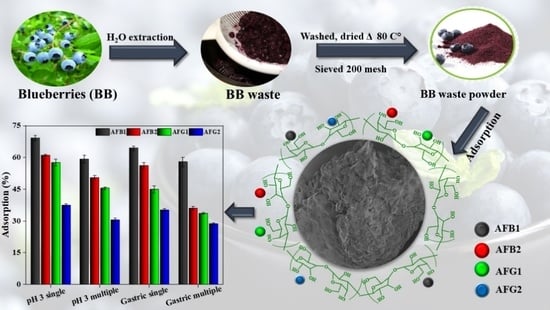
Assessing the Aflatoxins Mitigation Efficacy of Blueberry Pomace Biosorbent in Buffer, Gastrointestinal Fluids and Model Wine
Abstract
1. Introduction
2. Results and Discussion
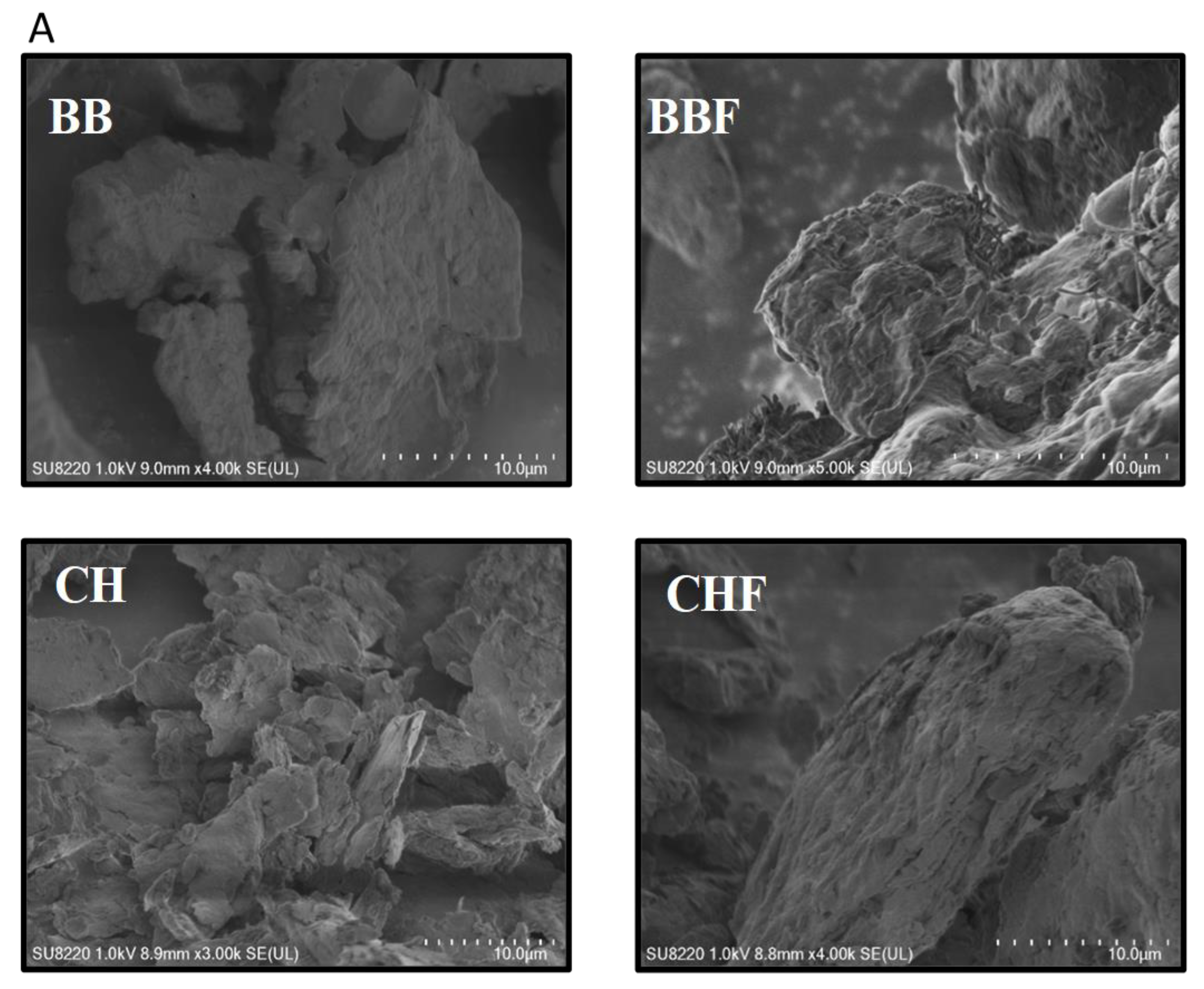
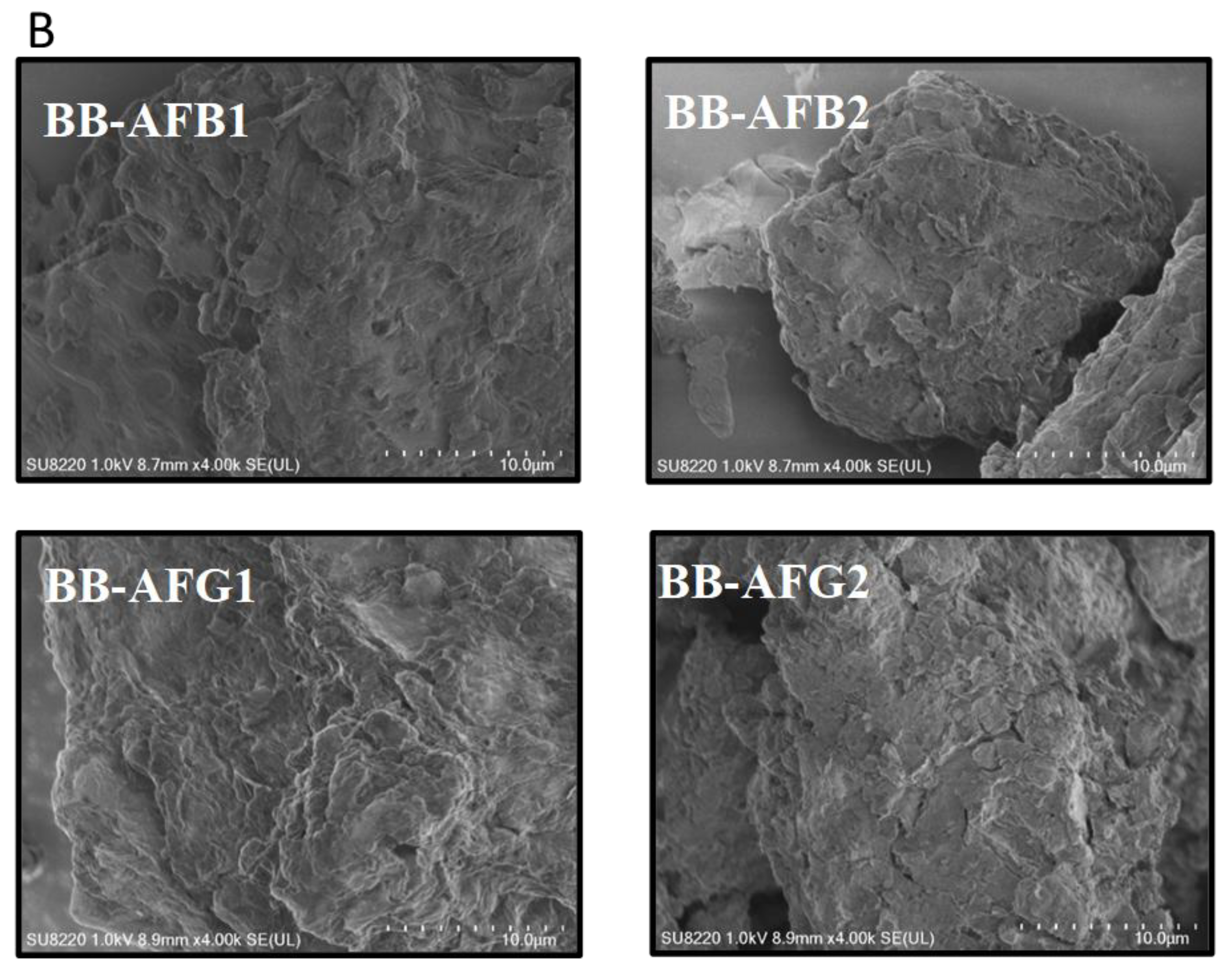
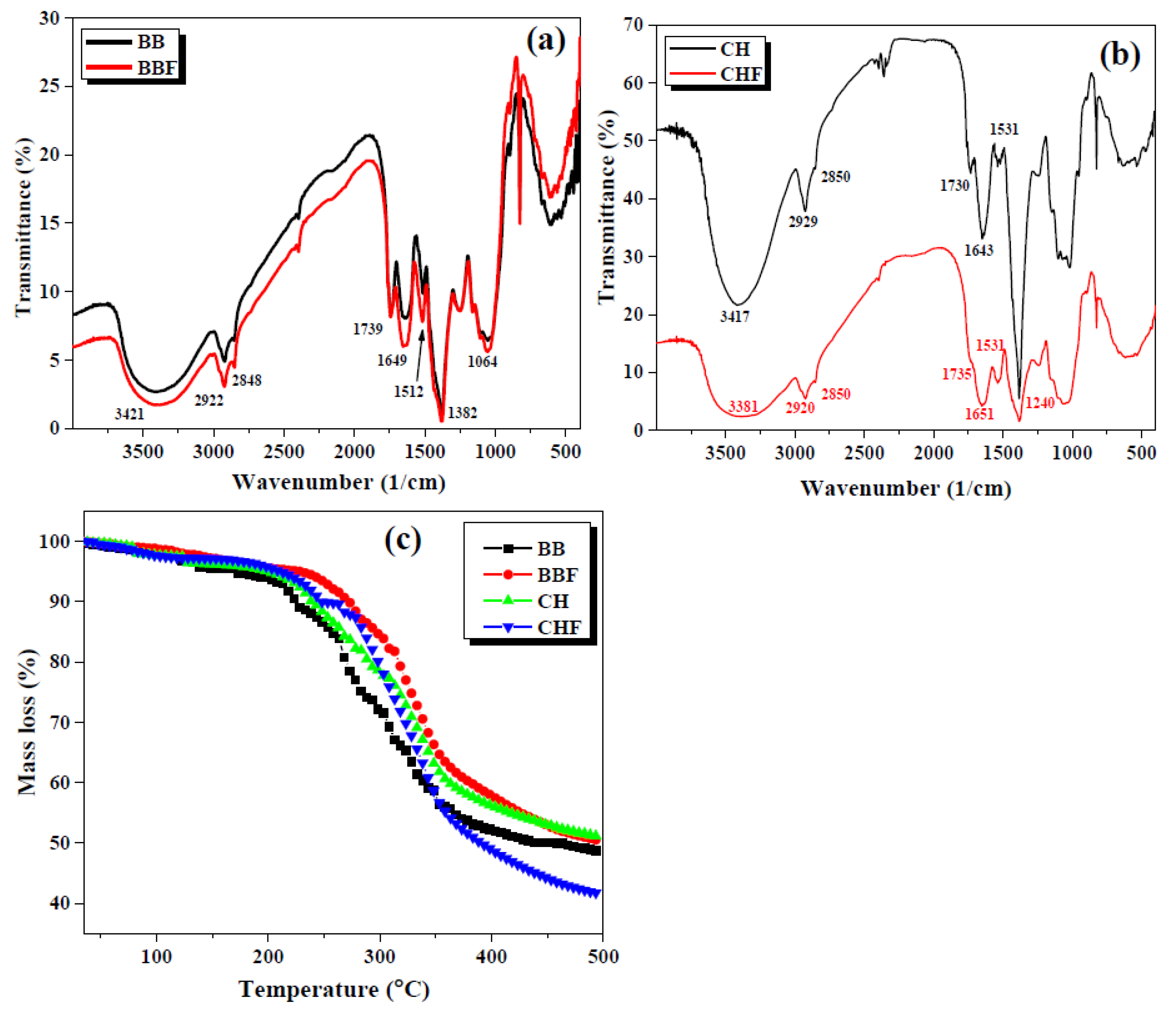
2.1. Textural Characterization of the Bio-Sorbents
2.2. Adsorption Performance of Blueberry
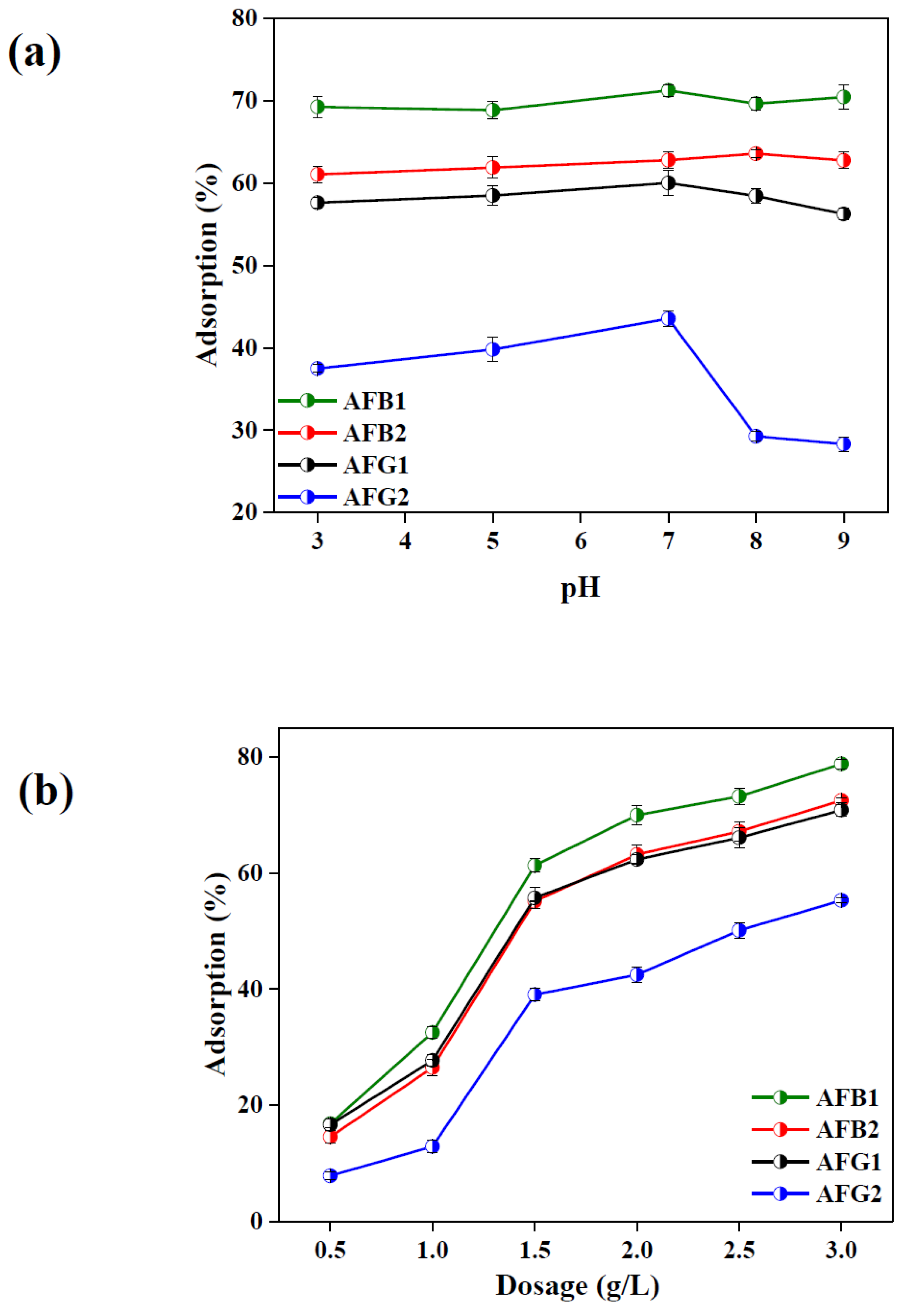
2.2.1. Effect of Solution pH
2.2.2. Effect of Adsorbent Dosage
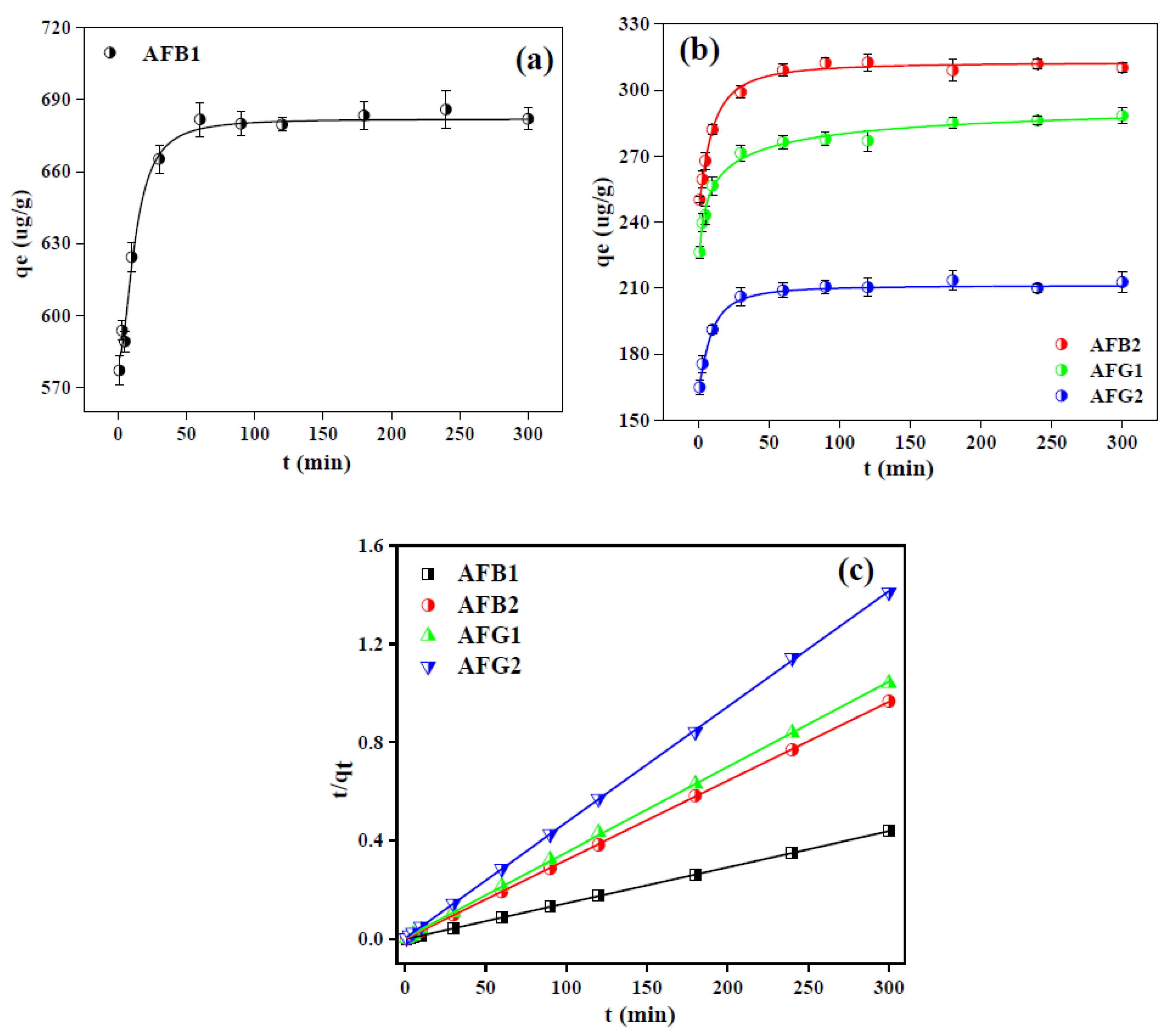
2.2.3. Adsorption Kinetics of Aflatoxins over Blueberry
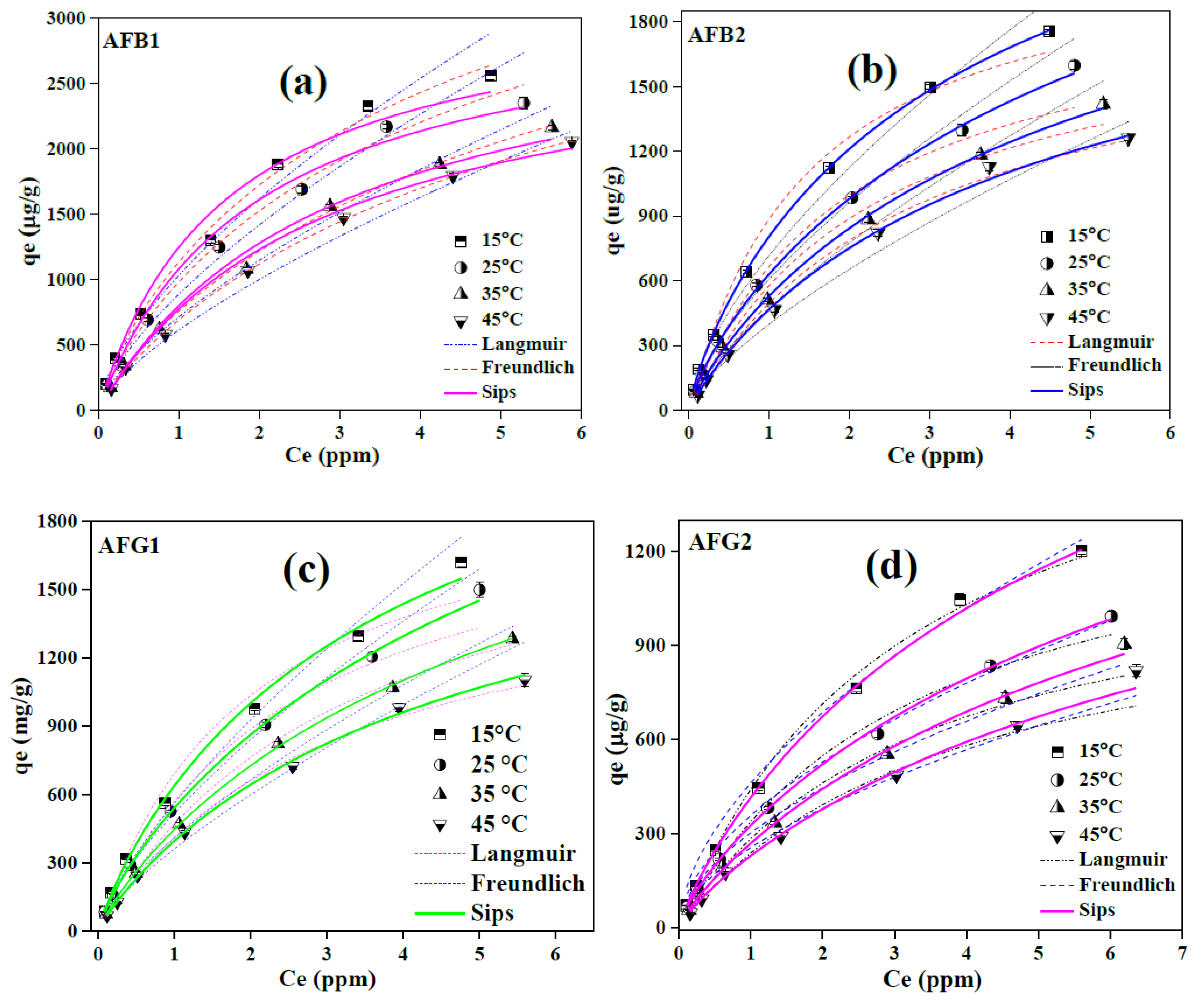
2.2.4. Isothermal Behavior of Aflatoxins Adsorption over Blueberry
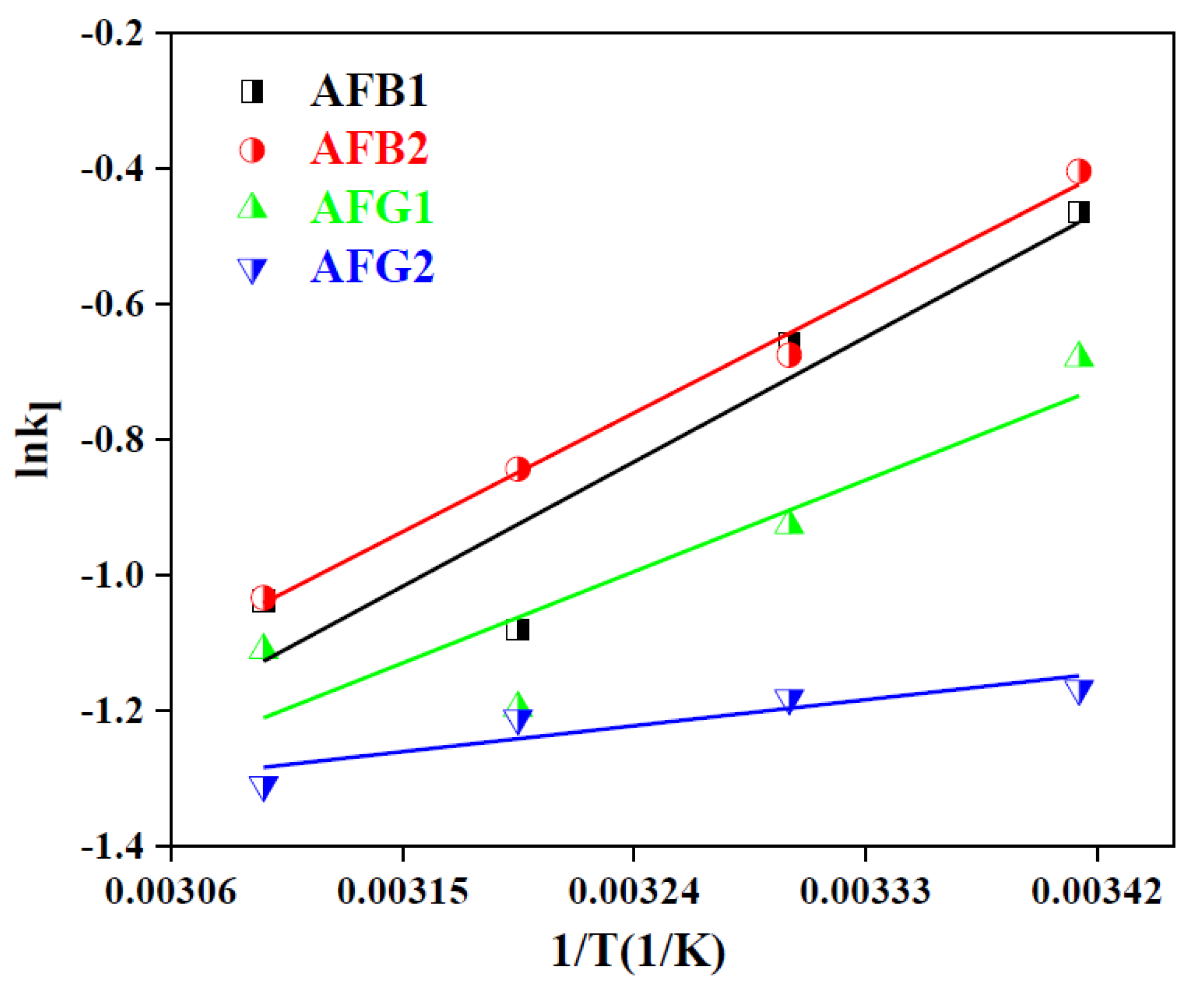
2.2.5. Adsorption Thermodynamics
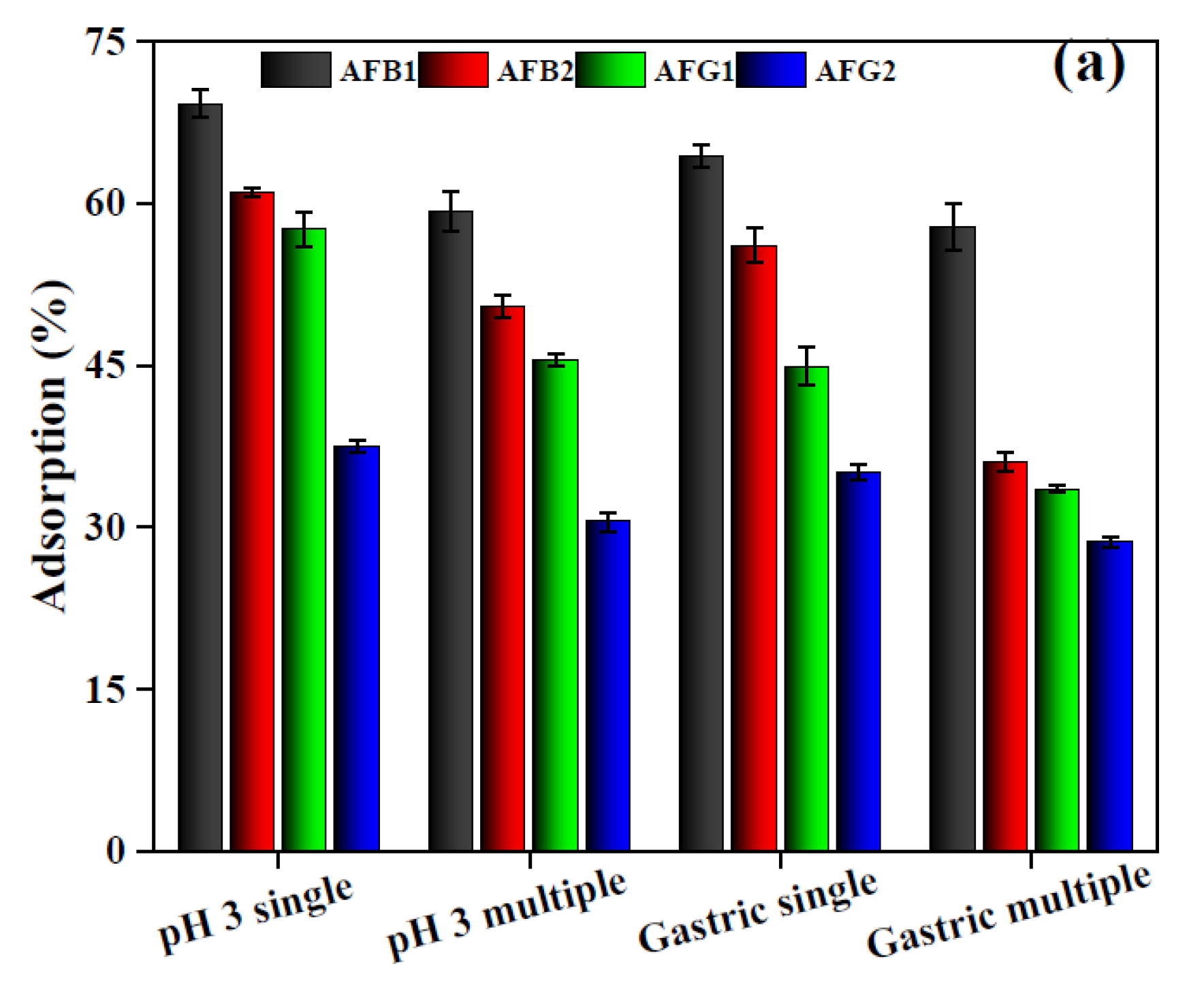
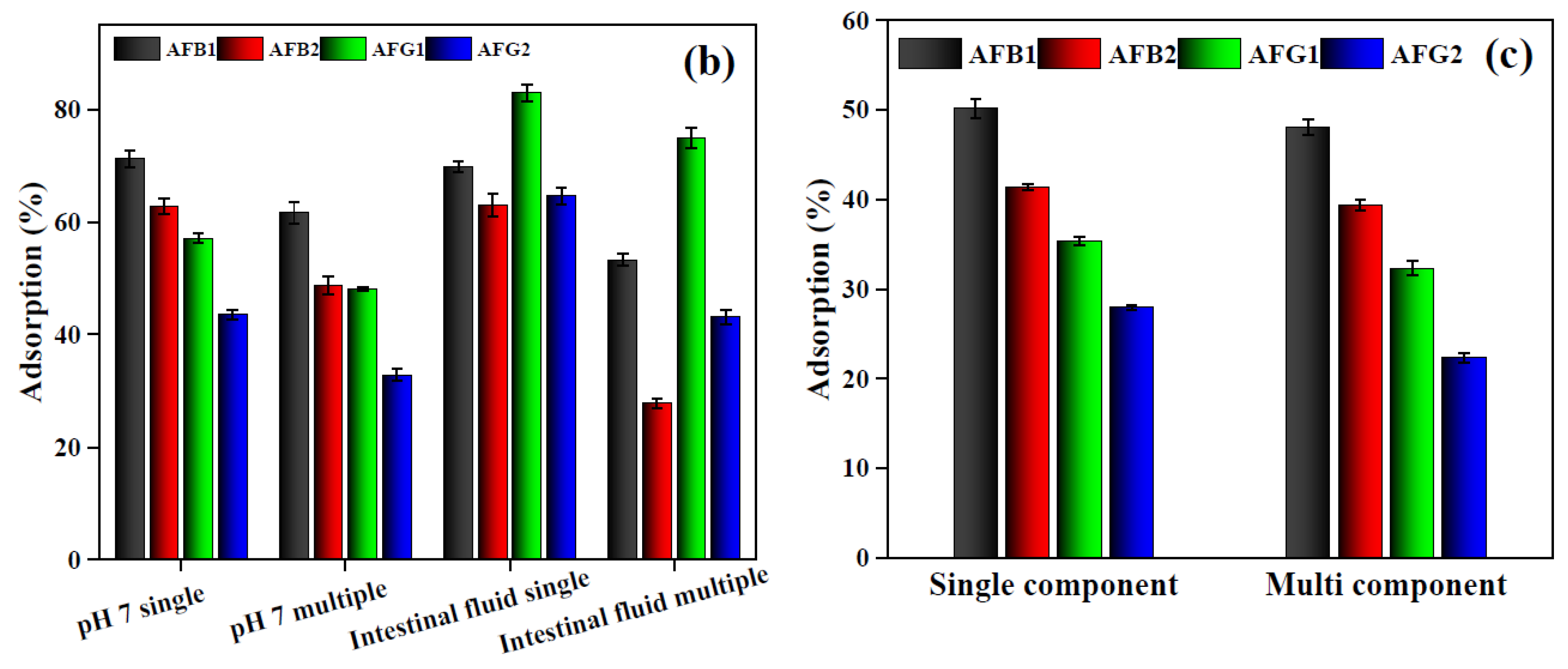
2.2.6. Adsorption in Gastrointestinal Fluids
2.2.7. Simultaneous Adsorption of Aflatoxins
2.2.8. Adsorption in Model Wine
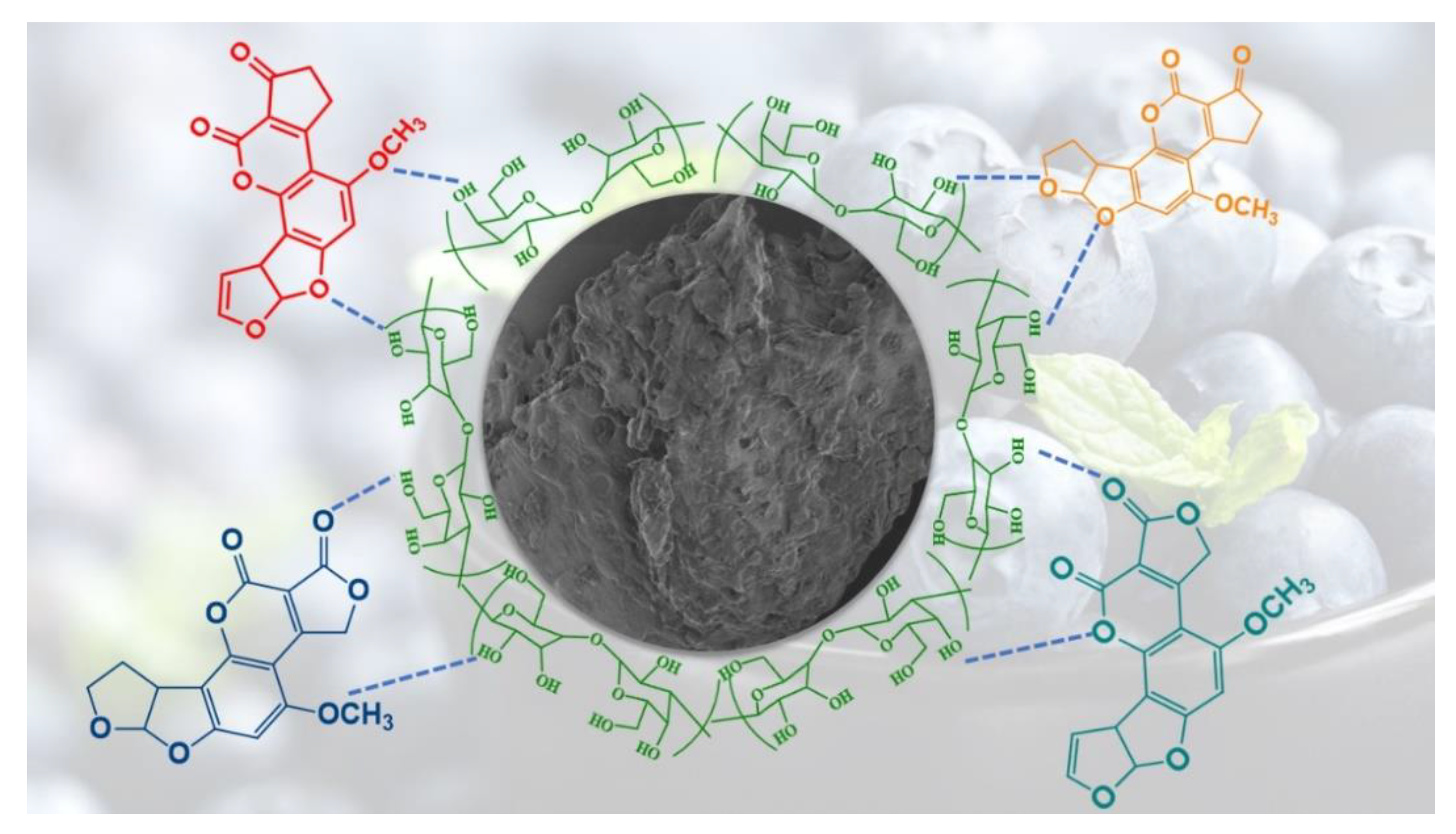
2.3. Proposed Adsorption Mechanism for Aflatoxins over Blueberry
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Material Synthesis
4.3. Characterization of Biosorbents
4.4. Initial Adsorbent Screening
4.5. High-Performance Liquid Chromatography (HPLC) Quantification of Aflatoxins
4.6. Batch Adsorption Experiments
4.7. Single and Multi-Toxin Adsorption in Simulated Gastrointestinal Fluid
4.8. Single and Multi-Toxin Adsorption in Model Wine
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coppock, R.W.; Christian, R.G.; Jacobsen, B.J. Chapter 69—Aflatoxins. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 983–994. ISBN 978-0-12-811410-0. [Google Scholar]
- Rasheed, U.; Wu, H.; Wei, J.; Ou, X.; Qin, P.; Yao, X.; Chen, H.; Chen, A.J.; Liu, B. A polyphasic study of Aspergillus section Flavi isolated from corn in Guangxi, China-a hot spot of aflatoxin contamination. Int. J. Food Microbiol. 2019, 310, 108307. [Google Scholar] [CrossRef]
- Inan, F.; Pala, M.; Doymaz, I. Use of ozone in detoxification of aflatoxin B1 in red pepper. J. Stored Prod. Res. 2007, 43, 425–429. [Google Scholar] [CrossRef]
- Severns, D.E.; Clements, M.J.; Lambert, R.J.; White, D.G. Comparison of Aspergillus ear rot and aflatoxin contamination in grain of high-oil and normal-oil corn hybrids. J. Food Prot. 2003, 66, 637–643. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Schenzel, J.; Meyer, M.T.; Phillips, P.J.; Hubbard, L.E.; Scott, T.-M.; Bucheli, T.D. Mycotoxins: Diffuse and point source contributions of natural contaminants of emerging concern to streams. Sci. Total Environ. 2014, 470, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.T.; Ferreira, J.P.; Oliveira, B.R.; Batoréu, M.C.; Crespo, M.T.B.; Pereira, V.J.; Bronze, M.R. Bottled water: Analysis of mycotoxins by LC–MS/MS. Food Chem. 2015, 176, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Jaimez, J.; Fente, C.A.; Vazquez, B.I.; Franco, C.M.; Cepeda, A.; Mahuzier, G.; Prognon, P. Application of the assay of aflatoxins by liquid chromatography with fluorescence detection in food analysis. J. Chromatogr. A 2000, 882, 1–10. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Ann. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Santos, R.R.; Vermeulen, S.; Haritova, A.; Fink-Gremmels, J. Isotherm modeling of organic activated bentonite and humic acid polymer used as mycotoxin adsorbents. Food Addit. Contam. Part A 2011, 28, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Franco, A.; Hernández-Patlán, D.; Solís-Cruz, B.; López-Arellano, R.; Tellez-Isaias, G.; Vázquez-Durán, A.; Méndez-Albores, A. Assessing the Aflatoxin B1 Adsorption capacity between biosorbents using an In Vitro multicompartmental model simulating the dynamic conditions in the gastrointestinal tract of poultry. Toxins 2018, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Murtaza, G.; Kunhikrishnan, A.; Seshadri, B.; Shahid, M.; Ali, S.; Bolan, N.S.; Ok, Y.S. Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Environ. Sci. Technol. 2016, 46, 467–499. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; García-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Wang, J.; Peng, S.; Wan, Z.; Yue, Z.; Wu, J.; Chen, T. Feasibility of anaerobic digested corn stover as biosorbent for heavy metal. Bioresour. Technol. 2013, 132, 453–456. [Google Scholar] [CrossRef]
- Daneshfozoun, S.; Abdullah, M.A.; Abdullah, B. Preparation and characterization of magnetic biosorbent based on oil palm empty fruit bunch fibers, cellulose and Ceiba pentandra for heavy metal ions removal Industrial Crops & Products Preparation and characterization of magnetic biosorbent based on oi. Ind. Crop. Prod. 2017, 105, 93–103. [Google Scholar]
- Pal, D.; Maiti, S.K. An approach to counter sediment toxicity by immobilization of heavy metals using waste fish scale derived biosorbent. Ecotoxicol. Environ. Saf. 2020, 187, 109833. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Kim, N.; Park, M.; Park, D. A new efficient forest biowaste as biosorbent for removal of cationic heavy metals. Bioresour. Technol. 2015, 175, 629–632. [Google Scholar] [CrossRef]
- Pap, S.; Radonić, J.; Trifunović, S.; Adamović, D.; Mihajlović, I.; Miloradov, M.V.; Sekulić, M.T. Evaluation of the adsorption potential of eco-friendly activated carbon prepared from cherry kernels for the removal of Pb2+, Cd2+ and Ni2+ from aqueous wastes. J. Environ. Manag. 2016, 184, 297–306. [Google Scholar] [CrossRef]
- Soleimani, M.; Kaghazchi, T. Agricultural waste conversion to activated carbon by chemical activation with phosphoric acid. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2007, 30, 649–654. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F.; Önal, Y. Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J. Clean. Prod. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of multi-mycotoxin adsorption efficacy of grape pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Ramales-Valderrama, R.A.; Vázquez-Durán, A.; Méndez-Albores, A. Biosorption of B-aflatoxins Using Biomasses Obtained from Formosa Firethorn. Toxins 2016, 8, 218. [Google Scholar] [CrossRef]
- Gambacorta, L.; Pinton, P.; Avantaggiato, G.; Oswald, I.P.; Solfrizzo, M. Grape pomace, an agricultural byproduct reducing mycotoxin absorption: In vivo assessment in pig using urinary biomarkers. J. Agric. Food Chem. 2016, 64, 6762–6771. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, R.; Antonissen, G.; De Boevre, M.; Detavernier, C.; Van de Velde, M.; De Saeger, S.; Ducatelle, R.; Hadj Ayed, M.; Ghorbal, A. Calcination enhances the aflatoxin and Zearalenone binding efficiency of a tunisian clay. Toxins 2019, 11, 602. [Google Scholar] [CrossRef]
- Chen, A.J.; Hubka, V.; Frisvad, J.C.; Visagie, C.M.; Houbraken, J.; Meijer, M.; Varga, J.; Demirel, R.; Jurjević, Ž.; Kubátová, A. Polyphasic taxonomy of Aspergillus section Aspergillus (formerly Eurotium), and its occurrence in indoor environments and food. Studies Mycol. 2017, 88, 37–135. [Google Scholar] [CrossRef]
- Rodrigues, P.; Venâncio, A.; Kozakiewicz, Z.; Lima, N. A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from Portuguese almonds. Int. J. Food Microbiol. 2009, 129, 187–193. [Google Scholar] [CrossRef]
- Liu, S.; Ge, H.; Wang, C.; Zou, Y.; Liu, J. Agricultural waste/graphene oxide 3D bio-adsorbent for highly efficient removal of methylene blue from water pollution. Sci. Total Environ. 2018, 628–629, 959–968. [Google Scholar] [CrossRef]
- Toumi, K.H.; Bergaoui, M.; Khalfaoui, M.; Benguerba, Y.; Erto, A.; Dotto, G.L.; Amrane, A.; Nacef, S.; Ernst, B. Computational study of acid blue 80 dye adsorption on low cost agricultural Algerian olive cake waste: Statistical mechanics and molecular dynamic simulations. J. Mol. Liq. 2018, 271, 40–50. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; El Ghoul, Y.; Alminderej, F.M. Populus tremula, Nerium oleander and Pergularia tomentosa seed fibers as sources of cellulose and lignin for the bio-sorption of methylene blue. Int. J. Biol. Macromol. 2019, 121, 655–665. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Mesoporous activated carbons of low-cost agricultural bio-wastes with high adsorption capacity: Preparation and artificial neural network modeling of dye removal from single and multicomponent (binary and ternary) systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Rasheed, U.; Ain, Q.U.; Yaseen, M.; Fan, X.; Yao, X.; Tong, Z.; Liu, B. Modification of bentonite with orange peels extract and its application as mycotoxins’ binder in buffered solutions and simulated gastrointestinal fluids. J. Clean. Prod. 2020, 267, 122105. [Google Scholar] [CrossRef]
- Tong, X.; Jiang, L.; Li, Y.; Chen, X.; Zhao, Y.; Hu, B.; Zhang, F. Function of agricultural waste montmorillonite-biochars for sorptive removal of 17β-estradiol. Bioresour. Technol. 2020, 296, 122368. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zheng, H.; Yu, Z.; Sun, Y.; Wang, Y.; Zhao, C.; Ding, W. Functioned hollow glass microsphere as a self-floating adsorbent: Rapid and high-efficient removal of anionic dye. J. Hazard. Mater. 2020, 381, 120971. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; He, R.; Tong, Z. Fabrication of magnetically separable 3-acrylamidopropyltrimethylammonium chloride intercalated bentonite composite for the efficient adsorption of cationic and anionic dyes. Appl. Surf. Sci. 2020, 514, 145929. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, P.; Zhao, L.; Han, B. Human hair-derived nitrogen and sulfur co-doped porous carbon materials for gas adsorption. RSC Adv. 2015, 5, 73980–73988. [Google Scholar] [CrossRef]
- Lee, L.Y.; Gan, S.; Yin Tan, M.S.; Lim, S.S.; Lee, X.J.; Lam, Y.F. Effective removal of Acid Blue 113 dye using overripe Cucumis sativus peel as an eco-friendly biosorbent from agricultural residue. J. Clean. Prod. 2016, 113, 194–203. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Azadeh, E.; Abdulkhani, A. Removal of Acid Orange 7 and Remazol Black 5 reactive dyes from aqueous solutions using a novel biosorbent. Mater. Sci. Eng. C 2012, 32, 1394–1400. [Google Scholar] [CrossRef]
- Sun, Z.; Song, A.; Wang, B.; Wang, G.; Zheng, S. Adsorption behaviors of aflatoxin B 1 and zearalenone by organo-rectorite modified with quaternary ammonium salts. J. Mol. Liq. 2018, 264, 645–651. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhang, Q.L.; Yao, S.J. A novel biosorbent formed of marine-derived Penicillium janthinellum mycelial pellets for removing dyes from dye-containing wastewater. Chem. Eng. J. 2015, 259, 837–844. [Google Scholar] [CrossRef]
- Kalaruban, M.; Loganathan, P.; Shim, W.G.; Kandasamy, J.; Ngo, H.H.; Vigneswaran, S. Enhanced removal of nitrate from water using amine-grafted agricultural wastes. Sci. Total Environ. 2016, 565, 503–510. [Google Scholar] [CrossRef]
- Rathod, M.; Mody, K.; Basha, S. Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alverezii. J. Clean. Prod. 2014, 84, 484–493. [Google Scholar] [CrossRef]
- Mokhtar, A.; Abdelkrim, S.; Djelad, A.; Sardi, A.; Boukoussa, B.; Sassi, M.; Bengueddach, A. Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads. Carbohydr. Polym. 2020, 229, 115399. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Duan, L.; Yang, H.; Gao, J. Tetracycline adsorption onto rice husk ash, an agricultural waste: Its kinetic and thermodynamic studies. J. Mol. Liq. 2016, 222, 487–494. [Google Scholar] [CrossRef]
- Ain, Q.U.; Zhang, H.; Yaseen, M.; Rasheed, U.; Liu, K.; Subhan, S.; Tong, Z. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb(II), Cd(II), and crystal violet from aqueous solution. J. Clean. Prod. 2019, 247, 119088. [Google Scholar] [CrossRef]
- Deniz, F.; Ersanli, E.T. Simultaneous bioremoval of two unsafe dyes from aqueous solution using a novel green composite biosorbent. Microchem. J. 2016, 128, 312–319. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhao, F.J. Bisorption of chromium(VI) from aqueous solutions by Sargassum thunbergii Kuntze. Biotechnol. Biotechnol. Equip. 2014, 28, 259–265. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; Tong, Z. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite. J. Hazard. Mater. 2020, 397, 122758. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.N.; Gogate, P.R. Efficient removal of Acid Green 25 dye from wastewater using activated Prunus Dulcis as biosorbent: Batch and column studies. J. Environ. Manag. 2018, 210, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of surfactants on sand surface in enhanced Oil Recovery: Isotherms, kinetics and thermodynamic studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Wang, G.; Miao, Y.; Sun, Z.; Zheng, S. Simultaneous adsorption of aflatoxin B1 and zearalenone by mono- and di-alkyl cationic surfactants modified montmorillonites. J. Colloid Interface Sci. 2017. [Google Scholar] [CrossRef]
- Barrientos-velázquez, A.L.; Marroquin, A.; Liu, L.; Phillips, T.; Deng, Y. Influence of layer charge origin and layer charge density of smectites on their a fl atoxin adsorption. Appl. Clay Sci. 2016, 132, 281–289. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Alonso, V.A.; Cavaglieri, L.R.; Dalcero, A.M.; Chiacchiera, S.M. Effect of monogastric and ruminant gastrointestinal conditions on in vitro aflatoxin B 1 adsorption ability by a montmorillonite. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2013, 30, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Al-Taher, F.; Banaszewski, K.; Jackson, L.; Zweigenbaum, J.; Ryu, D.; Cappozzo, J. Rapid method for the determination of multiple mycotoxins in wines and beers by LC-MS/MS using a stable isotope dilution assay. J. Agric. Food Chem. 2013, 61, 2378–2384. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Baeumner, A.J.; Feller, K.H. Rapid and sensitive inhibition-based assay for the electrochemical detection of Ochratoxin A and Aflatoxin M1 in red wine and milk. Electrochim. Acta 2017, 243, 82–89. [Google Scholar] [CrossRef]
- Sugita-Konishi, Y.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Aoyama, K.; Fujita, K.; Kai, S.; et al. Occurrence of aflatoxins, ochratoxin A, and fumonisins in retail foods in Japan. J. Food Prot. 2006, 69, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Rasch, C.; Böttcher, M.; Kumke, M. Determination of aflatoxin B1 in alcoholic beverages: Comparison of one- and two-photon-induced fluorescence. Anal. Bioanal. Chem. 2010, 397, 87–92. [Google Scholar] [CrossRef]
- Okaru, A.O.; Abuga, K.O.; Kibwage, I.O.; Hausler, T.; Luy, B.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Aflatoxin contamination in unrecorded beers from Kenya—A health risk beyond ethanol. Food Control 2017, 79, 344–348. [Google Scholar] [CrossRef]
- Kumagai, S.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Aoyama, K.; Fujita, K.; Kai, S.; et al. Aflatoxin and ochratoxin A contamination of retail foods and intake of these mycotoxins in Japan. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2008, 25, 1101–1106. [Google Scholar] [CrossRef]
- Beaver, J.W.; Miller, K.V.; Medina, P.C.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.; Oberholster, A. Heat-dependent desorption of proanthocyanidins from grape-derived cellwall material under variable ethanol concentrations in model wine systems. Molecules 2019, 24, 3561. [Google Scholar] [CrossRef]
- Beaver, J.W.; Medina, P.C.; Miller, K.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.; Oberholster, A. Effects of the temperature and ethanol on the kinetics of proanthocyanidin adsorption in model wine systems. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Nguyen, T.V.; Chao, H.P. Insight into the adsorption mechanism of cationic dye onto biosorbents derived from agricultural wastes. Chem. Eng. Commun. 2017, 204, 1020–1036. [Google Scholar] [CrossRef]
- Peng, B.; Chen, L.; Que, C.; Yang, K.; Deng, F.; Deng, X.; Shi, G.; Xu, G.; Wu, M. Adsorption of Antibiotics on graphene and biochar in aqueous solutions induced by π-π interactions. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Shar, Z.H.; Fletcher, M.T.; Sumbal, G.A.; Sherazi, S.T.H.; Giles, C.; Bhanger, M.I.; Nizamani, S.M. Banana peel: An effective biosorbent for aflatoxins. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2016, 33, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative efficacy of agricultural by-products in sequestering mycotoxins. J. Sci. Food Agric. 2019, 99, 1623–1634. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Gao, J.; Cheng, K.; Yuan, F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. LWT 2019, 109, 233–240. [Google Scholar] [CrossRef]
- Muhammad, Y.; Rahman, A.U.; Rashid, H.U.; Sahibzada, M.; Subhan, S.; Tong, Z. Hydrodesulfurization of dibenzothiophene using Pd-promoted Co–Mo/Al2O3 and Ni–Mo/Al2O3 catalysts coupled with ionic liquids at ambientoperating conditions. RSC Adv. 2019, 9, 10371–10385. [Google Scholar] [CrossRef]
- Muhammad, Y.; Ur, H.; Subhan, S.; Ur, A.; Sahibzada, M. Boosting the hydrodesulfurization of dibenzothiophene e ffi ciency of Mn decorated (Co/Ni)-Mo/Al2O3 catalysts at mild temperature and pressure by coupling with phosphonium based ionic liquids. Chem. Eng. J. 2019, 375, 121957. [Google Scholar] [CrossRef]
- Ingebrigtson, D.N.; Smith, A.L. lnfrared analvsis of solids by potassium bromide pellet technique. Anal. Chem. 1954, 26, 1765–1768. [Google Scholar] [CrossRef]
- Muhammad, Y.; Lu, Y.; Shen, C.; Li, C. Dibenzothiophene hydrodesulfurization over Ru promoted alumina based catalysts using in situ generated hydrogen. Energy Convers. Manag. 2011, 52, 1364–1370. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Song, L.; Mashayekhi, H.; Chefetz, B.; Xing, B. Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids. Environ. Sci. Technol. 2011, 45, 6018–6024. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Wilkinson, K.L.; Jiranek, V.; Taylor, D.K. Development and evaluation of a HS-SPME GC-MS method for determining the retention of volatile phenols by cyclodextrin in model wine. Molecules 2019, 24, 3432. [Google Scholar] [CrossRef] [PubMed]

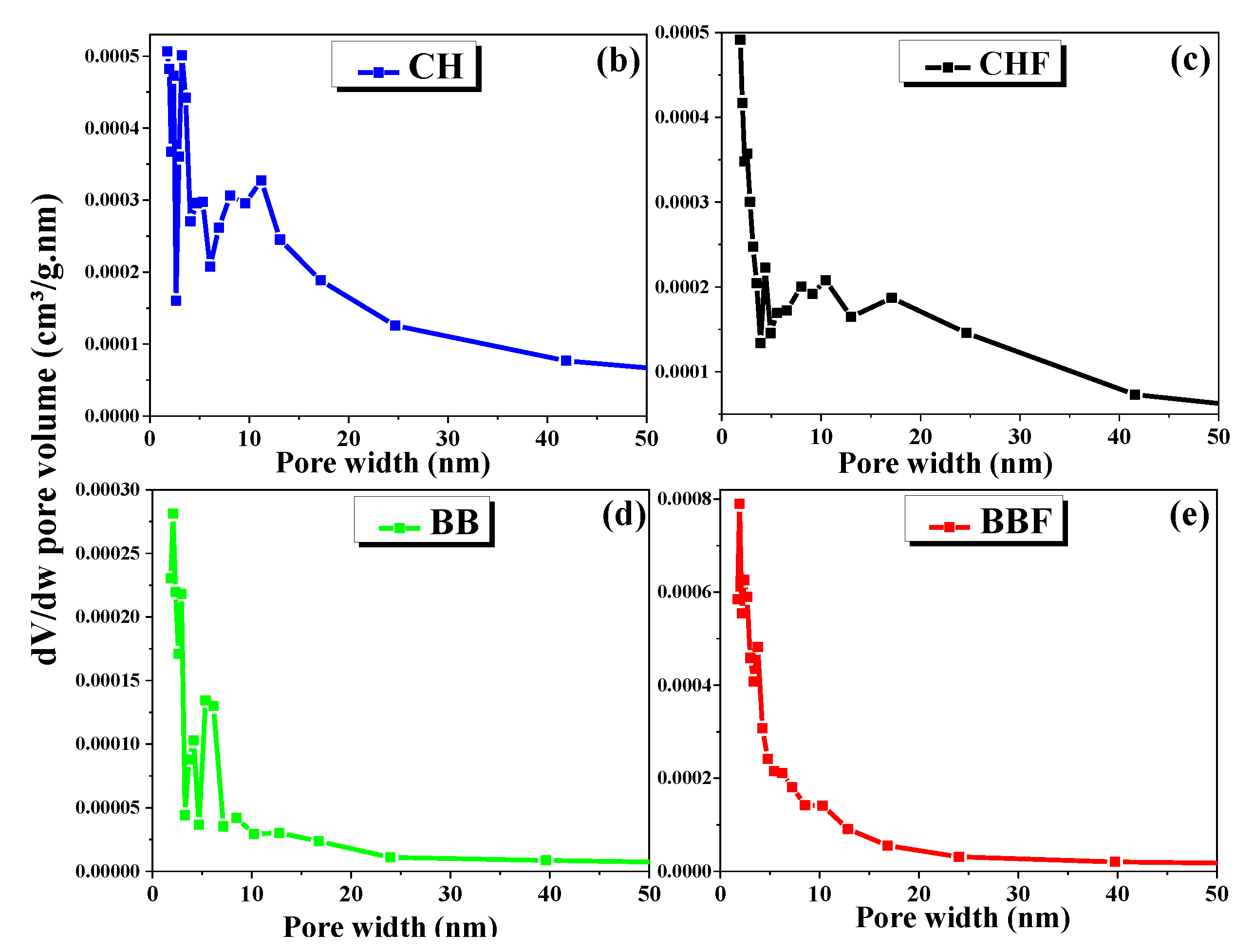


| Adsorbent | BET Surface Area (m²/g) | Langmuir Surface Area (m²/g) | BJH * Surface Area of Pores (m²/g) | BJH Volume of Pores (cm³/g) | BJH Average Pore Width (nm) | Maximum Pore Volume (cm³/g) | Median Pore Width (nm) | Average Particle Size (nm) |
|---|---|---|---|---|---|---|---|---|
| BB | 1.41 | 5.35 | 1.124 | 0.002 | 7.97 | 0.0004 | 1.067 | 4270 |
| BBF | 4.59 | 23.32 | 3.36 | 0.006 | 7.32 | 0.0011 | 1.117 | 1305 |
| CH | 3.913 | 43.41 | 3.989 | 0.015 | 14.66 | 0.0013 | 0.988 | 1533 |
| CHF | 3.607 | 30.5 | 3.802 | 0.013 | 16.555 | 0.0011 | 1.005 | 1663 |
| Type of AF | Pseudo Second Order | Pseudo First Order | ||||
|---|---|---|---|---|---|---|
| qe (µg/g) | k2 (min−1) | R2 | qe (µg/g) | k1 (g/(mg.min)) | R2 | |
| AFB1 | 1.14E+03 | 0.00088 | 0.999 | 44.53253 | 0.01217 | 0.557 |
| AFB2 | 5.46E+02 | 0.00183 | 0.999 | 21.4246 | 0.01205 | 0.713 |
| AFG1 | 1.66E+02 | 0.00602 | 0.999 | 40.42264 | 0.01312 | 0.912 |
| AFG2 | 2.52E+02 | 0.00397 | 0.999 | 21.25049 | 0.01095 | 0.681 |
| Type of AF | Langmuir Isotherm | Freundlich Isotherm | Sips | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm (µg/g) | kl (L/mg) | R2 | kf (L/mg) | n | R2 | ks | bs (L/mg) | qs (µg/g) | R2 | |
| AFB1 | 3229 | 0.629 | 0.985 | 887 | 1.48 | 0.989 | 0.194 | 0.77 | 4603 | 0.995 |
| AFB2 | 2215 | 0.668 | 0.989 | 718 | 1.54 | 0.989 | 0.229 | 0.78 | 2989 | 0.999 |
| AFG1 | 2053 | 0.507 | 0.989 | 561 | 1.38 | 0.983 | 0.226 | 0.86 | 2716 | 0.995 |
| AFG2 | 1860 | 0.311 | 0.995 | 461 | 1.74 | 0.988 | 0.144 | 0.84 | 2375 | 0.999 |
| ΔG° (KJ/mol) at (°C) | AFB1 | AFB2 | AFG1 | AFG2 |
|---|---|---|---|---|
| 15 | −1.531 | −1.626 | −1.235 | −0.758 |
| 25 | −1.305 | −1.282 | −0.998 | −0.773 |
| 35 | −0.883 | −1.119 | −0.786 | −0.775 |
| 45 | −0.951 | −0.956 | −0.884 | −0.725 |
| ΔH° (kJ/mol) | −16.98 | −16.21 | −12.47 | −3.542 |
| ΔS° (kJ/molK) | −0.062 | −0.059 | −0.049 | −0.022 |
| Adsorbent Type | Aflatoxin | qm (exp) | Reaction Conditions (Dose and Time) | References |
|---|---|---|---|---|
| Grape pomace | AFB1 | 4.7 mg/g | 1 g/L, 90 min | [23] |
| Formosa Firethorn biomass | AFB1 | 0.016 mg/g | 250 g/L, 240 min | [24] |
| Banana peel | AFB1 | 0.0084 mg/g | 60 g/L, 15 min | [65] |
| AFB2 | 0.0095 mg/g | |||
| AFG1 | 0.0004 mg/g | |||
| AFG2 | 0.0011 mg/g | |||
| Sangiovese grape pomace | AFB1 | 2.93 mg/g | 2 g/L, 90 min | [66] |
| Malvasia grape pomace | AFB1 | 1.43 mg/g | 10 g/L, 90 min | |
| Almond hull | AFB1 | 2.28 mg/g | 15 g/L, 90 min | |
| Artichoke | AFB1 | 1.79 mg/g | 15 g/L, 90 min | |
| Blueberry fruit waste | AFB1 | 4.60 mg/g | 2 g/L, 90 min | This study |
| AFB2 | 2.98 mg/g | This study | ||
| AFG1 | 2.71 mg/g | This study | ||
| AFG2 | 2.37 mg/g | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, U.; Ain, Q.U.; Yaseen, M.; Santra, S.; Yao, X.; Liu, B. Assessing the Aflatoxins Mitigation Efficacy of Blueberry Pomace Biosorbent in Buffer, Gastrointestinal Fluids and Model Wine. Toxins 2020, 12, 466. https://doi.org/10.3390/toxins12070466
Rasheed U, Ain QU, Yaseen M, Santra S, Yao X, Liu B. Assessing the Aflatoxins Mitigation Efficacy of Blueberry Pomace Biosorbent in Buffer, Gastrointestinal Fluids and Model Wine. Toxins. 2020; 12(7):466. https://doi.org/10.3390/toxins12070466
Chicago/Turabian StyleRasheed, Usman, Qurat Ul Ain, Muhammad Yaseen, Sayantan Santra, Xiaohua Yao, and Bin Liu. 2020. "Assessing the Aflatoxins Mitigation Efficacy of Blueberry Pomace Biosorbent in Buffer, Gastrointestinal Fluids and Model Wine" Toxins 12, no. 7: 466. https://doi.org/10.3390/toxins12070466
APA StyleRasheed, U., Ain, Q. U., Yaseen, M., Santra, S., Yao, X., & Liu, B. (2020). Assessing the Aflatoxins Mitigation Efficacy of Blueberry Pomace Biosorbent in Buffer, Gastrointestinal Fluids and Model Wine. Toxins, 12(7), 466. https://doi.org/10.3390/toxins12070466