Nature-Identical Compounds and Organic Acids Reduce E. coli K88 Growth and Virulence Gene Expression In Vitro
Abstract
1. Introduction
2. Results
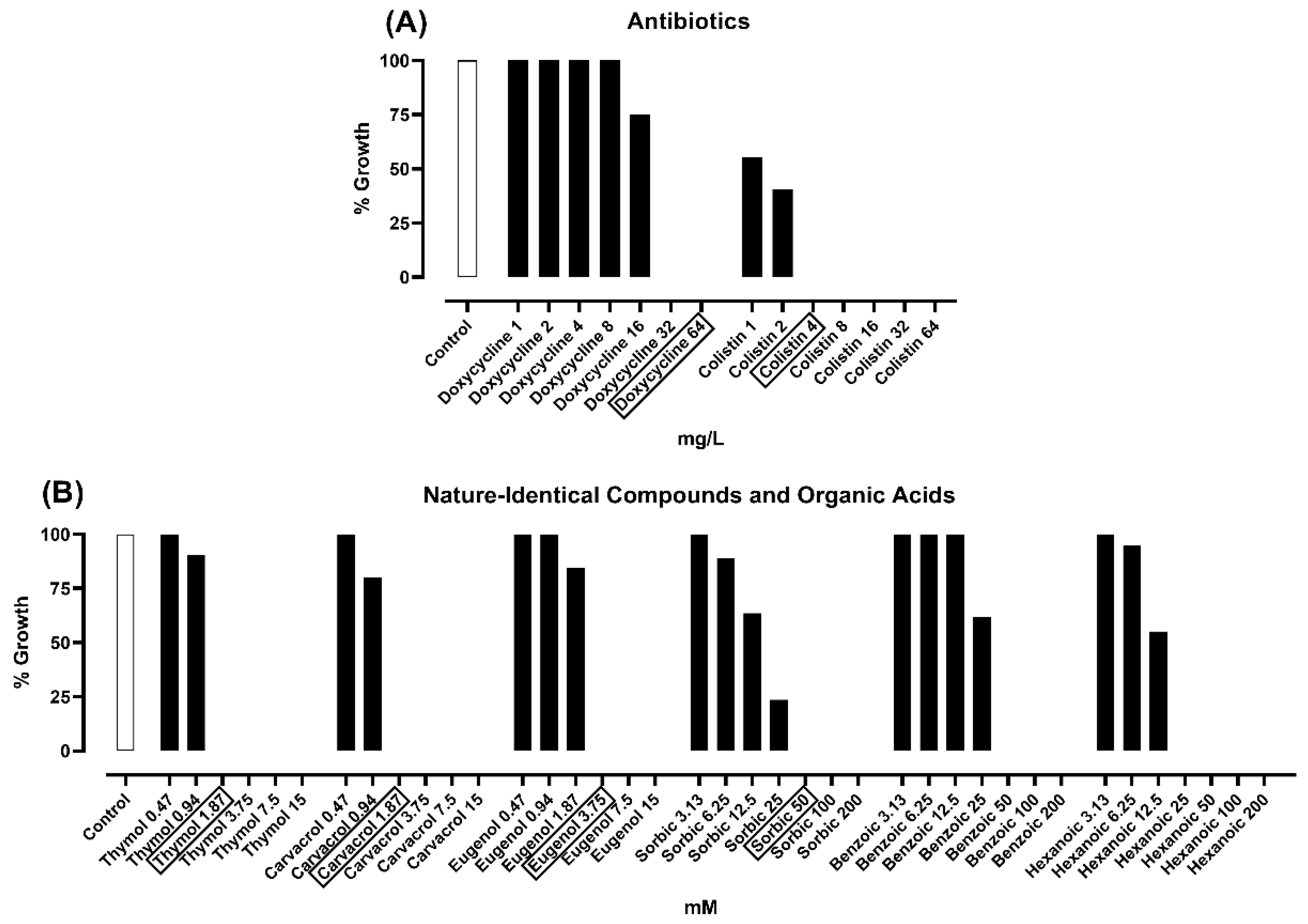
2.1. Minimal Inhibitory Concentration (MIC) Assay
2.2. Minimal Bactericidal Concentration (MBC) Assay
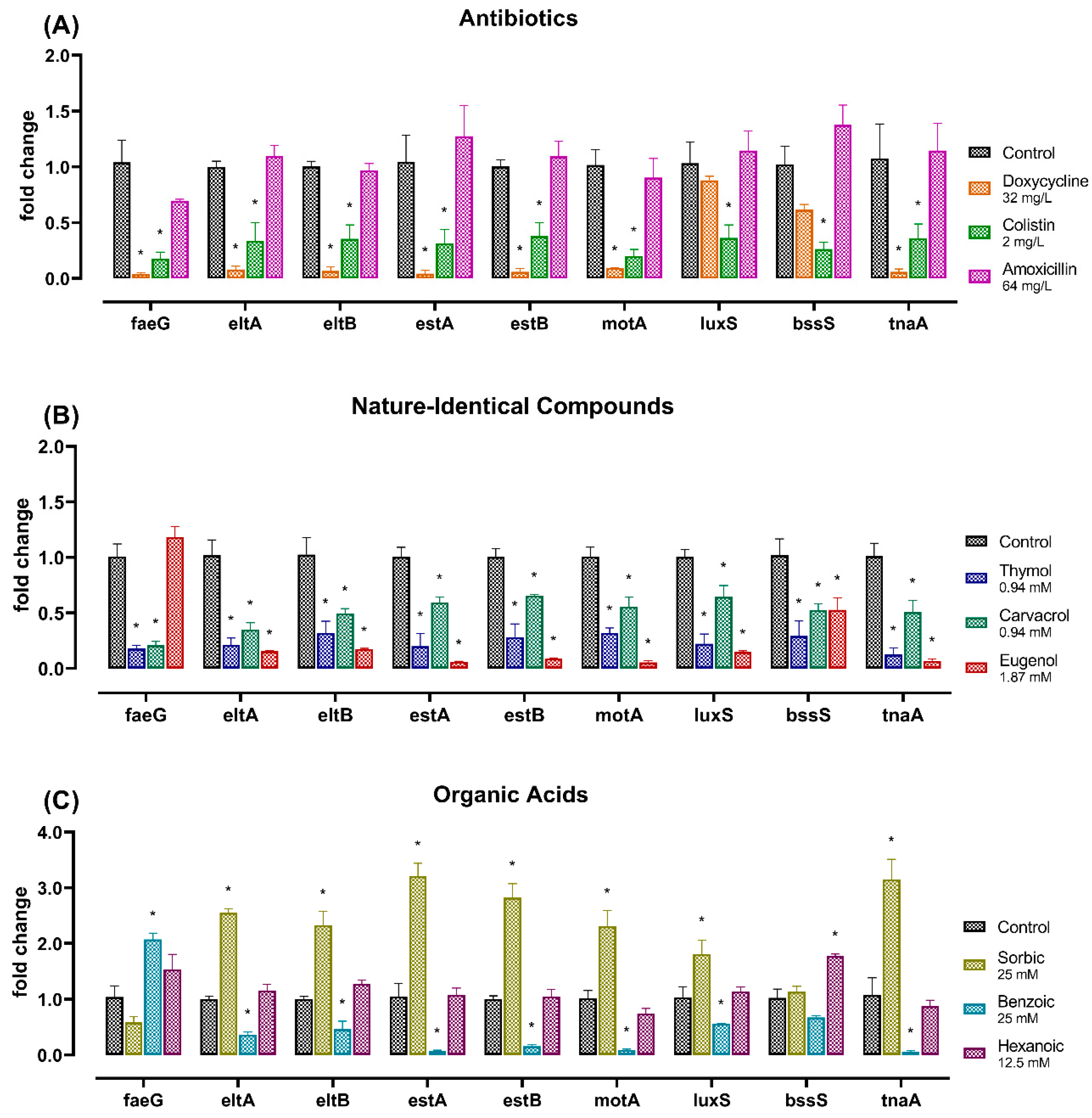
2.3. Gene Expression Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strain and Culture Conditions
5.2. Chemicals and Stock Solutions
5.3. Minimal Inhibitory Concentration (MIC) Assay
5.4. Minimal Bactericidal Concentration (MBC) Assay
5.5. Gene Expression Analysis
5.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, Á. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porc. Health Manag. 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Cordonnier, C.; Livrelli, V.; Van de Wiele, T.; Blanquet-Diot, S. Enterotoxigenic and Enterohemorrhagic Escherichia coli: Survival and Modulation of Virulence in the Human Gastrointestinal Tract. In Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; Amidou, S., Ed.; InTech: London, UK, 2017; pp. 3–24. [Google Scholar]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 1–47. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli targeting intestinal epithelial tight junctions: An effective way to alter the barrier integrity. Microb. Pathog. 2017, 113, 129–134. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Antibacterial and antidiarrheal activities of plant products against enterotoxinogenic Escherichia coli. Toxins 2013, 5, 2009–2041. [Google Scholar] [CrossRef]
- Giovagnoni, G.; Tugnoli, B.; Piva, A.; Grilli, E. Organic Acids and Nature Identical Compounds Can Increase the Activity of Conventional Antibiotics Against Clostridium Perfringens and Enterococcus Cecorum In Vitro. J. Appl. Poult. Res. 2019, 28, 1398–1407. [Google Scholar] [CrossRef]
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Control of pathogenic and spoilage microorganisms in fresh-cut fruits and fruit juices by traditional and alternative natural antimicrobials. Compr. Rev. Food Sci. Food Saf. 2009, 8, 157–180. [Google Scholar] [CrossRef]
- Russell, J.B.; Diez-Gonzalez, F. The effects of fermentation acids on bacterial growth. Adv. Microb. Physiol. 1998, 39, 205–234. [Google Scholar] [CrossRef] [PubMed]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From acidifiers to intestinal health enhancers: How organic acids can improve growth efficiency of pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Toschi, A.; Piva, A.; Grilli, E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr. Res. Rev. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Grilli, E.; Tugnoli, B.; Passey, J.L.; Stahl, C.H.; Piva, A.; Moeser, A.J. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet. Res. 2015, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Amezcua, R.; Friendship, R.M.; Dewey, C.E.; Gyles, C.; Fairbrother, J.M. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can. J. Vet. Res. 2002, 66, 73–78. [Google Scholar]
- Klein, U.; Thomas, V.; de Jong, A.; Simjee, S.; Moyaert, H.; Siegwart, E.; El Garch, F.; Butty, P.; Marion, H.; Rigaut, D.; et al. Antimicrobial susceptibility monitoring of respiratory and enteric tract pathogens isolated from diseased pigs across Europe between 2009 and 2012. In Proceedings of the 7th European Symposium of Porcine Health Management, Nantes, France, 22–24 April 2015. [Google Scholar]
- Choi, C.; Ham, H.J.; Kwon, D.; Kim, J.; Cheon, D.S.; Min, K.; Cho, W.S.; Chung, H.K.; Jung, T.; Jung, K.; et al. Antimicrobial susceptibility of pathogenic Escherichia coli isolated from pigs in Korea. J. Vet. Med. Sci. 2002, 64, 71–73. [Google Scholar] [CrossRef]
- van Breda, L.K.; Dhungyel, O.P.; Ward, M.P. Antibiotic resistant Escherichia coli in southeastern Australian pig herds and implications for surveillance. Zoonoses Public Health 2018, 65, e1–e7. [Google Scholar] [CrossRef]
- Docic, M.; Bilkei, G. Differences in antibiotic resistance in Escherichia coli, isolated from East-European swine herds with or without prophylactic use of antibiotics. J. Vet. Med. Ser. B 2003, 50, 27–30. [Google Scholar] [CrossRef]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Beaudry, F.; Thériault, W.; Letellier, A. Colistin in pig production: Chemistry, mechanism of antibacterial action, microbial resistance emergence, and one health perspectives. Front. Microbiol. 2016, 7, 1789. [Google Scholar] [CrossRef]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact on Human and Animal Health; European Medicines Agency (EMA): London, UK, 2006.
- Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2020.
- Chopra, I.; Hodgson, J.; Metcalf, B.; Poste, G. The search for antimicrobial agents effective against bacteria resistant to multiple antibiotics. Antimicrob. Agents Chemother. 1997, 41, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, K.; Holley, R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Rivas, L.; Burgess, C.M.; Fanning, S.; Duffy, G. Evaluation of carvacrol for the control of Escherichia coli O157 on cattle hide and carcass cuts. Foodborne Pathog. Dis. 2012, 9, 1049–1052. [Google Scholar] [CrossRef]
- Rivas, L.; McDonnell, M.J.; Burgess, C.M.; O’Brien, M.; Navarro-Villa, A.; Fanning, S.; Duffy, G. Inhibition of verocytotoxigenic Escherichia coli in model broth and rumen systems by carvacrol and thymol. Int. J. Food Microbiol. 2010, 139, 70–78. [Google Scholar] [CrossRef]
- Jiménez, M.J.; Berrios, R.; Stelzhammer, S.; Bracarense, A.P.F.R.L. Ingestion of organic acids and cinnamaldehyde improves tissue homeostasis of piglets exposed to enterotoxic Escherichia coli (ETEC). J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef]
- Silveira, H.; Amaral, L.G.M.; Garbossa, C.A.P.; Rodrigues, L.M.; Silva, C.C.D.; Cantarelli, V.S. Benzoic acid in nursery diets increases the performance from weaning to finishing by reducing diarrhoea and improving the intestinal morphology of piglets inoculated with Escherichia coli K88. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.J.; Park, J.W.; Baek, D.H.; Kim, J.K.; Kim, I.H. Feeding the blend of organic acids and medium chain fatty acids reduces the diarrhea in piglets orally challenged with enterotoxigenic Escherichia coli K88. Anim. Feed Sci. Technol. 2017, 224, 46–51. [Google Scholar] [CrossRef]
- Sun, Y.; Kim, S.W. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim. Nutr. 2017, 3, 322–330. [Google Scholar] [CrossRef]
- Mukiza, C.N.; Dubreuil, D.J. Escherichia coli heat-stable toxin b impairs intestinal epithelial barrier function by altering tight junction proteins. Infect. Immun. 2013, 81, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, J.M.; Hardwidge, P.R.; Munson, G.P.; Rasko, D.A.; Sommerfelt, H.; Steinsland, H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010, 12, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Duan, Q.; Zhu, X.; Guo, Z.; Li, Y.; Hardwidge, P.R.; Zhu, G. Both flagella and F4 fimbriae from F4ac+ enterotoxigenic Escherichia coli contribute to attachment to IPEC-J2 cells in vitro. Vet. Res. 2013, 44, 30. [Google Scholar] [CrossRef]
- Yuan, W.; Yuk, H.-G. Effects of sublethal thymol, carvacrol, and trans-cinnamaldehyde adaptation on virulence properties of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2019, 85, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Lu, X.; Hu, P.; Chen, H.; Li, Z.; Bu, Z.; Lang, X.; Wang, X. Rapid method of luxS and pfs gene inactivation in enterotoxigenic Escherichia coli and the effect on biofilm formation. Mol. Med. Rep. 2016, 13, 257–264. [Google Scholar] [CrossRef][Green Version]
- Hu, M.; Zhang, C.; Mu, Y.; Shen, Q.; Feng, Y. Indole affects biofilm formation in bacteria. Indian J. Microbiol. 2010, 50, 362–368. [Google Scholar] [CrossRef]
- Di Martino, P.; Fursy, R.; Bret, L.; Sundararaju, B.; Phillips, R.S. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can. J. Microbiol. 2003, 49, 443–449. [Google Scholar] [CrossRef]
- Domka, J.; Lee, J.; Wood, T.K. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol. 2006, 72, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2019, 16, 1–12. [Google Scholar] [CrossRef]
- Bialvaei, A.Z.; Samadi Kafil, H. Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin. 2015, 31, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Kocić, B.D.; Ćirić, V.M.; Nikolić, D.M. Antibacterial Investigation of Thyme Essential Oil and Its Main Constituents in Combination with Tetracycline. J. Med. Food 2015, 18, 935–937. [Google Scholar] [CrossRef]
- Cirino, I.C.S.; Menezes-Silva, S.M.P.; Silva, H.T.D.; de Souza, E.L.; Siqueira-Júnior, J.P. The Essential Oil from Origanum vulgare L. and Its Individual Constituents Carvacrol and Thymol Enhance the Effect of Tetracycline against Staphylococcus aureus. Chemotherapy 2014, 60, 290–293. [Google Scholar] [CrossRef]
- Stone, K.J.; Strominger, J.L. Mechanism of Action of Bacitracin: Complexation with Metal Ion and C55-Isoprenyl Pyrophosphate. Proc. Natl. Acad. Sci. USA 1971, 68, 3223–3227. [Google Scholar] [CrossRef]
- Tovaglieri, A.; Sontheimer-Phelps, A.; Geirnaert, A.; Prantil-Baun, R.; Camacho, D.M.; Chou, D.B.; Jalili-Firoozinezhad, S.; De Wouters, T.; Kasendra, M.; Super, M.; et al. Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome 2019, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.W.; Freese, E. Lipopolysaccharide layer protection of Gram negative bacteria against inhibition by long chain fatty acids. J. Bacteriol. 1973, 115, 869–875. [Google Scholar] [CrossRef]
- Ter Beek, A.; Keijser, B.J.F.; Boorsma, A.; Zakrzewska, A.; Orij, R.; Smits, G.J.; Brul, S. Transcriptome analysis of sorbic acid-stressed Bacillus subtilis reveals a nutrient limitation response and indicates plasma membrane remodeling. J. Bacteriol. 2008, 190, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Bearson, S.; Bearson, B.; Foster, J.W. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 1997, 147, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Svensson, S.L.; Gaynor, E.C.; Swanson, M.S. ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 2010, 74, 171–199. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Casey, T.A.; Bosworth, B.T. Design and evaluation of a multiplex polymerase chain reaction assay for the simultaneous identification of genes for nine different virulence factors associated with Escherichia coli that cause diarrhea and edema disease in swine. J. Vet. Diagn. Investig. 2009, 21, 25–30. [Google Scholar] [CrossRef]
| Antibiotics (AB) | Nature-Identical Compounds (NIC) | Organic Acids (OA) | |||
|---|---|---|---|---|---|
| Substance | MIC (mg/L) | Substance | MIC (mM) | Substance | MIC (mM) |
| Amoxicillin | >64 | α-pinene | >7.5 | Benzoic | 50 |
| Ampicillin | >64 | Carvacrol | 1.87 | Butyric | >100 |
| Colistin | 4 | Eucalyptol | >7.5 | Citric | >100 |
| Doxycycline | 32 | Eugenol | 3.75 | Decanoic | >100 |
| Lincomycin | >64 | Limonene | >7.5 | Dodecanoic | >100 |
| Neomycin | >64 | Linalool | >7.5 | Formic | >100 |
| Penicillin G | >64 | Menthol | >7.5 | Fumaric | >100 |
| Thymol | 1.87 | Hexanoic | 25 | ||
| Vanillin | >7.5 | Lactic | >100 | ||
| Malic | >100 | ||||
| Octanoic | >100 | ||||
| Propionic | >100 | ||||
| Sorbic | 50 | ||||
| Function | Gene | Sequence (5′ → 3′) | Product Length (bp) | AN 1 | Ref 2 |
|---|---|---|---|---|---|
| Adhesion to cells | faeG | F: ACGTCGCAGGTTCTTACAGG R: GCTCCACTGAGTGCTGGTAG | 140 | M35954 | This study |
| LT toxin production | eltA | F: TTGGTGATCCGGTGGGAAAC R: AGGAGGTTTCTGCGTTAGGTG | 185 | LN870273 | This study |
| eltB | F: CACGGAGCTCCCCAGACTAT R: GCCTGCCATCGATTCCGTAT | 105 | M17873 | This study | |
| STa toxin production | estA | F: CAACTGAATCACTTGACTCTT R: TTAATAACATCCAGCACAGG | 158 | V00612 | [64] |
| STb toxin production | estB | F: TGCCTATGCATCTACACAA R: CTCCAGCAGTACCATCTC | 113 | M35586 | [64] |
| Flagellar movement | motA | F: TGAACGACCCCCATTACAGC R: AGCGGTCACATGAACACCTT | 155 | NZ_LBBN01000002 | This study |
| Quorum sensing | luxS | F: CAGTGCCAGTTCTTCGTTGC R: TGAACGTCTACCAGTGTGGC | 116 | HQ538844 | This study |
| Biofilm formation | bssS | F: TCCCTTCCTGCTCGGACTTA R: CAGACTCATCCGCTCGTAGG | 106 | NZ_LBBN01000031 | This study |
| tnaA | F: CGCCAAGAAAGATGCGATGG R: CGTCATACAGACCTACCGCC | 173 | NZ_LBBN01000006 | This study | |
| Housekeeping genes | ihfB | F: CCCGTCAAGACGGTTGAAGA R: TCGCCAGTCTTCGGATTACG | 152 | NC_017641 | This study |
| 16S | F: GAGGGCGCTTACCACTTTGT R: GTAAGGAGGTGATCCAACCG | 90 | NC_017641 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetti, A.; Tugnoli, B.; Rossi, B.; Giovagnoni, G.; Piva, A.; Grilli, E. Nature-Identical Compounds and Organic Acids Reduce E. coli K88 Growth and Virulence Gene Expression In Vitro. Toxins 2020, 12, 468. https://doi.org/10.3390/toxins12080468
Bonetti A, Tugnoli B, Rossi B, Giovagnoni G, Piva A, Grilli E. Nature-Identical Compounds and Organic Acids Reduce E. coli K88 Growth and Virulence Gene Expression In Vitro. Toxins. 2020; 12(8):468. https://doi.org/10.3390/toxins12080468
Chicago/Turabian StyleBonetti, Andrea, Benedetta Tugnoli, Barbara Rossi, Giulia Giovagnoni, Andrea Piva, and Ester Grilli. 2020. "Nature-Identical Compounds and Organic Acids Reduce E. coli K88 Growth and Virulence Gene Expression In Vitro" Toxins 12, no. 8: 468. https://doi.org/10.3390/toxins12080468
APA StyleBonetti, A., Tugnoli, B., Rossi, B., Giovagnoni, G., Piva, A., & Grilli, E. (2020). Nature-Identical Compounds and Organic Acids Reduce E. coli K88 Growth and Virulence Gene Expression In Vitro. Toxins, 12(8), 468. https://doi.org/10.3390/toxins12080468






