Rattlesnake (Crotalus simus) Venom Pharmacokinetics in Lymph and Blood Using an Ovine Model
Abstract
1. Introduction
2. Results
2.1. Venom Characterization
2.2. Size-exclusion Chromatography and Toxicity of Protein Fractions
2.3. Purification of SVMPs, SVSPs, Crotoxin and Their Respective Antibodies
2.4. Coagulation of Whole Blood
2.5. Fibrinogen Degradation and Quantification
2.6. Antibodies and Their Recognition
2.7. Pharmacokinetic Parameters of WV, SVMPs, SVSPs and Crotoxin
3. Discussion
3.1. Purification of Protein Families and Antibodies
3.2. Whole Venom
3.3. SVSPs
3.4. SVMPs
3.5. Crotoxin
3.6. Pharmacokinetics and Its Clinical Implications
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Animals
5.3. Venom
5.4. Protein Quantification
5.5. SDS-PAGE
5.6. Lethality
5.7. Procoagulant Activity
5.8. Local Hemorrhagic Activity
5.9. Separation and Purification of SVMPs, SVSPs, and Crotoxin from Pooled C. simus Venom
5.10. Amino Acid Sequence Determination
5.11. Production of Antibodies
5.12. Antibody Purification
5.13. Biotinylation of Antibodies
5.14. Quantification of WV, SVSPs, SVMPs, Crotoxin and BSA by ELISA
5.15. Ovine Model
5.16. Blood and Lymph Processing
5.17. Surgical Technique for G3 Sheep
5.18. Purification and Quantification of Fibrinogen
5.19. Pharmacokinetic Analysis
5.20. Relative Abundance of the Main Venom Components
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peter Uetz, J.H. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 2 June 2020).
- Campbell, J.; Lamar, W.W. The Venomous Reptiles of the Western Hemisphere; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Wüster, W.; Ferguson, J.E.; Quijada-Mascareñas, J.A.; Pook, C.E.; Salomão, M.D.G.; Thorpe, R.S. Tracing an invasion: Landbridges, refugia, and the phylogeography of the Neotropical rattlesnake (Serpentes: Viperidae: Crotalus durissus). Mol. Ecol. 2005, 14, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.N.; Lomonte, B.; del Carmen Gutiérrez, M.; Alagón, A.; Gutiérrez, J.M. Intraspecies variation in the venom of the rattlesnake Crotalus simus from Mexico: Different expression of crotoxin results in highly variable toxicity in the venoms of three subspecies. J. Proteom. 2013, 87, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Márquez, R.A.; Cedeño-Vázquez, J.R.; Martínez-Arce, A.; Neri-Castro, E.; Rabet, S.C.M.-M. Accessing cryptic diversity in Neotropical rattlesnakes (Serpentes: Viperidae: Crotalus) with the description of two new species. Zootaxa 2020, 4729, 451–481. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Cid, P.; de la Torre, P.; Flores-Diaz, M.; Dos Santos, M.C.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B.; et al. Snake Venomics of the Central American Rattlesnake Crotalus simus and the South American Crotalus durissus Complex Points to Neurotoxicity as an Adaptive Paedomorphic Trend along Crotalus Dispersal in South America. J. Proteome Res. 2010, 9, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Durban, J.; Sanz, L.; Trevisan-Silva, D.; Neri-Castro, E.; Alagón, A.; Calvete, J.J. Integrated Venomics and Venom Gland Transcriptome Analysis of Juvenile and Adult Mexican Rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus Revealed miRNA-modulated Ontogenetic Shifts. J. Proteome Res. 2017, 16, 3370–3390. [Google Scholar] [CrossRef] [PubMed]
- Saravia, P.; Rojas, E.; Arce, V.; Guevara, C.; López, J.C.; Chaves, E.; Velásquez, R.; Rojas, G.; Gutiérrez, J.M. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: Pathophysiological and therapeutic implications. Rev. Biol. Trop. 2002, 50, 337–346. [Google Scholar]
- Segura, Á.; Herrera, M.; Reta Mares, F.; Jaime, C.; Sánchez, A.; Vargas, M.; Villalta, M.; Gómez, A.; Gutiérrez, J.M.; León, G. Proteomic, toxicological and immunogenic characterization of Mexican west-coast rattlesnake (Crotalus basiliscus) venom and its immunological relatedness with the venom of Central American rattlesnake (Crotalus simus). J. Proteom. 2017, 158, 62–72. [Google Scholar] [CrossRef]
- Neri-Castro, E.; Hernández-Dávila, A.; Olvera-Rodríguez, A.; Cardoso-Torres, H.; Bénard-Valle, M.; Bastiaans, E.; López-Gutierrez, O.; Alagón, A. Detection and quantification of a β-neurotoxin (crotoxin homologs) in the venom of the rattlesnakes Crotalus simus, C. culminatus and C. tzabcan from Mexico. Toxicon X 2019, 2, 100007. [Google Scholar] [CrossRef]
- Faure, G.; Saliou, B.; Bon, C.; Guillaume, J.L.; Camoin, L. Multiplicity of Acidic Subunit Isoforms of Crotoxin, the Phospholipase A2 Neurotoxin from Crotalus durissus terrificus Venom, Results from Posttranslational Modifications. Biochemistry 1991, 30, 8074–8083. [Google Scholar] [CrossRef]
- Faure, G.; Harvey, A.L.; Thomson, E.; Saliou, B.; Radvanyi, F.; Bon, C. Comparison of crotoxin isoforms reveals that stability of the complex plays a major role in its pharmacological action. Eur. J. Biochem. 1993, 214, 491–496. [Google Scholar] [CrossRef]
- Faure, G.; Choumet, V.; Bouvhier, C.; Camoin, L.; Guillaume, J.-L.; Monegier, B.; Vuilhorgne, M.; Bon, C. The origin of the diversity of crotoxin isoforms in the venom of Crotalus durissus terrificus. Eur. J. Biochem. 1994, 223, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Faure, G.; Xu, H.; Saul, F.A. Crystal structure of crotoxin reveals key residues involved in the stability and toxicity of this potent heterodimeric β-neurotoxin. J. Mol. Biol. 2011, 412, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M. Comprendiendo los venenos de serpientes: 50 Años de investigaciones en América Latina. Rev. Biol. Trop. 2002, 50, 377–394. [Google Scholar] [PubMed]
- Supersaxo, A.; Hein, W.; Steffen, H. Effect of Molecular Weight on the Lymphatic Absorption of Water-Soluble Compounds Following Subcutaneous Administration. Pharm. Res. 1990, 7, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M.T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Takeda, S.; Takeya, H.; Iwanaga, S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 164–176. [Google Scholar] [CrossRef]
- Boyer, L.V.; Seifert, S.A.; Cain, J.S. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 2. Guidelines for clinical management with crotaline fab antivenom. Ann. Emerg. Med. 2001, 37, 196–201. [Google Scholar] [CrossRef]
- Bush, S.P.; Seifert, S.A.; Oakes, J.; Smith, S.D.; Phan, T.H.; Pearl, S.R.; Reibling, E.T. Continuous IV Crotalidae Polyvalent Immune Fab (Ovine) (FabAV) for selected North American Rattlesnake bite patients. Toxicon 2013, 69, 29–37. [Google Scholar] [CrossRef]
- Theakston, R.D.G.; Warrell, D.A.; Griffiths, E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon 2003, 41, 541–557. [Google Scholar] [CrossRef]
- Audebert, F.; Urtizberea, M.; Sabouraud, A.; Scherrmann, J.M.; Bon, C. Pharmacokinetics of Vipera aspis venom after experimental envenomation in rabbits. J. Pharmacol. Exp. Ther. 1994, 268, 1512–1517. [Google Scholar]
- Moreno, E.; Gutiérrez, J. Body distribution of Bothrops asper (terciopelo) snake venom myotoxin and its relationship to pathological changes. Toxicon 1988, 26, 403–409. [Google Scholar] [CrossRef]
- Sim, S.M.; Saremi, K.; Tan, N.H.; Fung, S.Y. Pharmacokinetics of Cryptelytrops purpureomaculatus (mangrove pit viper) venom following intravenous and intramuscular injections in rabbits. Int. Immunopharmacol. 2013, 17, 997–1001. [Google Scholar] [CrossRef]
- Tan, C.H.; Sim, S.M.; Gnanathasan, C.A.; Fung, S.Y.; Tan, N.H. Pharmacokinetics of the Sri Lankan hump-nosed pit viper (Hypnale hypnale) venom following intravenous and intramuscular injections of the venom into rabbits. Toxicon 2014, 79, 37–44. [Google Scholar] [CrossRef]
- Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of snake venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.K.K.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Tan, C.H. Pharmacokinetics of Naja sumatrana (Equatorial Spitting Cobra) Venom and Its Major Toxins in Experimentally Envenomed Rabbits. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, D.; Jiménez, L.; Romero, C.; Vergara, I.; Calderón, A.; Benard, M.; Bernas, M.J.; Rilo, H.; de Roodt, A.; D’Suze, G.; et al. Lymphatic route of transport and pharmacokinetics of Micrurus fulvius (coral snake) venom in sheep. Lymphology 2012, 45, 144–153. [Google Scholar] [PubMed]
- Boyer, L.V.; Chase, P.B.; Degan, J.A.; Figge, G.; Buelna-Romero, A.; Luchetti, C.; Alagón, A. Subacute coagulopathy in a randomized, comparative trial of Fab and F(ab’)2 antivenoms. Toxicon 2013, 74, 101–108. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; León, G.; Lomonte, B. Pharmacokinetic-Pharmacodynamic Relationships of Immunoglobulin Therapy for Envenomation. Clin. Pharmacokinet. 2003, 42, 721–741. [Google Scholar] [CrossRef]
- Dart, R.C.; Hurlbut, K.M.; Garcia, R.; Boren, J. Validation of a severity score for the assessment of crotalid snakebite. Ann. Emerg. Med. 1996, 27, 321–326. [Google Scholar] [CrossRef]
- Lavonas, E.J.; Gerardo, C.J.; Arcuri, K.; Daugherty, C.A.; Temu, A.; Anderson, V.E.; Bartelson, B.B.; Coulter, M.S.; Gillman, S.M.; Goodman, E.M.; et al. Prospective study of recovery from copperhead snake envenomation: An observational study. BMC Emerg. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.J.; Hodgson, W.C.; O’Leary, M.; Isbister, G.K. Pharmacokinetics and pharmacodynamics of the myotoxic venom of Pseudechis australis (mulga snake) in the anesthetised rat. Clin. Toxicol. 2014, 52, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Borja, M.; Neri-Castro, E.; Castañeda-Gaytán, G.; Strickland, J.L.; Parkinson, C.L.; Castañeda-Gaytán, J.; Ponce-López, R.; Lomonte, B.; Olvera-Rodríguez, A.; Alagón, A.; et al. Biological and proteolytic variation in the venom of crotalus scutulatus scutulatus from Mexico. Toxins 2018, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, D.; Vergara, I.; Román, R.; Romero, C.; Benard-Valle, M.; Calderón, A.; Jiménez, L.; Bernas, M.J.; Witte, M.H.; Boyer, L.V.; et al. Antivenom effect on lymphatic absorption and pharmacokinetics of coral snake venom using a large animal model. Clin. Toxicol. 2019, 57, 1–8. [Google Scholar] [CrossRef]
- Camacho, E.; Villalobos, E.; Sanz, L.; Pérez, A.; Escalante, T.; Lomonte, B.; Calvete, J.J.; Gutiérrez, J.M.; Rucavado, A. Understanding structural and functional aspects of PII snake venom metalloproteinases: Characterization of BlatH1, a hemorrhagic dimeric enzyme from the venom of Bothriechis lateralis. Biochimie 2014, 101, 145–155. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Romero, M.; Díaz, C.; Borkow, G.; Ovadia, M. Isolation and characterization of a metalloproteinase with weak hemorrhagic activity from the venom of the snake Bothrops asper (terciopelo). Toxicon 1995, 33, 19–29. [Google Scholar] [CrossRef]
- Herrera, C.; Escalante, T.; Voisin, M.B.; Rucavado, A.; Morazán, D.; Macêdo, J.K.A.; Calvete, J.J.; Sanz, L.; Nourshargh, S.; Gutiérrez, J.M.; et al. Tissue Localization and Extracellular Matrix Degradation by PI, PII and PIII Snake Venom Metalloproteinases: Clues on the Mechanisms of Venom-Induced Hemorrhage. PLoS Negl. Trop. Dis. 2015, 9, 1–20. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, P.; Li, Z.; Roy, P.; Sahajwalla, C.G. The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J. Clin. Pharmacol. 2013, 53, 314–325. [Google Scholar] [CrossRef]
- Helden, D.F.; Van Dosen, P.J.; Leary, M.A.O.; Isbister, G.K. Two pathways for venom toxin entry consequent to injection of an Australian elapid snake venom. Sci. Rep. 2019, 1–10. [Google Scholar] [CrossRef]
- Markland, F.S. Snake venom fibrinogenolytic and fibrinolytic enzymes: An updated inventory. Registry of Exogenous Hemostatic Factors of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb. Haemost. 1998, 79, 668–674. [Google Scholar] [CrossRef]
- Fox, J.W.; Gutiérrez, J.M. Understanding the snake venom metalloproteinases: An interview with jay fox and josé maría gutiérrez. Toxins 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A comprehensive view of the structural and functional alterations of extracellular matrix by snake venom metalloproteinases (SVMPs): Novel perspectives on the pathophysiology of envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Barral-Netto, M.; von Sohsten, R.L. Serum kinetics of crotoxin from Crotalus durissus terrificus venom in mice: Evidence for a rapid clearance. Toxicon 1991, 29, 527–531. [Google Scholar] [CrossRef][Green Version]
- Mora, J.; Mora, R.; Lomonte, B.; Gutiérrez, J.M. Effects of Bothrops asper snake venom on lymphatic vessels: Insights into a hidden aspect of envenomation. PLoS Negl. Trop. Dis. 2008, 2. [Google Scholar] [CrossRef]
- Román-Domínguez, L.; Neri-Castro, E.; Vázquez López, H.; García-Osorio, B.; Archundia, I.G.; Ortiz-Medina, J.A.; Petricevich, V.L.; Alagón, A.; Bénard-Valle, M. Biochemical and immunochemical characterization of venoms from snakes of the genus Agkistrodon. Toxicon X 2019, 4, 100013. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Casasola, A.; Ramos-Cerrillo, B.; de Roodt, A.R.; Saucedo, A.C.; Chippaux, J.P.; Alagón, A.; Stock, R.P. Paraspecific neutralization of the venom of African species of cobra by an equine antiserum against Naja melanoleuca: A comparative study. Toxicon 2009, 53, 602–608. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Ransnas, L.A. Fitting curves nonlinear regression: Review a practical. FASEB J. 1987, 1, 365–374. [Google Scholar] [CrossRef]
- Theakston, R.D.G.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venoms. Bull. W. Health Organ. 1983, 61, 949–956. [Google Scholar]
- Gutiérrez, J.M.; Gené, A.; Rodas, G.; Cerdas, L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon 1985, 23, 887–893. [Google Scholar] [CrossRef]
- Calvete, J.J. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev. Proteom. 2011, 8, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Tsai, W.C.; Bonilla, F.; Solórzano, A.; Solano, G.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics and toxicological profiling of the arboreal pitviper Bothriechis supraciliaris from Costa Rica. Toxicon 2012, 59, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Progr. Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]






| Activity | FI | FII | FIII | FIV | WV |
|---|---|---|---|---|---|
| LD50 (µg/g) | >11.84 | 11.5 | 0.52 | 0.1 | 0.16 |
| (10.6 ± 12.5) | (0.46 ± 0.57) | (0.07 ± 0.1) | (0.13 ± 0.18) | ||
| MCD-P (µg) | >100 | 29 ± 2 | 15.3 ± 1 | 50 ± 3.6 | 23 ± 1 |
| MHD (µg) | >100 | 10.4 ± 1.6 | >50 | >50 | 29 ± 2 |
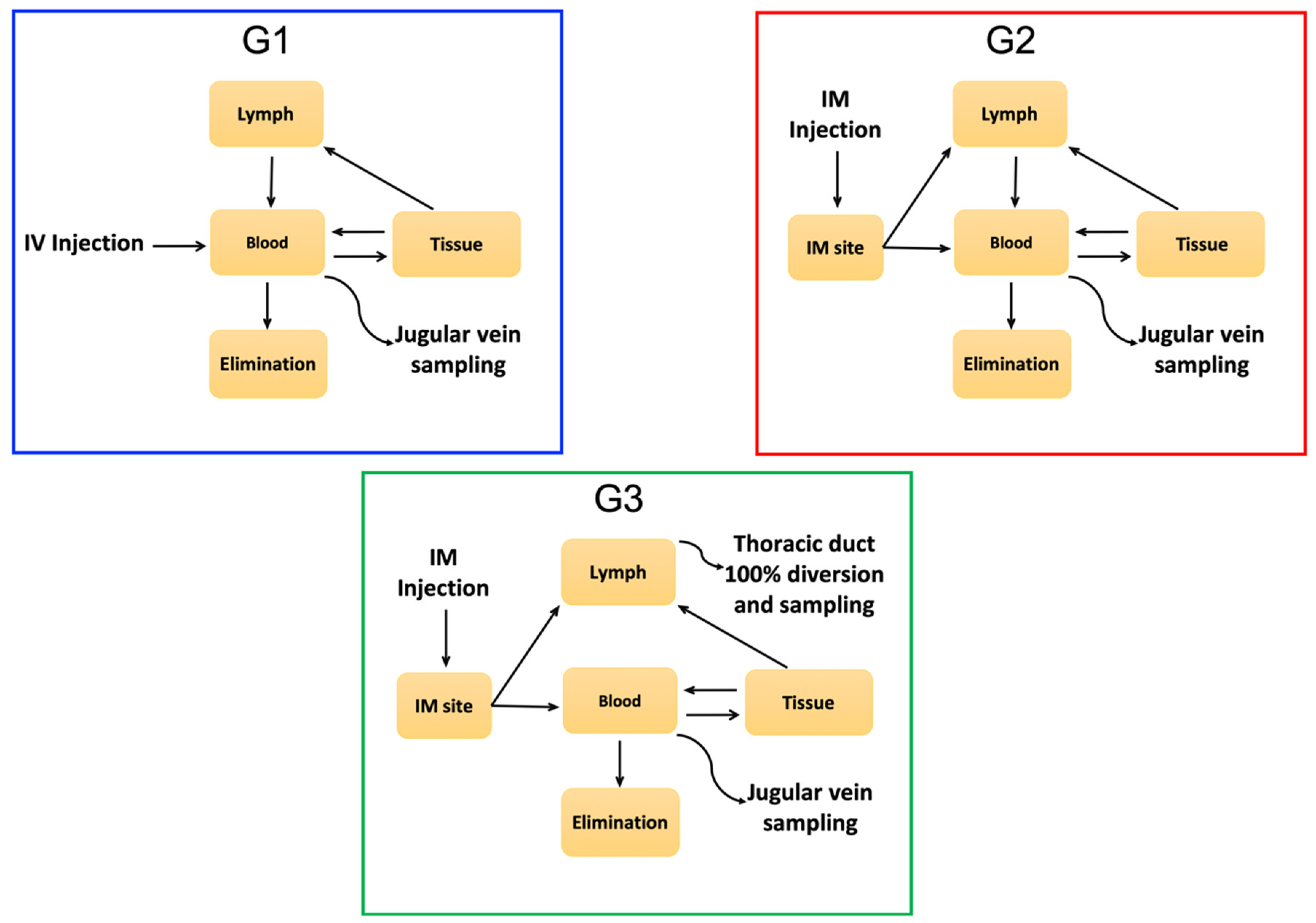
| Experimental | Sheep ID | Route of Venom | Sample | Venom | Duration of Experiment |
|---|---|---|---|---|---|
| Group | Administration | Dose (mg/kg) | (h) | ||
| G1 | 1A, 1B, 1C | I.V. | Blood | 0.02 | 48, 48, 48 |
| G2 | 2A, 2B, 2C | I.M. | Blood | 0.15 * | 240, 72, 36 |
| G3 | 3A, 3B, 3C | I.M. | Blood and lymph | 0.15 * | 3, 12, 12 |
| Parameter | WV | SVMP | SVSP |
|---|---|---|---|
| Administration route | I.V. | I.V. | I.V. |
| Dose (mg) | 1.04 | 0.27 | 0.38 (±0.00) |
| tz (h) | 48 | 48 | 40.00 (±6.93) |
| k10 (1/h) | 1.91 (±0.44) | 2.26 (±0.42) | 0.81 (±0.14) |
| k12 (1/h) | 3.25 (±0.36) | 2.40 (±0.49) | 0.69 (±0.58) |
| k21 (1/h) | 1.61 (±0.46) | 2.68 (±0.38) | 1.27 (±1.78) |
| t1/2 α (h) | 0.11 (±0.02) | 0.11 (±0.02) | 0.45 (±0.28) |
| t1/2 β (h) | 1.52 (±0.49) | 0.75 (±0.10) | 4.06 (±3.28) |
| C0 (ng/mL) | 97.88 (±57.79) | 21.34 (±10.72) | 28.61 (±6.82) |
| V1 (l) | 13.14 (±6.65) | 14.40 (±5.80) | 13.77 (±2.93) |
| CL1 (l/h) | 25.73 (±13.81) | 34.10 (±18.26) | 11.24 (±3.68) |
| V2 (l) | 25.47 (±7.70) | 12.86 (±5.55) | 16.67 (±11.93) |
| CL2 (l/h) | 43.11 (±21.83) | 34.19 (±15.90) | 10.10 (±9.96) |
| AUC 0-t (ng × h/mL) | 55.76 (±42.98) | 10.17 (±7.35) | 36.03 (±10.75) |
| AUC 0-∞ (ng × h/mL) | 55.77 (±43.00) | 10.32 (±7.23) | 36.19 (±10.79) |
| AUMC (ng × h2/mL) | 110.10 (±116.87) | 9.66 (±8.59) | 112.39 (±67.40) |
| MRT (h) | 1.69 (±0.55) | 0.86 (±0.17) | 2.93 (±1.47) |
| Vss (l) | 38.61 (±13.92) | 27.27 (±11.29) | 30.43 (±12.36) |
| F0-t | 1 | 1 | 1 |
| F0-∞ | 1 | 1 | 1 |
| Parameter | WV | SVMP | SVSP |
|---|---|---|---|
| Administration route | I.M. | I.M. | I.M. |
| Dose (mg) | 8.80 (±0.35) | 2.25 (±0.09) | 3.22 (±0.13) |
| tz (h) | 60.00 (±21.00) | 60.00 (±21.00) | 58.00 (±24.25) |
| ʎz (1/h) | 0.16 (±0.16) | 0.06 (±0.06) | 0.08 (±0.05) |
| t1/2 (h) | 8.05 (±5.63) | 19.84 (±18.67) | 12.09 (±7.34) |
| tmax (h) | 9.00 (±3.00) | 22 (±3.46) | 13.00 (±9.64) |
| Cmax (ng/mL) | 17.36 (±4.83) | 1.94 (±1.17) | 16.56 (±4.17) |
| CL/F (l/h) | 23.35 (±11.48) | 43.56 (±22.55) | 10.09 (±6.51) |
| AUC 0-t (ng × h/mL) | 400.01 (±141.76) | 43.90 (±27.24) | 337.98 (±134.62) |
| AUC 0-∞ (ng × h /mL) | 431.24 (±166.82) | 61.10 (±31.62) | 408.78 (±222.47) |
| AUMC (ng × h2/mL) | 8603.77 (±4878.09) | 1798.63 (±1493.23) | 10,041.29 (±7825.81) |
| MRT (h) | 19.49 (±4.90) | 26.70 (±10.63) | 21.89 (±7.42) |
| Vss (l) | 445.49 (±219.54) | 1043.13 (±139.17) | 189.60 (±498.8) |
| Vz/F (l) | 268.15 (±208.32) | 943.25 (±527.95) | 134.30 (±12.58) |
| F0-t | 0.85 (±0.32) | 0.51 (±0.34) | 1.11 (±0.45) |
| F0-∞ | 0.92 (±0.38) | 0.72 (±0.40) | 1.34 (±0.71) |
| Blood Absorption | |||
|---|---|---|---|
| Parameter | WV | SVMP | SVSP |
| Administration route | I.M. | I.M. | I.M. |
| Dose (mg) | 9.65 (±0.17) | 2.47 (±0.44) | 3.53 (±0.06) |
| tz (h) | 8.90 (±5.37) | 9.00 (±5.20) | 9.00 (±5.20) |
| ʎz (1/h) | 0.03 (±0.03) | 0.05 c | 0.07 (±0.08) |
| t1/2 (h) | 37.96 (±34.52) | 14.50 c | 32.16 (±37.26) |
| tmax (h) | 2.40 (±0.79) | 5.5 (±5.68) | 2.67 (±0.58) |
| Cmax (ng/mL) | 31.57 (±17.30) | 1.42 (±0.78) | 17.25 (±11.55) |
| CL/F (l/h) | 14.19 (±11.39) | 91.53 c | 18.13 (±18.10) |
| AUC 0-t (ng × h/mL) | 156.45 (±53.29) | 6.61 (±2.50) | 69.23 (±15.50) |
| AUC 0-∞ (ng × h/mL) | 1006.90 (±823.01) | 26.43 c | 390.17 (±393.58) |
| AUMC (ng × h2/mL) | 76,644.93 (±96,707.77) | 624.10 c | 29,250.31 (±39,833.31) |
| MRT (h) | 55.36 (±50.80) | 23.61 c | 47.79 (±53.88) |
| Vss (l) | 496.26 (±90.33) | 2161.29 c | 379.04 (±112.4) |
| Vz/F (l) | 493.32 (±83.19) | 1914.45 c | 354.86 (±135.38) |
| F0-t | 0.30 (±0.03) | 0.07 (±0.03) | 0.08 (±0.02) |
| F0-∞ | 1.94 (±1.56) | 0.28 c | 0.46 (±0.46) |
| Lymphatic Absorption | |||
| Venom in lymph (mg) | 0.187 (±0.08) | 0.01 (±0.006) | 0.05 (±0.02) |
| F(lymph) 0-t | 0.02 (±0.01) | 0.004 (±0.003) | 0.01 (±0.01) |
| Total Absorption | |||
| F total 0-t | 0.32 (±0.11) | 0.074 (±0.03) | 0.10 (±0.02) |
| Blood Absorption | ||||||
|---|---|---|---|---|---|---|
| Parameter | G2.WV | G3.WV | G2.SVMP | G3.SVMP | G2.SVSP | G3.SVSP |
| Administration route | I.M. | I.M. | I.M. | I.M. | I.M. | I.M. |
| Dose (mg) | 8.80 (±0.35) | 9.65 (±0.17) | 2.25 (±0.09) | 2.47 (±0.44) | 3.22 (±0.13) | 3.53 (±0.06) |
| tz (h) | 12 | 8.90 (±5.37) | 12 | 9.00 (±5.20) | 12.00 (±0.00) | 9.00 (±5.20) |
| ʎz (1/h) | 0.02 c | 0.03 (±0.03) | 0.005 c | 0.05 c | ND | 0.07 (±0.08) |
| t1/2 (h) | 34.78 c | 37.96 (±34.52) | 151.10 c | 14.50 c | ND | 32.16 (±37.26) |
| tmax (h) | 9.00 (±3.00) | 2.40 (±0.79) | 9.33 (±4.62) | 5.5 (±5.68) | 9.00 (±3.00) | 2.67 (±0.58) |
| Cmax (ng/mL) | 17.36 (±4.83) | 31.57 (±17.30) | 1.72 (±1.07) | 1.42 (±0.78) | 14.07 (±4.63) | 17.25 (±11.55) |
| CL/F (l/h) | 8.36 c | 14.19 (±11.39) | 13.50 c | 91.53 c | ND | 18.13 (±18.10) |
| AUC 0-t (ng × h/mL) | 151.83 (±62.13) | 156.45 (±53.29) | 10.75 (±2.90) | 6.61 (±2.50) | 115.25 (±50.63) | 69.23 (±15.50) |
| AUC 0-∞ (ng × h/mL) | 1076.16 c | 1006.90 (±823.01) | 170.78 c | 26.43 c | ND | 390.17 (±393.58) |
| AUMC (ng × h2/mL) | 55,943.52 c | 76,644.93 (±96,707.77) | 37,576.65 c | 624.10 c | ND | 29,250.31 (±39,833.31) |
| MRT (h) | 51.98 c | 55.36 (±50.80) | 220.03 c | 23.61 c | ND | 47.79 (±53.88) |
| Vss (l) | 434.75 c | 496.26 (±90.33) | 2968 c | 2161.29 c | ND | 379.04 (±112.4) |
| Vz/F (l) | 419.61 c | 493.32 (±83.19) | 2940 c | 1914.45 c | ND | 354.86 (±135.38) |
| F0-t | 0.32 (±0.13) | 0.30 (±0.03) | 0.11 (±0.03) | 0.07 (±0.03) | 0.37 (±0.16) | 0.08 (±0.02) |
| F0-∞ | 2.23 c | 1.94 (±1.56) | 1.82 c | 0.28 c | ND | 0.46 (±0.46) |
| Lymphatic Absorption | ||||||
| Venom in lymph (mg) | NA | 0.187 (±0.08) | NA | 0.01 (±0.006) | NA | 0.05 (±0.02) |
| F(lymph) 0-t | NA | 0.02 (±0.01) | NA | 0.004 (±0.003) | NA | 0.01 (±0.01) |
| Total Absorption | ||||||
| F total 0-t | 0.32 (±0.13) | 0.32 (±0.11) | 0.11 (±0.03) | 0.074 (±0.03) | 0.37 (±0.16) | 0.10 (±0.02) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri-Castro, E.; Bénard-Valle, M.; Paniagua, D.; V. Boyer, L.; D. Possani, L.; López-Casillas, F.; Olvera, A.; Romero, C.; Zamudio, F.; Alagón, A. Neotropical Rattlesnake (Crotalus simus) Venom Pharmacokinetics in Lymph and Blood Using an Ovine Model. Toxins 2020, 12, 455. https://doi.org/10.3390/toxins12070455
Neri-Castro E, Bénard-Valle M, Paniagua D, V. Boyer L, D. Possani L, López-Casillas F, Olvera A, Romero C, Zamudio F, Alagón A. Neotropical Rattlesnake (Crotalus simus) Venom Pharmacokinetics in Lymph and Blood Using an Ovine Model. Toxins. 2020; 12(7):455. https://doi.org/10.3390/toxins12070455
Chicago/Turabian StyleNeri-Castro, Edgar, Melisa Bénard-Valle, Dayanira Paniagua, Leslie V. Boyer, Lourival D. Possani, Fernando López-Casillas, Alejandro Olvera, Camilo Romero, Fernando Zamudio, and Alejandro Alagón. 2020. "Neotropical Rattlesnake (Crotalus simus) Venom Pharmacokinetics in Lymph and Blood Using an Ovine Model" Toxins 12, no. 7: 455. https://doi.org/10.3390/toxins12070455
APA StyleNeri-Castro, E., Bénard-Valle, M., Paniagua, D., V. Boyer, L., D. Possani, L., López-Casillas, F., Olvera, A., Romero, C., Zamudio, F., & Alagón, A. (2020). Neotropical Rattlesnake (Crotalus simus) Venom Pharmacokinetics in Lymph and Blood Using an Ovine Model. Toxins, 12(7), 455. https://doi.org/10.3390/toxins12070455







