Cytotoxicity of the Sesquiterpene Lactones, Ivalin and Parthenolide in Murine Muscle Cell Lines and Their Effect on Desmin, a Cytoskeletal Intermediate Filament
Abstract
1. Introduction
2. Results
2.1. In Vitro Cytotoxicity
2.1.1. Cytotoxicity of Ivalin and Parthenolide in C2C12 Cell Lines
2.1.2. Cytotoxicity of Ivalin and Parthenolide in H9c2 Cell Lines
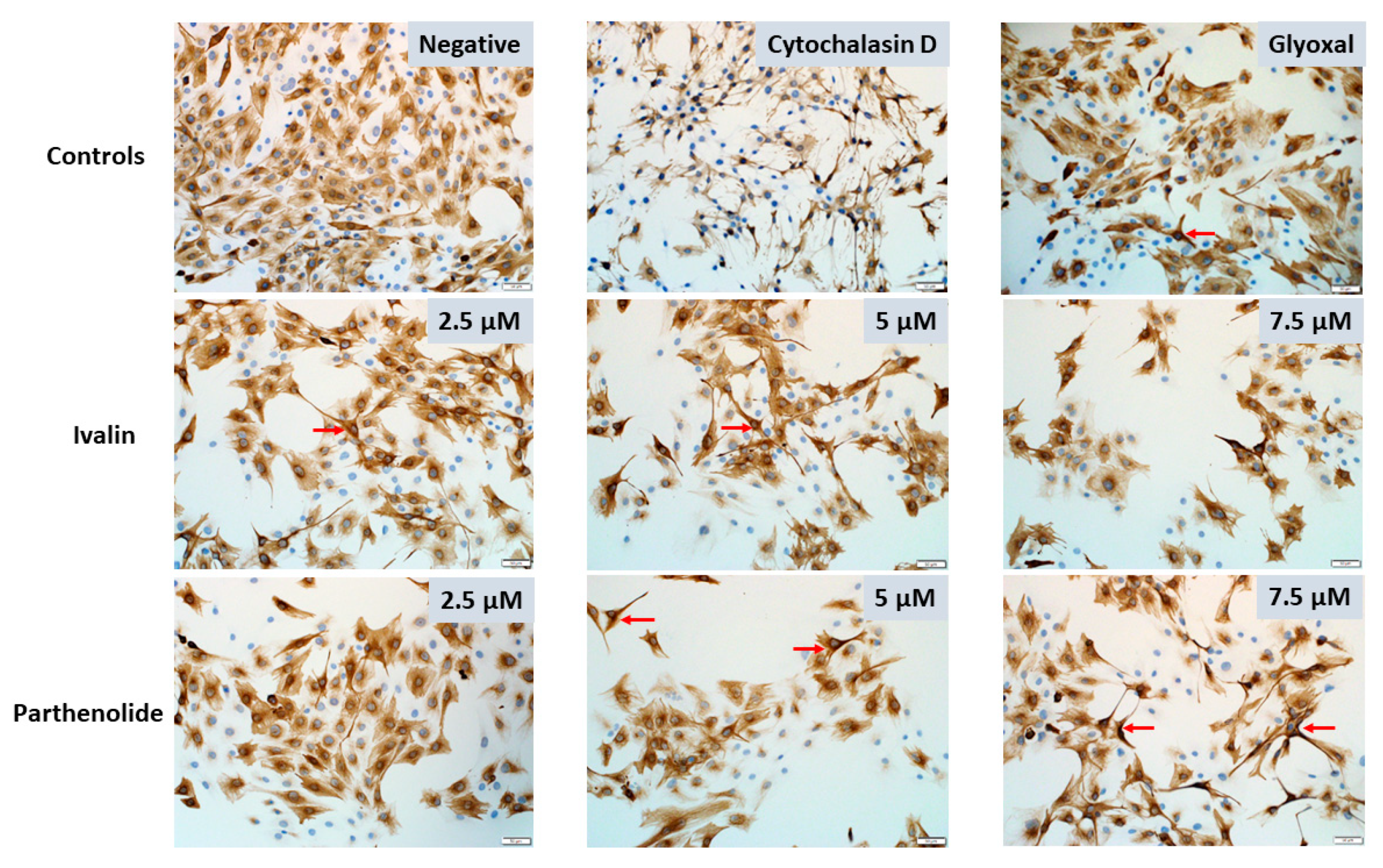
2.2. Desmin Immunocytochemistry
3. Discussion
4. Conclusions
5. Materials and Methods
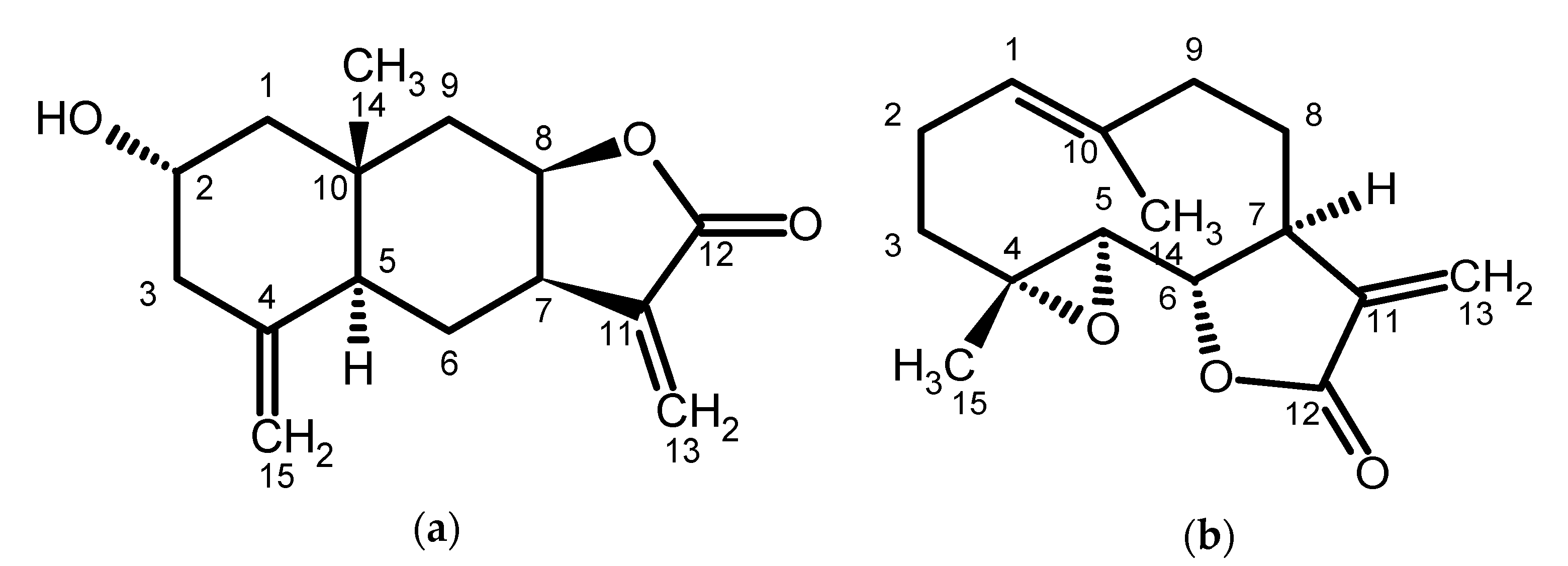
5.1. Plant-Derived Sesquiterpene Lactones
5.2. Cell Culture
5.2.1. Chemicals, Reagents, and Plastic Ware
5.2.2. Cell Lines
5.3. Exposure Studies
5.3.1. MTT Cytotoxicity Assay
5.3.2. Desmin Immunocytochemistry
5.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kellerman, T.S.; Coetzer, J.A.W.; Naudé, T.W.; Botha, C.J. Plant Poisonings and Mycotoxicoses of Livestock in Southern Africa, 2nd ed.; Oxford University Press: Cape Town, South Africa, 2005; pp. 184–191. [Google Scholar]
- Van Heerden, J.; Van der Lugt, J.J.; Durante, E. Experimental vermeersiekte (Geigeria ornativa O. Hoffm. poisoning) in sheep. I: An evaluation of diagnostic aids and an assessment of the preventive effect of ethoxyquin. J. S. Afr. Vet. Assoc. 1993, 64, 76–81. [Google Scholar] [PubMed]
- Grosskopf, J.F.W. Our Present Knowledge of “Vermeersiekte” (Geigeria Poisoning); Technical Communication 21; Department of Agricultural Technical Services: Pretoria, South Africa, 1964. [Google Scholar]
- Botha, C.J.; Clift, S.J.; Ferreira, G.C.H.; Masango, M.M. Geigerin-induced cytotoxicity in a murine myoblast cell line (C2C12). Onderstepoort J. Vet. Res. 2017, 84, a1465. [Google Scholar] [CrossRef] [PubMed]
- Van der Lugt, J.J.; Van Heerden, J. Experimental vermeersiekte (Geigeria ornativa O. Hoffm. poisoning) in sheep. II: Histological and ultrastructural lesions. J. S. Afr. Vet. Assoc. 1993, 64, 82–88. [Google Scholar] [PubMed]
- Merrill, J.; Kim, H.; Safe, S. Hymenoxon: Biologic and toxic effects. Biochem. Pharmacol. 1985, 34, 3383–3386. [Google Scholar] [CrossRef]
- Burrows, G.E.; Tyrl, R.J. Toxic Plants of North America, 1st ed.; Iowa State University Press: Ames, IA, USA, 2001; pp. 170–178. [Google Scholar]
- Snyman, L.D.; Carstens, A.; Schultz, R.A.; Joubert, J.P.J.; Labuschagne, L. Changes in sheep oesophageal diameter and function during Geigeria ornativa (vermeerbos) poisoning and subsequent recovery. J. S. Afr. Vet. Assoc. 2008, 79, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, J.G.; Kriek, N.P.J.; Naudé, T.W.; Adelaar, T.F.; Ellis, S.D. Lesions in sheep skeletal and oesophageal muscle in vermeersiekte (Geigeria ornativa O. Hoffm. poisoning. Onderstepoort J. Vet. Res. 1973, 40, 127–138. [Google Scholar] [PubMed]
- Botha, C.J.; Venter, E.A.; Ferreira, G.C.; Phaswane, R.M.; Clift, S.J. Geigerin-induced disorganization of desmin, an intermediate filament of the cytoskeleton, in a murine myoblast cell line (C2C12). Toxicon 2019, 167, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Paulin, D.; Huet, A.; Khanamyrian, L.; Xue, Z. Desminopathies in muscle disease. J. Pathol. 2004, 204, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Clemen, C.S.; Herrmann, H.; Strelkov, S.V.; Schröder, R. Desminopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Diguet, N.; Mallat, Y.; Ladouce, R.; Clodic, G.; Prola, A.; Tritsch, E.; Blanc, J.; Larcher, J.-C.; Delcayre, C.; Samuel, J.-L.; et al. Muscle creatine kinase deficiency triggers both actin depolymerization and desmin disorganization by advanced glycation end products in dilated cardiomyopathy. J. Biol. Chem. 2011, 286, 35007–35019. [Google Scholar] [CrossRef] [PubMed]
- Fouché, G.; Ackerman, L.G.; Venter, E.A.; Mathe, Y.Z.; Liles, D.C.; Botha, C.J. Sesquiterpene lactones from Geigeria aspera Harv. and their cytotoxicity. Nat. Prod. Res. 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Towers, G.H.N.; Mitchell, J.C. Biological activities of sesquiterpene lactones. Phytochemistry 1976, 15, 1573–1580. [Google Scholar] [CrossRef]
- Hussien, T.A.; El-Toumy, S.; Hassan, H.; Hetta, M. Cytotoxic and antioxidant activities of secondary metabolites from Pulicaria undulata. Int. J. Pharm. Pharm. Sci. 2016, 8, 150–155. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Chan, P.; Gong, C.-L.; Wong, K.-L.; Su, T.-H.; Shen, P.-C.; Leung, Y.-M.; Liu, Z.-M. Parthenolide-induced cytotoxicity in H9c2 cardiomyoblasts involves oxidative stress. Acta Cardiol. Sin. 2015, 31, 33–41. [Google Scholar] [PubMed]
- Van Aswegen, C.-H.; Potgieter, D.J.J.; Vermeulen, N.M.J. Site of respiratory inhibition by sesquiterpene lactones from Geigeria. S. Afr. J. Sci. 1982, 78, 125–127. [Google Scholar]
- Van Aswegen, C.-H.; Vermeulen, N.M.J.; Potgieter, D.J.J. Inhibition of oxidative phosphorylation by sesquiterpene lactones from Geigeria aspera. S. Afr. J. Sci. 1979, 75, 84–85. [Google Scholar]
- Costa, M.L.; Escaleira, R.; Cataldo, A.; Oliveira, F.; Mermelstein, C.S. Desmin: Molecular interactions and putative functions of the muscle intermediate filament protein. Braz. J. Med. Biol. Res. 2004, 37, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S.; Leeuwenburgh, C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim. Biophys. Acta 2002, 1588, 94–101. [Google Scholar] [CrossRef]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Motulsky, H.; Christopoulus, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting, 1st ed.; Oxford University Press: New York, NY, USA, 2004; pp. 260–264. [Google Scholar]
- Payne, R.A. Guide to Regression, Nonlinear and Generalized Linear Models, 19th ed.; VSN International: Hemel Hempstead, UK, 2017; p. 43. [Google Scholar]
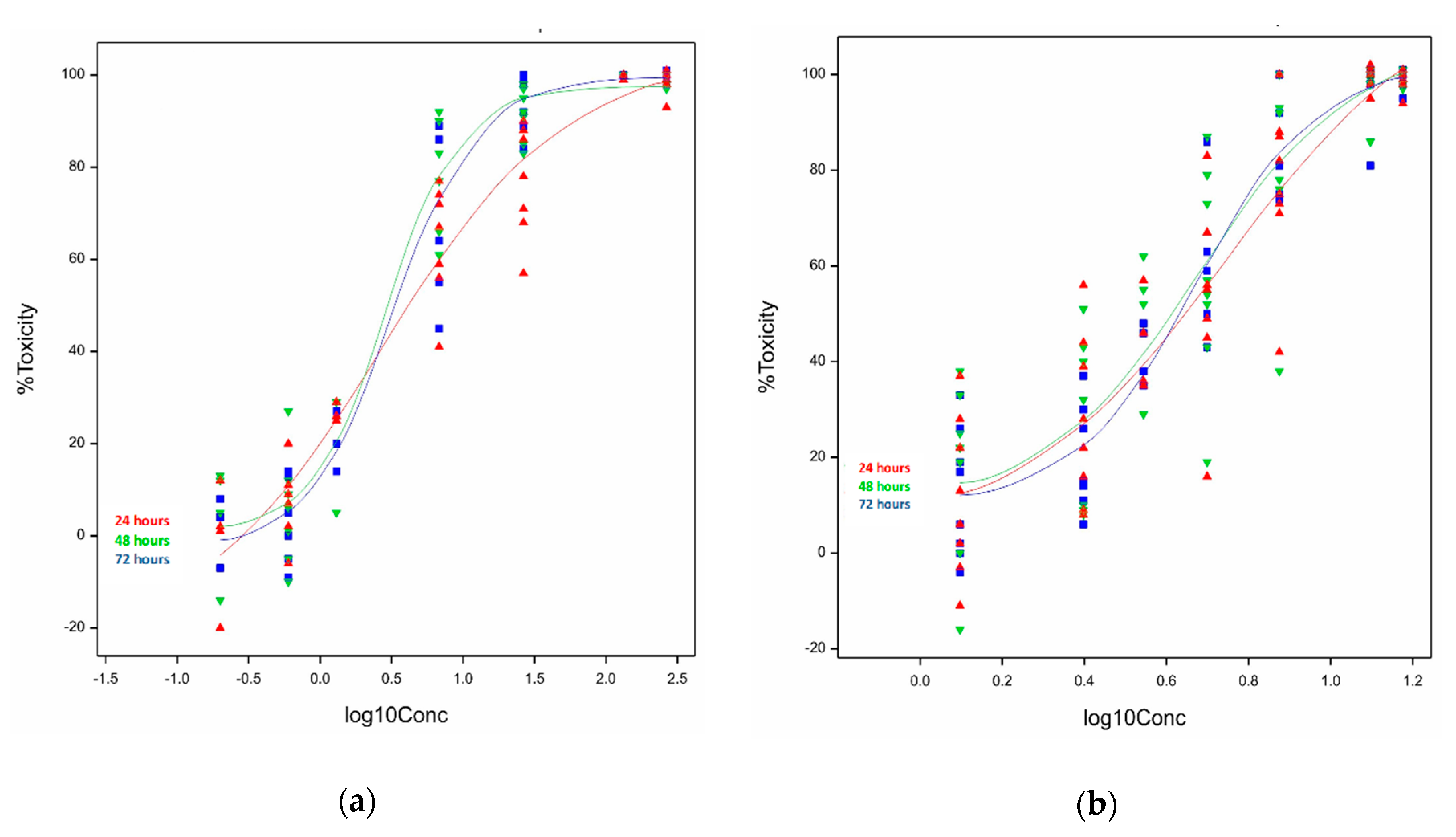
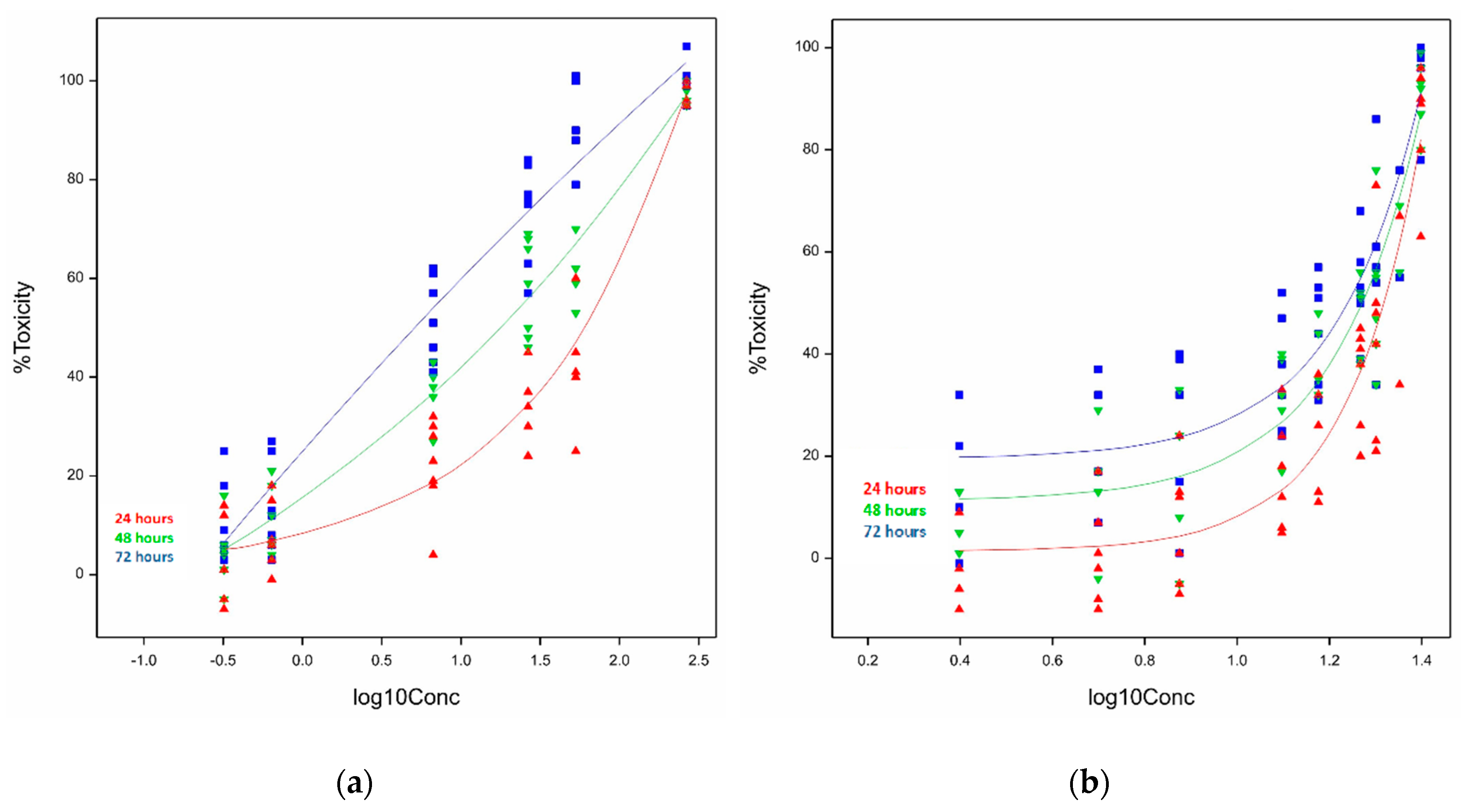
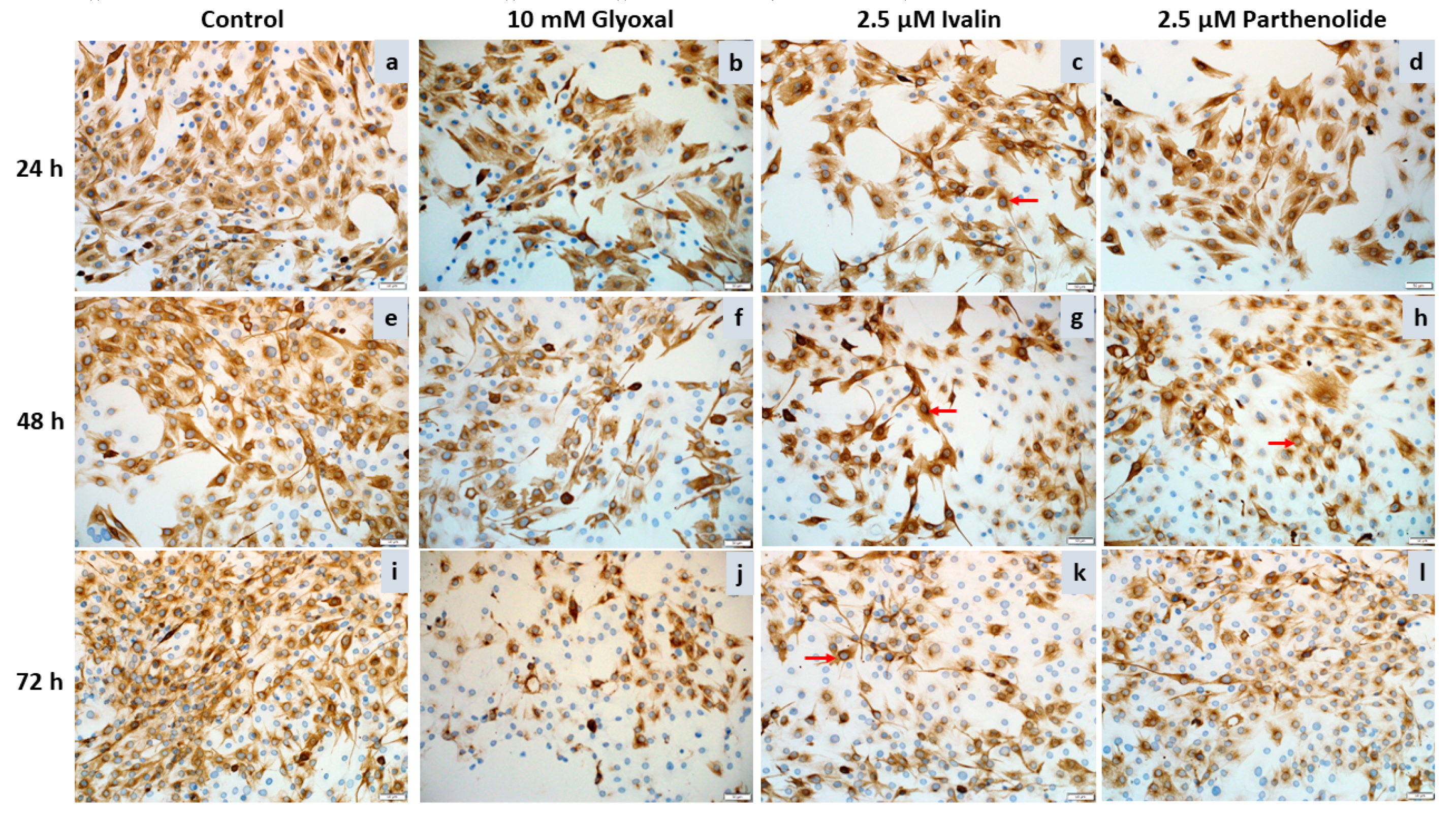
| Cell Line. | Exposure Time (h) | Ivalin 1 (µM) | Parthenolide 1 (µM) |
|---|---|---|---|
| C2C12 | 24 | 2.7 ± 1.6 n = 7 | 5.6 ± 1.2 n = 8 |
| 48 | 3.0 ± 1.2 n = 7 | 4.8 ± 1.1 n = 8 | |
| 72 | 3.3 ± 1.2 n = 7 | 4.7 ± 1.1 n = 8 | |
| H9c2 | 24 | 60.7 ± 1.1 n = 7 | 20.8 ± 1.0 n = 6 |
| 48 | 17.8 ± 1.1 n = 7 | 18.4 ± 1.0 n = 6 | |
| 72 | 8.5 ± 1.3 n = 7 | 17.4 ± 1.0 n = 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botha, C.J.; Mathe, Y.Z.; Ferreira, G.C.H.; Venter, E.A. Cytotoxicity of the Sesquiterpene Lactones, Ivalin and Parthenolide in Murine Muscle Cell Lines and Their Effect on Desmin, a Cytoskeletal Intermediate Filament. Toxins 2020, 12, 459. https://doi.org/10.3390/toxins12070459
Botha CJ, Mathe YZ, Ferreira GCH, Venter EA. Cytotoxicity of the Sesquiterpene Lactones, Ivalin and Parthenolide in Murine Muscle Cell Lines and Their Effect on Desmin, a Cytoskeletal Intermediate Filament. Toxins. 2020; 12(7):459. https://doi.org/10.3390/toxins12070459
Chicago/Turabian StyleBotha, Christo J., Y. Zethu Mathe, Gezina C. H. Ferreira, and E. Annette Venter. 2020. "Cytotoxicity of the Sesquiterpene Lactones, Ivalin and Parthenolide in Murine Muscle Cell Lines and Their Effect on Desmin, a Cytoskeletal Intermediate Filament" Toxins 12, no. 7: 459. https://doi.org/10.3390/toxins12070459
APA StyleBotha, C. J., Mathe, Y. Z., Ferreira, G. C. H., & Venter, E. A. (2020). Cytotoxicity of the Sesquiterpene Lactones, Ivalin and Parthenolide in Murine Muscle Cell Lines and Their Effect on Desmin, a Cytoskeletal Intermediate Filament. Toxins, 12(7), 459. https://doi.org/10.3390/toxins12070459





