Effects of Dietary Ochratoxin A on Growth Performance and Intestinal Apical Junctional Complex of Juvenile Grass Carp (Ctenopharyngodon idella)
Abstract
:1. Introduction
2. Results
2.1. Growth Performance and OTA Intestinal Residue
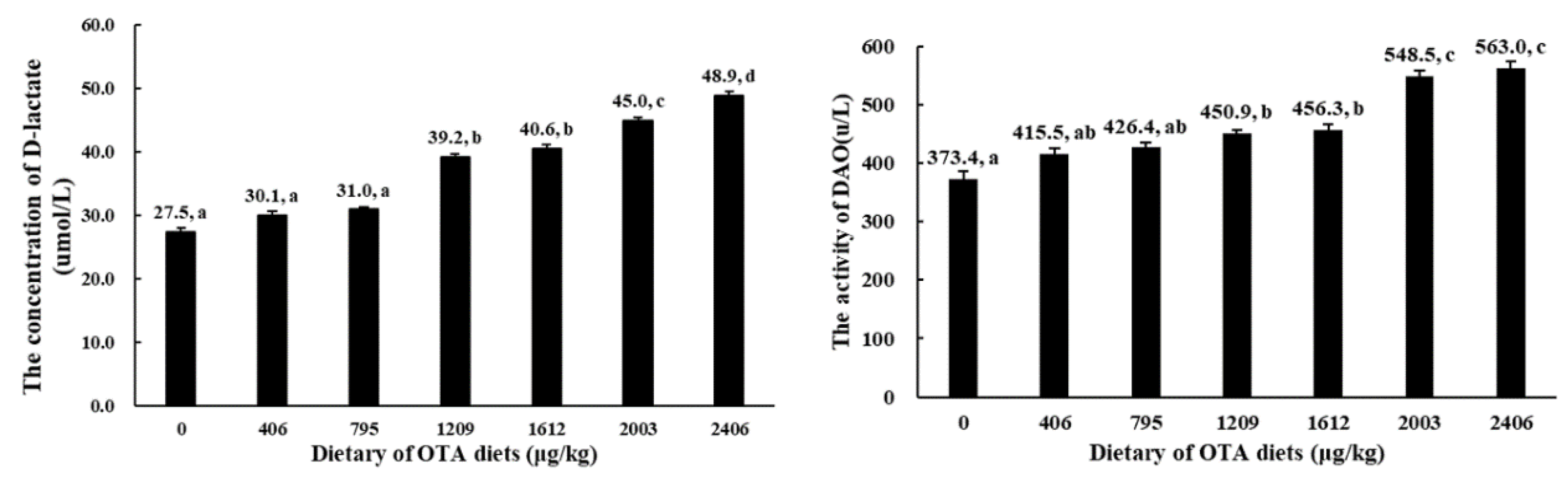
2.2. Serum D-Lactate Concentration and Diamine Oxidase (DAO) Activity
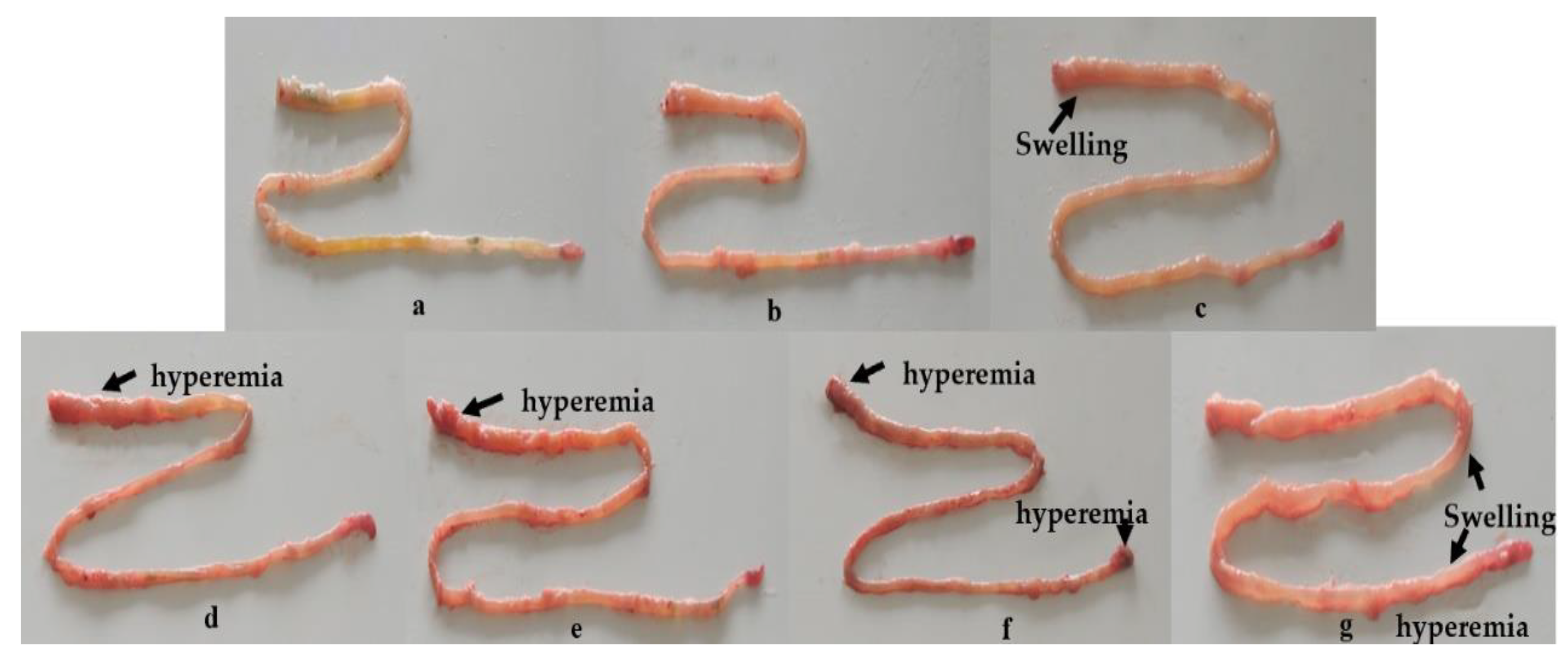
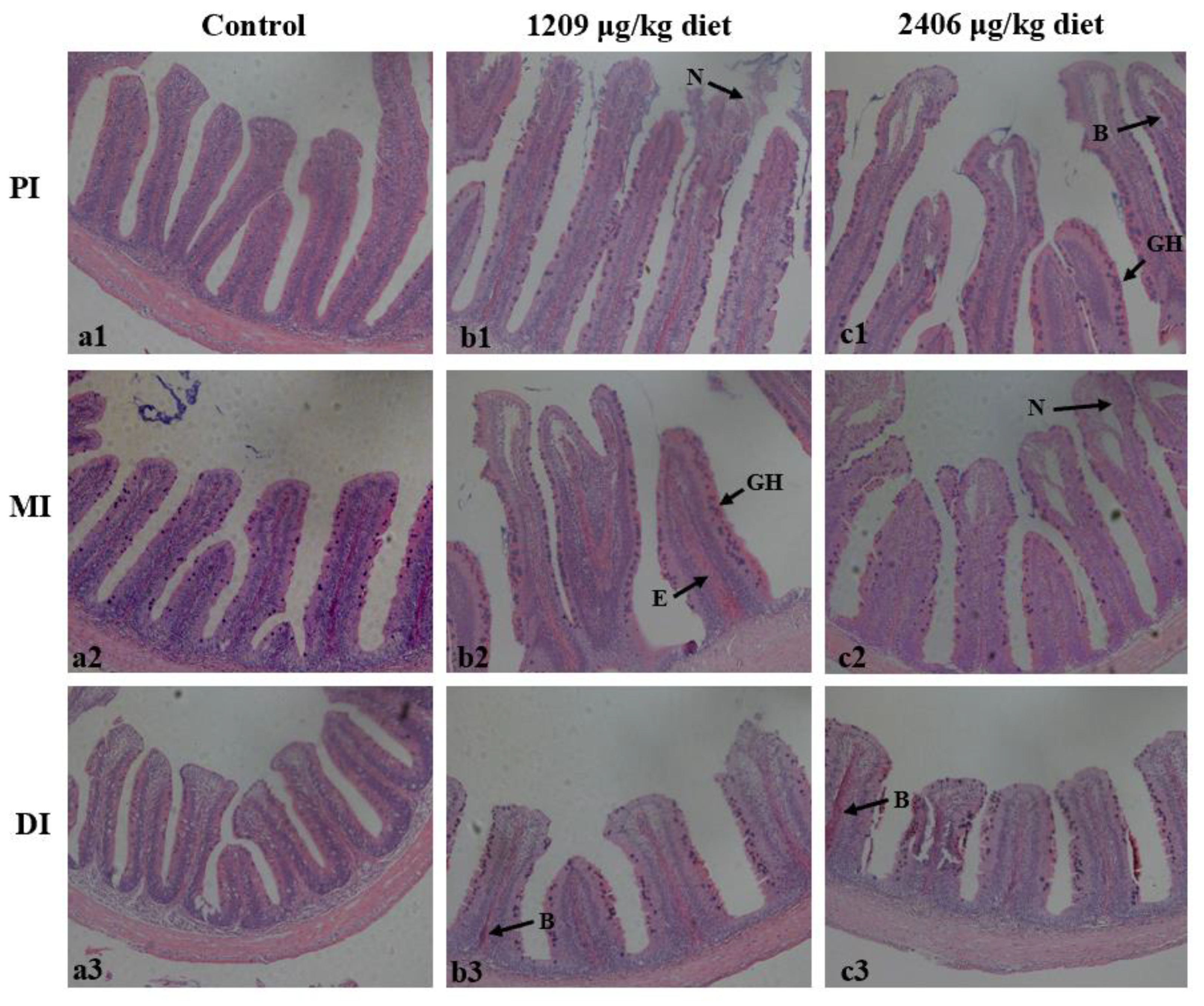
2.3. Dietary OTA-Induced Intestinal Hyperemia and Histopathological Changes
2.4. Antioxidant-Related Parameters
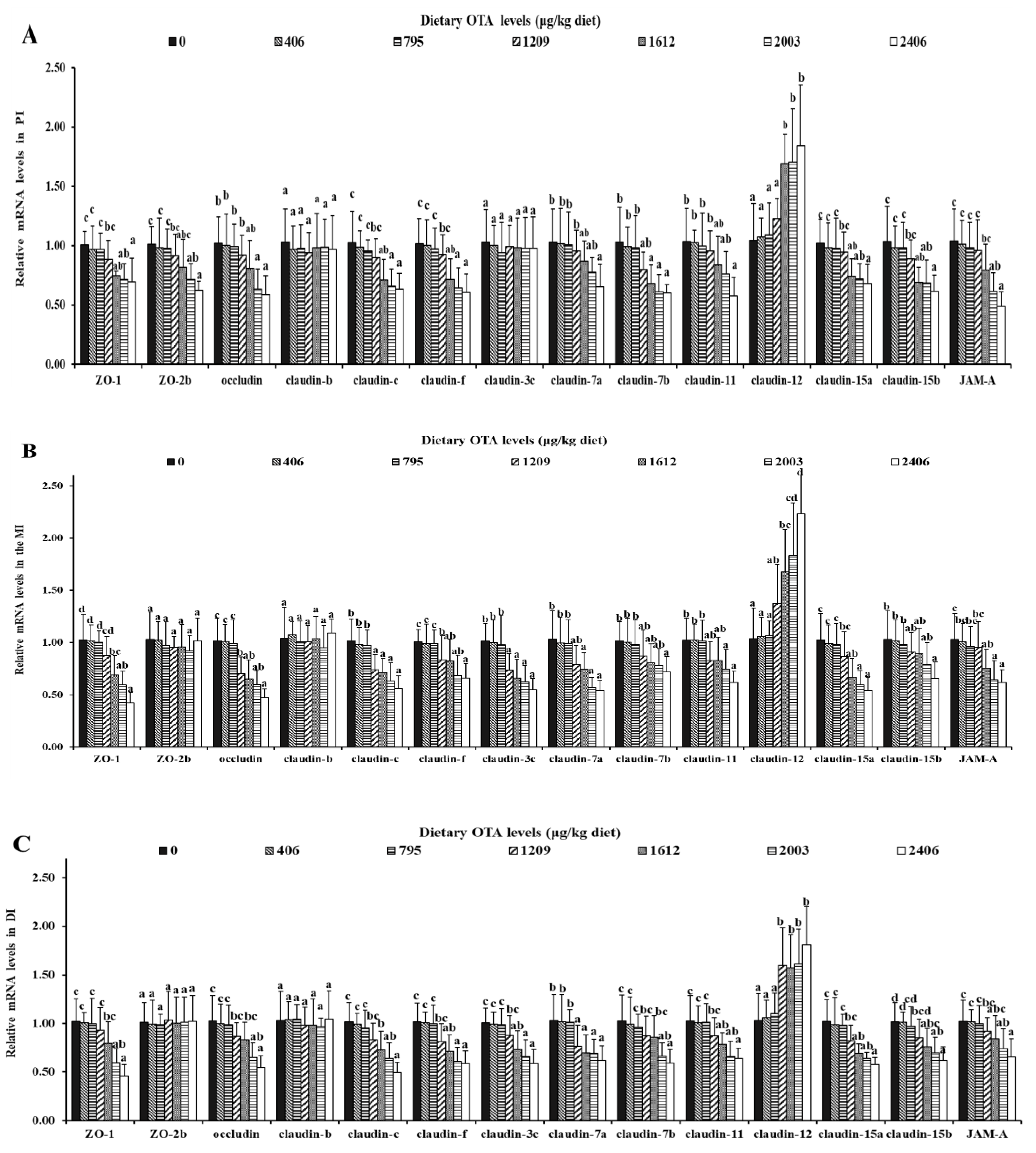
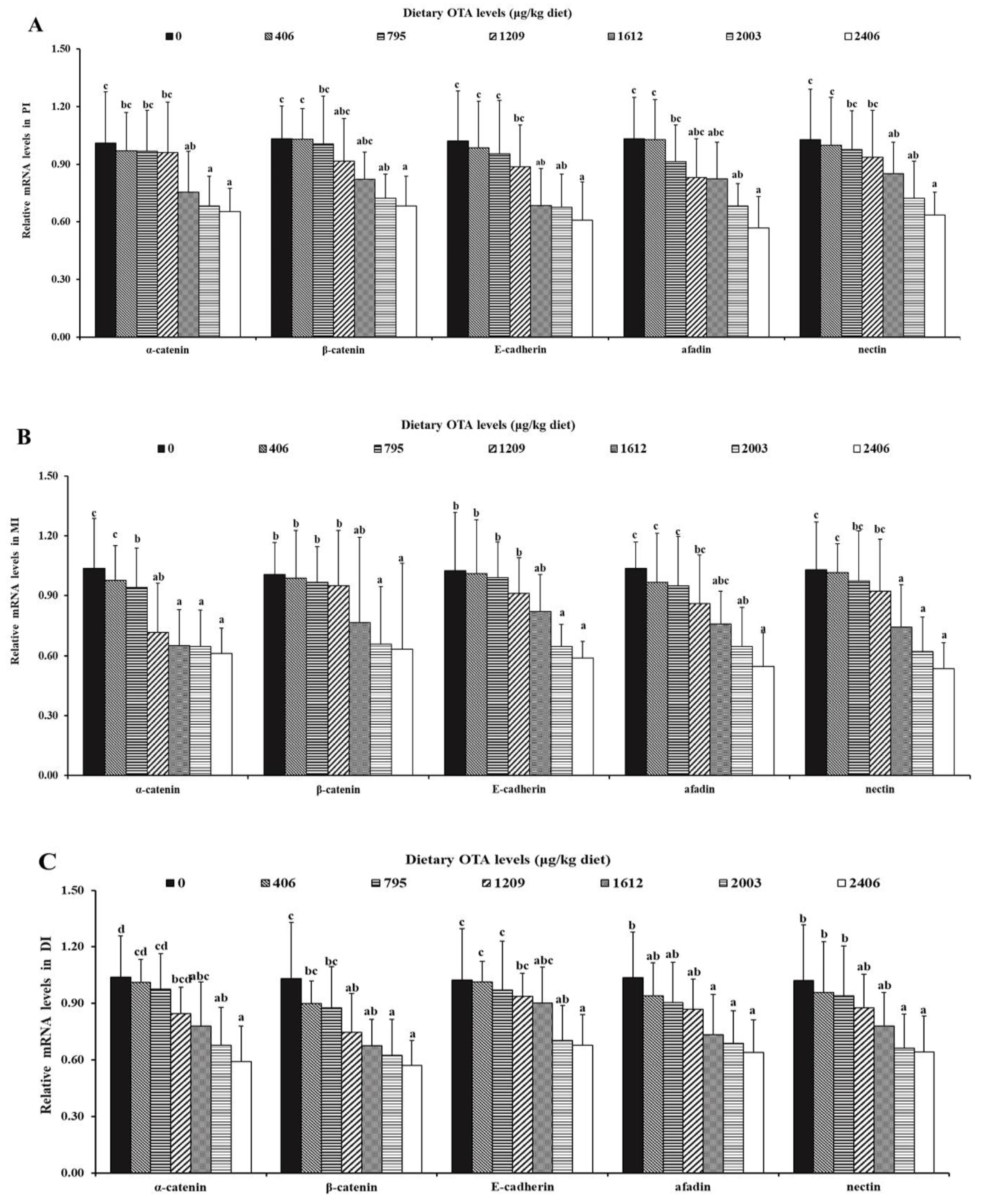
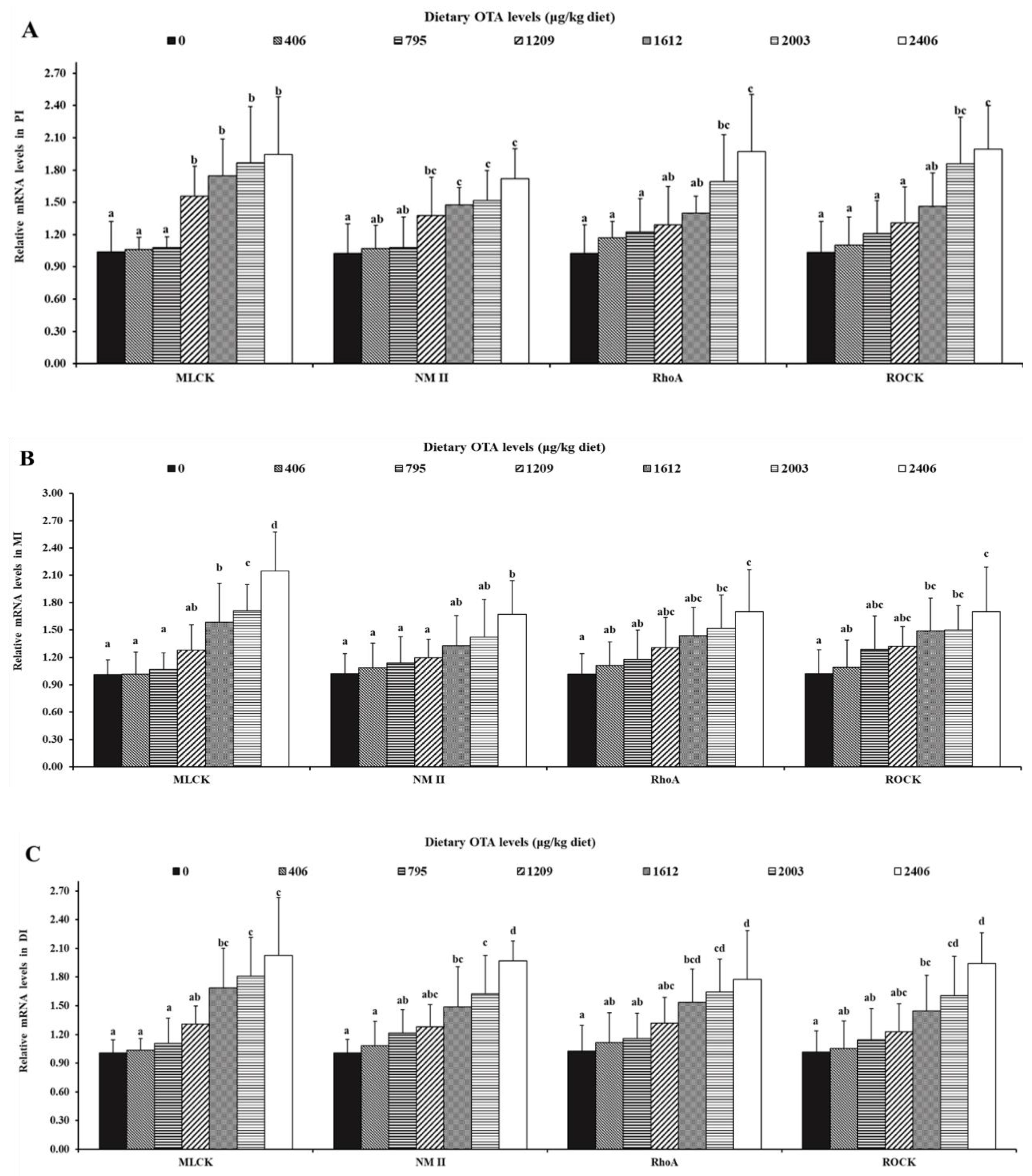
2.5. Relative mRNA Levels of AJC-Related Parameters in the Intestine
2.5.1. OTA Decreased the mRNA Expression Levels of Tight Junctions
2.5.2. OTA Decreased the mRNA Expression Levels of Adhesive Junctions
2.5.3. OTA Increased the mRNA Expression Levels of AJC and Relevant Signaling Molecules
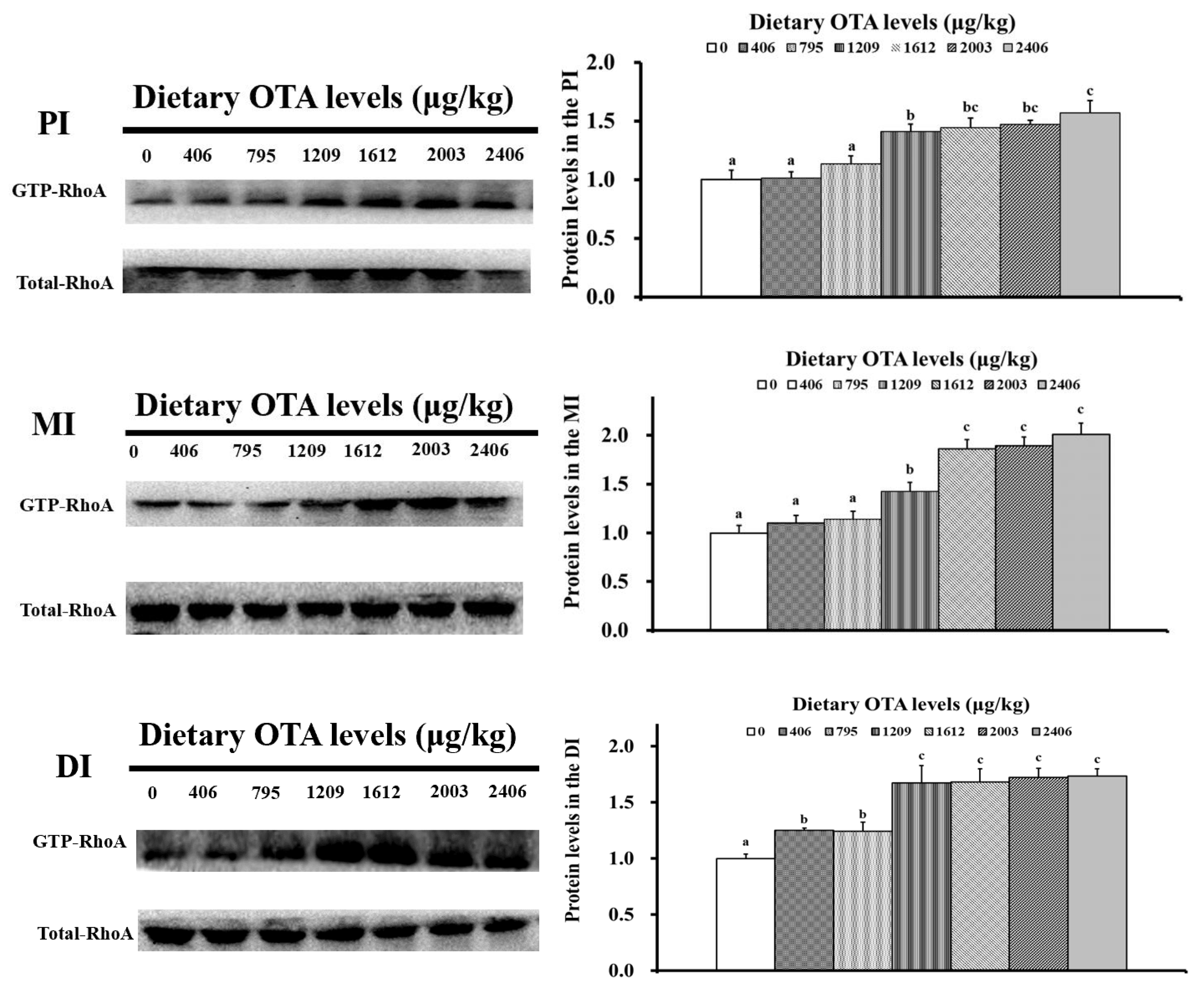
2.6. OTA Decreased the Protein Levels of GTP-RhoA
2.7. Correlation Analyses
3. Discussion
3.1. OTA Caused Poor Growth Performance in Fish
3.2. OTA Led to Fish Intestine Histopathological Lesions
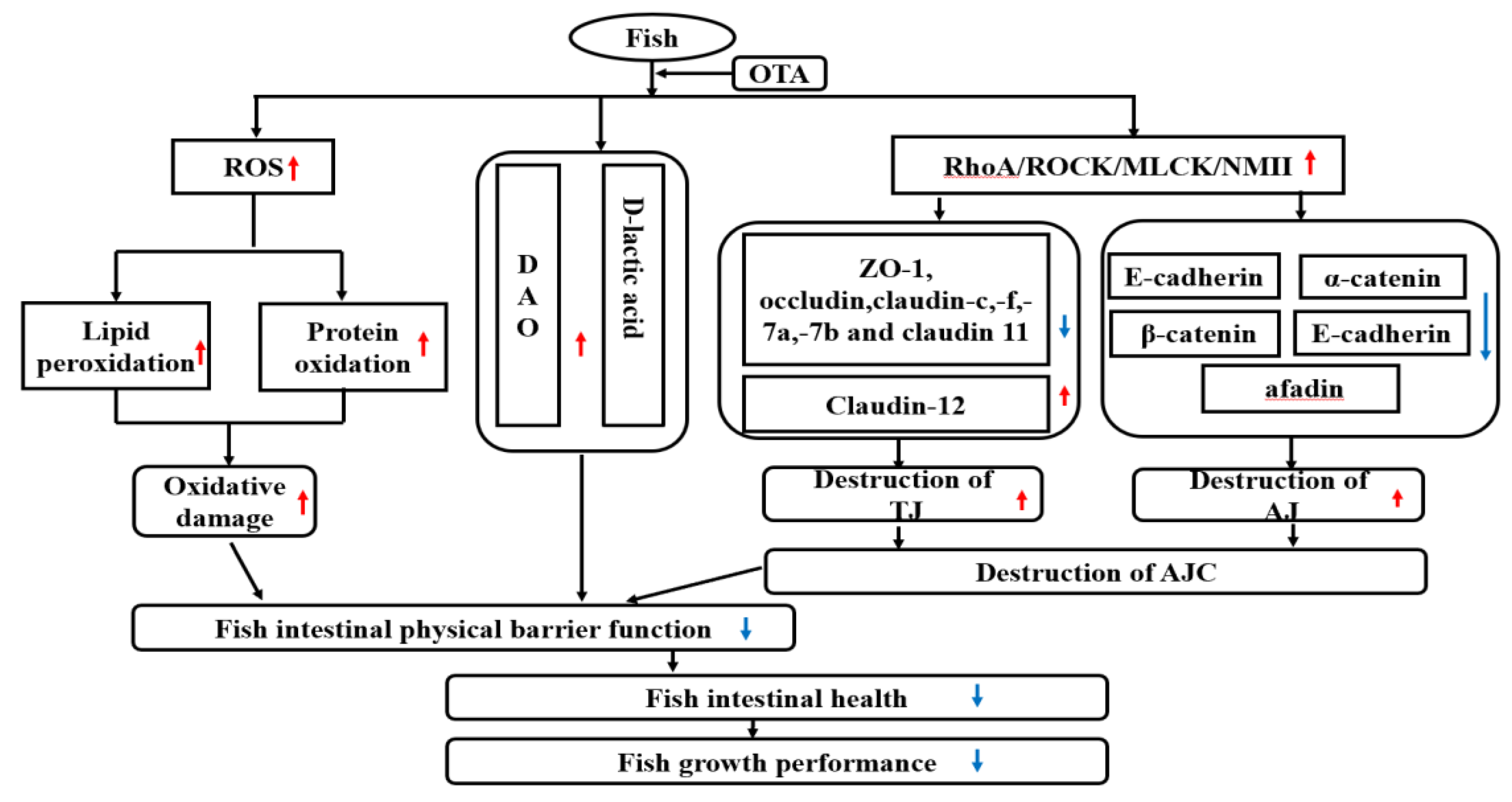
3.3. OTA Disrupted Fish Intestine AJC, Partly via RhoA/ROCK Pathway
3.4. The Maximum Controlled Dose of OTA for Juvenile Grass Carp
4. Conclusions
5. Materials and Methods
5.1. Diets
5.2. Feeding Trial and Sample Collection
5.3. Biochemical Analysis
5.4. Histological Examination
5.5. RT-PCR Analysis
5.6. Western Blot Analysis
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schrenk, D.; Bodin, L.; Chipman, J.K.; Mazo, J.d.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Naehrer, K.; Santos, A.G. Occurrence of mycotoxins in commercial aquafeeds in Asia and Europe: A real risk to aquaculture? Rev. Aquac. 2018, 10, 263–280. [Google Scholar] [CrossRef]
- Battacone, G.; Nudda, A.; Pulina, G. Effects of Ochratoxin A on Livestock Production. Toxins 2010, 2, 1796–1824. [Google Scholar] [CrossRef] [Green Version]
- Matejova, I.; Faldyna, M.; Modra, H.; Blahova, J.; Palikova, M.; Markova, Z.; Franc, A.; Vicenova, M.; Vojtek, L.; Bartonkova, J. Effect of T-2 toxin-contaminated diet on common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2017, 60, 458–465. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Rached, E.; Hoffmann, D.; Blumbach, K.; Weber, K.; Dekant, W.; Mally, A. Evaluation of putative biomarkers of nephrotoxicity after exposure to ochratoxin a in vivo and in vitro. Toxicol. Sci. 2008, 103, 371–381. [Google Scholar] [CrossRef]
- Duarte, S.C.; Lino, C.M.; Pena, A. Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Vet. Microbiol. 2011, 154, 1–13. [Google Scholar] [CrossRef]
- Manning, B.B.; Ulloa, R.M.; Li, M.H.; Robinson, E.H.; Rottinghaus, E.G. Ochratoxin A fed to channel catfish (Ictalurus punctatus) causes reduced growth and lesions of hepatopancreatic tissue. Aquaculture 2003, 219, 739–750. [Google Scholar] [CrossRef]
- Zahran, E.; Manning, B.; Seo, J.K.; Noga, E.J. The effect of Ochratoxin A on antimicrobial polypeptide expression and resistance to water mold infection in channel catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2016, 57, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Sundh, H.; Sundell, K.S. 7—Environmental impacts on fish mucosa. Mucosal Health Aquac. 2015, 561, 171–197. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Bouhet, S.; Oswald, P.I. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007, 51, 925–931. [Google Scholar] [CrossRef]
- Szczech, G.M.; Carlton, W.W.; Tuite, J. Ochratoxin A Toxicosis in Swine. Vet. Pathol. 1973, 10, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Kanisawa, M.; Suzuki, S.; Kozuka, Y.; Yamazaki, M. Histopathological studies on the toxicity of ochratoxin A in rats. I. Acute oral toxicity. Toxicol. Appl. Pharmacol. 1977, 42, 55–64. [Google Scholar] [CrossRef]
- Stoev, S.D.; Goundasheva, D.; Mirtcheva, T.; Mantle, P.G. Susceptibility to secondary bacterial infections in growing pigs as an early response in ochratoxicosis. Exp. Toxicol. Pathol. 2000, 52, 287–296. [Google Scholar] [CrossRef]
- Yuwei, H. The study on damage of intestinal mucosa barrier structure with oxidized fish oil diets in. J. Fish. China 2015, 79, 1620–1631. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.I.; Bachar, M.; Babbin, B.A.; Adelstein, R.S.; Nusrat, A.; Parkos, C.A. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS ONE 2007, 2, e658. [Google Scholar] [CrossRef]
- Miyauchi, E.; Morita, H.; Tanabe, S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J. Dairy Sci. 2009, 92, 2400–2408. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014, 36, 157–165. [Google Scholar]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, I.S.; Hulpiau, P.; Saeys, Y.; Van Roy, F. Evolution and diversity of cadherins and catenins. Exp. Cell Res. 2017, S0014482717301064. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, N.; Sarpal, R.; Wood, M.N.; Barrick, S.K.; Nishikawa, T.; Hayashi, H.; Kobb, A.B.; Flozak, A.S.; Yemelyanov, A.; Fernandez-Gonzalez, R. Force-dependent allostery of the α-catenin actin-binding domain controls adherens junction dynamics and functions. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Sahai, E.; Marshall, C.J. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat. Cell Biol. 2002, 4, 408–415. [Google Scholar] [CrossRef]
- Chen, W.; Mao, K.; Liu, Z.; Dinh-Xuan, A.T. The role of the RhoA/Rho kinase pathway in anti-angiogenesis and its potential value in prostate cancer (Review). Oncol. Lett. 2014. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martinez, M.; Martinez-Larranaga, M.R.; Anadon, A.; Martinez, M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353–354, 21–33. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Wang, J.; Luo, C.; Zhao, S.; Zheng, N. Modulation of Intestinal Epithelial Permeability in Differentiated Caco-2 Cells Exposed to Aflatoxin M1 and Ochratoxin A Individually or Collectively. Toxins 2017, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhai, N.; Chen, Y.; Fu, C.; Huang, K. OTA induces intestinal epithelial barrier dysfunction and tight junction disruption in IPEC-J2 cells through ROS/Ca(2+)-mediated MLCK activation. Environ. Pollut. 2018, 242, 106–112. [Google Scholar] [CrossRef]
- Mally, A.; Decker, M.; Bekteshi, M.; Dekant, W. Ochratoxin A alters cell adhesion and gap junction intercellular communication in MDCK cells. Toxicology 2006, 223, 15–25. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Wang, S.W.; Kuang, S.Y.; Tang, L.; Feng, L. Effects of Dietary Zearalenone on Oxidative Stress, Cell Apoptosis, and Tight Junction in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-A.; Jiang, W.-D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A. Dietary myo-inositol deficiency decreased the growth performances and impaired intestinal physical barrier function partly relating to nrf2, jnk, e2f4 and mlck signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 67, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-Z.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhang, Y.-A.; Zhou, X.-Q.; Feng, L. Gossypol reduced the intestinal amino acid absorption capacity of young grass carp (Ctenopharyngodon idella). Aquaculture 2018, 492, 46–58. [Google Scholar] [CrossRef]
- Tashjian, D.H.; Teh, S.J.; Sogomonyan, A.; Hung, S.S. Bioaccumulation and chronic toxicity of dietary l-selenomethionine in juvenile white sturgeon (Acipenser transmontanus). Aquat. Toxicol. 2006, 79, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracarense, A.-P.F.; Lucioli, J.; Grenier, B.; Pacheco, G.D.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [Green Version]
- Manning, B.B.; Abbas, H.K.; Wise, D.J.; Greenway, T. The effect of feeding diets containing deoxynivalenol contaminated corn on channel catfish (Ictalurus punctatus) challenged with Edwardsiella ictaluri. Aquac. Res. 2014, 45, 1782–1786. [Google Scholar] [CrossRef]
- Min, L.; Rui, G.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A.; Kartik, S. Toxic Effects of Maternal Zearalenone Exposure on Intestinal Oxidative Stress, Barrier Function, Immunological and Morphological Changes in Rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Feng, L.; Jiang, W.-D.; Wu, P.; Jiang, J.; Tang, L.; Kuang, S.-Y.; Zhou, X.-Q.; Liu, Y. Dimethyl-β-propiothetine (DMPT) supplementation under the all-plant protein diet enhances growth performance, digestive capacity and intestinal structural integrity for on-growing grass carp (Ctenopharyngodon idella). Aquaculture 2019, 513, 734421. [Google Scholar] [CrossRef]
- Jiang, W.D.; Hu, K.; Zhang, J.X.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Soyabean glycinin depresses intestinal growth and function in juvenile Jian carp (Cyprinus carpio var Jian): Protective effects of glutamine. Br. J. Nutr. 2015, 114, 1569–1583. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-S.; Wu, F.; Long, L.-N.; Li, T.-J.; Xiong, X.; Liao, P.; Liu, H.-N.; Yin, Y.-L. Effects of yeast products on the intestinal morphology, barrier function, cytokine expression, and antioxidant system of weaned piglets. J. Zhejiang Univ. Sci. B 2016, 17, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qin, G.; Sun, Z.; Che, D.; Bao, N.; Zhang, X. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int. J. Mol. Sci. 2011, 12, 8502–8512. [Google Scholar] [CrossRef] [PubMed]
- Cai-hong, L.H.-P.H.; Yong, X. Effects of Early Weaning on Intestinal Permeability and Tight Junction Protein Occludin mRNA Expression Levels of Piglets. Chin. J. Anim. Nutr. 2008, 20, 442–446. [Google Scholar]
- Yu, Q.-H.; Yang, Q. Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol. Int. 2009, 33, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.J.; Mallick, E.M.; Scanlon, K.M.; Turner, J.R.; Donnenberg, M.S.; Leong, J.M.; Snapper, S.B. Attaching-and-Effacing Pathogens Exploit Junction Regulatory Activities of N-WASP and SNX9 to Disrupt the Intestinal Barrier. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 273–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 2008, 19, 1912–1921. [Google Scholar] [CrossRef] [Green Version]
- Nagar, I. Experimentally Induced Toxicity of Ochratoxin A and Endosulfan in Male Wistar Rats: A Hormonal Disorder. J. Anim. Vet. Adv. 2011, 10, 1750–1755. [Google Scholar] [CrossRef] [Green Version]
- Chasiotis, H.; Kelly, S.P. Effect of cortisol on permeability and tight junction protein transcript abundance in primary cultured gill epithelia from stenohaline goldfish and euryhaline trout. Gen. Comp. Endocrinol. 2011, 172, 494–504. [Google Scholar] [CrossRef]
- Frizzell, C.; Verhaegen, S.; Ropstad, E.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of ochratoxin A at the level of nuclear receptor activation and steroidogenesis. Toxicol. Lett. 2013, 217, 243–250. [Google Scholar] [CrossRef]
- Mercado, A.M.; Padgett, D.A.; Sheridan, J.F.; Marucha, P.T. Altered kinetics of IL-1α, IL-1β, and KGF-1 gene expression in early wounds of restrained mice. Brain Behav. Immun. 2002, 16, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Watson, R.; Poddar, R.; Walker, J.; McGuill, I.; Hoare, L.; Griffiths, C.; O’neill, C. Altered claudin expression is a feature of chronic plaque psoriasis. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2007, 212, 450–458. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakamoto, T.; Hyodo, S.; Shepherd, B.S.; Kaneko, T.; Grau, E.G. Expression of glucocorticoid receptor in the intestine of a euryhaline teleost, the Mozambique tilapia (Oreochromis mossambicus): Effect of seawater exposure and cortisol treatment. Life Sci. 2006, 78, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Stachurska, A.; Sobczak, M.; Podkalicka, P.; Mucha, O.; Jozkowicz, A.; Dulak, J. Nrf2 deficiency exacerbates ochratoxin A-induced toxicity in vitro and in vivo. Toxicology 2017, 389, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.-M.; Elner, S.G.; Elner, V.M. Regulation of VEGF mRNA expression and protein secretion by TGF-β2 in human retinal pigment epithelial cells. Exp. Eye Res. 2007, 84, 812–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiros, M.; Alarcon, L.; Ponce, A.; Giannakouros, T.; Gonzalez-Mariscal, L. The intracellular fate of zonula occludens 2 is regulated by the phosphorylation of SR repeats and the phosphorylation/O-GlcNAcylation of S257. Mol. Biol. Cell 2013, 24, 2528–2543. [Google Scholar] [CrossRef]
- Wang, K.Z.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q. Dietary gossypol reduced intestinal immunity and aggravated inflammation in on-growing grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2019, 86, 814–831. [Google Scholar] [CrossRef]
- Gan, L.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. Erucic acid impairs intestinal immune function of on-growing grass carp (Ctenopharyngodon idella). Aquaculture 2020, 519, 734916. [Google Scholar] [CrossRef]
- Gan, L.; Wu, P.; Feng, L.; Jiang, W.-D.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. Erucic acid inhibits growth performance and disrupts intestinal structural integrity of on-growing grass carp (Ctenopharyngodon idella). Aquaculture 2019, 513, 734437. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Mukherjee, S.C. Immunosuppressive effect of aflatoxin B1 in Indian major carp (Labeo rohita). Comp. Immunol. Microbiol. Infect. Dis. 2001, 24, 143–149. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Jiang, W.D.; Liu, Y.; Wu, P.; Zhao, J.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A. Optimal dietary alpha-linolenic acid/linoleic acid ratio improved digestive and absorptive capacities and target of rapamycin gene expression of juvenile grass carp (Ctenopharyngodon idellus). Aquac. Nutr. 2016, 22, 1251–1266. [Google Scholar] [CrossRef]
- Liang, J.J.; Liu, Y.J.; Tian, L.X.; Yang, H.J.; Liang, G.Y. Dietary available phosphorus requirement of juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2012, 18, 181–188. [Google Scholar] [CrossRef]
- Pan, F.-Y.; Feng, L.; Jiang, W.-D.; Jiang, J.; Wu, P.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q. Methionine hydroxy analogue enhanced fish immunity via modulation of NF-κB, TOR, MLCK, MAPKs and Nrf2 signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 56, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.W.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Threonine deficiency decreased intestinal immunity and aggravated inflammation associated with NF-kappaB and target of rapamycin signalling pathways in juvenile grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Br. J. Nutr. 2017, 118, 92–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Feng, L.; Jiang, W.D.; Wu, P.; Zhou, X.Q. Condensed tannins decreased the growth performance and impaired intestinal immune function in on-growing grass carp (Ctenopharyngodon idella). Br. J. Nutr. 2019, 123, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-Y.; Jiang, W.-D.; Liu, Y.; Wu, P.; Zhao, J.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A. Dietary alpha-linolenic acid/linoleic acid ratios modulate intestinal immunity, tight junctions, anti-oxidant status and mRNA levels of NF-kappa B p65, MLCK and Nrf2 in juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 51, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Nagel, F.; Danwitz, A.V.; Tusche, K.; Kroeckel, S.; Bussel, C.G.J.V.; Schlachter, M.; Adem, H.; Tressel, R.P.; Schulz, C. Nutritional evaluation of rapeseed protein isolate as fish meal substitute for juvenile turbot (Psetta maxima L.)—Impact on growth performance, body composition, nutrient digestibility and blood physiology. Aquaculture 2012, 356, 357–364. [Google Scholar] [CrossRef]
- Zheng, L.; Feng, L.; Jiang, W.D.; Wu, P.; Tang, L.; Kuang, S.Y.; Zeng, Y.Y.; Zhou, X.Q.; Liu, Y. Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 77, 53–70. [Google Scholar] [CrossRef]
- Deng, J.; Kang, B.; Tao, L.; Rong, H.; Zhang, X. Effects of dietary cholesterol on antioxidant capacity, non-specific immune response, and resistance to Aeromonas hydrophila in rainbow trout (Oncorhynchus mykiss) fed soybean meal-based diets. Fish Shellfish Immunol. 2013, 34, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Salazar, S.; Oliver, C.; Yáñez, A.J.; Avendaño-Herrera, R. Comparative analysis of innate immune responses to Streptococcus phocae strains in Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 51, 97–103. [Google Scholar] [CrossRef]
- Yu, H.; Deng, W.; Zhang, D.; Gao, Y.; Yang, Z.; Shi, X.; Sun, J.; Zhou, J.; Ji, H. Antioxidant defenses of Onychostoma macrolepis in response to thermal stress: Insight from mRNA expression and activity of superoxide dismutase and catalase. Fish Shellfish Immunol. 2017, 66, 50–61. [Google Scholar] [CrossRef]
- Perši, N.; Pleadin, J.; Vulić, A.; Kmetić, I.; Šimić, B. Determination of ochratoxin A in serum and urine of pigs. World Mycotoxin J. 2012, 5, 351–356. [Google Scholar] [CrossRef]
- Morán, P.; Marco-Rius, F.; Megías, M.; Covelo-Soto, L.; Pérez-Figueroa, A. Environmental induced methylation changes associated with seawater adaptation in brown trout. Aquaculture 2013, 392, 77–83. [Google Scholar] [CrossRef]
- Holen, E.; Olsvik, P.A. β-naphthoflavone interferes with cyp1c1, cox2 and IL-8 gene transcription and leukotriene B4 secretion in Atlantic cod (Gadus morhua) head kidney cells during inflammation. Fish Shellfish Immunol. 2016, 54, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Niu, J.; Profirovic, J.; Pan, H.; Vaiskunaite, R.; Voyno-Yasenetskaya, T. G protein βγ subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: Regulation of cell shape and reactive oxygen species production. Circ. Res. 2003, 93, 848–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Searcy-Bernal, R. Statistical power and aquacultural research. Aquaculture 1994, 127, 371–388. [Google Scholar] [CrossRef]
- Grey, E.K.; Chiasson, S.C.; Williams, H.G.; Troeger, V.J.; Taylor, C.M. Evaluation of blue crab, Callinectes sapidus, megalopal settlement and condition during the Deepwater Horizon oil spill. PLoS ONE 2015, 10, e0135791. [Google Scholar] [CrossRef]
- Li, S.Q.; Feng, L.; Jiang, W.D.; Liu, Y.; Wu, P.; Zhao, J.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N. Deficiency of dietary niacin decreases digestion and absorption capacities via declining the digestive and brush border enzyme activities and downregulating those enzyme gene transcription related to TOR pathway of the hepatopancreas and intestine in young grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2016, 22, 1267–1282. [Google Scholar] [CrossRef]
- Jiang, W.D.; Feng, L.; Liu, Y.; Jiang, J.; Zhou, X.Q. Growth, digestive capacity and intestinal microflora of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary inositol. Aquac. Res. 2009, 40, 955–962. [Google Scholar] [CrossRef]
| Growth Indicators | Dietary OTA Levels (µg/kg Diet) | ||||||
|---|---|---|---|---|---|---|---|
| 0.0 | 406 | 795 | 1209 | 1612 | 2003 | 2406 | |
| IBW 1 | 25.73 ± 0.07 a | 25.74 ± 0.03 a | 25.70 ± 0.08 a | 25.79 ± 0.07 a | 25.76 ± 0.09 a | 25.68 ± 0.04 a | 25.75 ± 0.13 a |
| FBW 1 | 125.22 ± 2.14 e | 125.00 ± 1.20 e | 124.67 ± 0.88 e | 117.22 ± 3.53 d | 108.78 ± 3.40 c | 99.89 ± 1.26 b | 92.44 ± 2.14 a |
| PWG 1 | 386.59 ± 7.10 e | 385.63 ± 4.64 e | 385.07 ± 3.23 e | 354.48 ± 14.25 d | 322.34 ± 13.75 c | 289.01 ± 5.48 b | 259.05 ± 10.10 a |
| SGR 1 | 2.637 ± 0.024 e | 2.634 ± 0.015 e | 2.632 ± 0.011 e | 2.523 ± 0.052 d | 2.406 ± 0.053 c | 2.264 ± 0.023 b | 2.130 ± 0.0472 a |
| FI 1 | 122.19 ± 0.12 e | 122.15 ± 0.14 e | 122.08 ± 0.17 e | 115.21 ± 0.11 d | 109.97 ± 0.18 c | 101.67 ± 0.18 b | 95.56 ± 0.20 a |
| FE 1 | 0.81 ± 0.02 c | 0.81 ± 0.01 c | 0.81 ± 0.01 c | 0.79 ± 0.05 c | 0.75 ± 0.05 b | 0.73 ± 0.02 b | 0.70 ± 0.05 a |
| IL 2 | 33.13 ± 2.47 c | 32.39 ± 1.20 c | 31.72 ± 1.03 c | 28.02 ± 0.71 b | 26.78 ± 0.47 b | 23.88 ± 0.57 a | 23.62 ± 0.18 a |
| ILI 2 | 167.59 ± 5.50 c | 162.28 ± 8.67 c | 161.96 ± 0.49 c | 152.39 ± 5.34 bc | 143.32 ± 6.48 b | 132.38 ± 3.29 a | 124.16 ± 2.18 a |
| IW 2 | 3.30 ± 0.05 d | 3.21 ± 0.06 d | 3.16 ± 0.08 d | 2.84 ± 0.14 c | 2.49 ± 0.12 b | 2.17 ± 0.07 a | 2.00 ± 0.11 a |
| ISI 2 | 2.69 ± 0.07 c | 2.60 ± 0.05 bc | 2.60 ± 0.15 bc | 2.46 ± 0.08 b | 2.21 ± 0.10 a | 2.21 ± 0.12 a | 2.15 ± 0.15 a |
| OTA Content (μg/kg tissue) | |||||||
| PI 3 | n.d 4 | n.d 4 | n.d 4 | 8.02 ± 0.20 a | 16.68 ± 1.26 b | 28.68 ± 2.85 c | 39.24 ± 3.55 d |
| MI 3 | n.d 4 | n.d 4 | n.d 4 | 15.14 ± 1.09 a | 26.13 ± 1.54 b | 32.67 ± 2.93 c | 41.19 ± 1.73 d |
| DI 3 | n.d 4 | n.d 4 | 9.33 ± 0.77 a | 21.22 ± 2.03 b | 28.61 ± 2.74 c | 34.17 ± 3.28 d | 49.56 ± 2.75 e |
| Item | Dietary OTA Levels (µg/kg Diet) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 406 | 795 | 1209 | 1612 | 2003 | 2406 | |
| PI | |||||||
| ROS | 100.00 ± 7.74 a | 102.41 ± 3.42 a | 102.61 ± 1.84 a | 115.69 ± 3.54 b | 120.34 ± 6.68 bc | 124.56 ± 3.28 c | 125.32 ± 7.80 c |
| MDA | 17.82 ± 1.52 a | 17.99 ± 0.90 a | 18.08 ± 0.27 a | 23.70 ± 2.04 b | 24.86 ± 1.95 bc | 25.32 ± 2.08 bc | 26.74 ± 1.82 c |
| PC | 2.94 ± 0.28 a | 3.00 ± 0.21 a | 3.04 ± 0.19 a | 4.74 ± 0.26 b | 5.10 ± 0.40 b | 7.30 ± 0.70 c | 8.89 ± 0.57 d |
| MI | |||||||
| ROS | 100.00 ± 9.76 a | 103.70 ± 2.81 a | 104.43 ± 2.88 a | 110.95 ± 7.34 b | 114.62 ± 4.20 bc | 116.01 ± 4.06 bc | 120.90 ± 2.46 c |
| MDA | 16.53 ± 1.06 a | 16.66 ± 0.91 a | 16.85 ± 0.71 a | 22.94 ± 1.40 b | 23.60 ± 0.80 b | 25.76 ± 1.89 c | 26.65 ± 1.11 c |
| PC | 2.99 ± 0.18 a | 3.03 ± 0.16 a | 3.09 ± 0.19 a | 3.92 ± 0.36 b | 4.34 ± 0.25 b | 6.88 ± 0.37 c | 7.00 ± 0.68 c |
| DI | |||||||
| ROS | 100.00 ± 3.45 a | 104.83 ± 2.60 a | 105.13 ± 3.38 a | 112.30 ± 7.24 b | 114.31 ± 6.05 b | 115.11 ± 7.03 b | 116.84 ± 6.75 b |
| MDA | 17.21 ± 0.43 a | 17.61 ± 1.58 a | 17.89 ± 0.74 a | 23.94 ± 0.90 b | 25.29 ± 1.61 bc | 26.77 ± 2.11 c | 26.80 ± 1.33 c |
| PC | 2.95 ± 0.24 a | 2.99 ± 0.10 a | 3.06 ± 0.24 a | 5.08 ± 0.44 b | 5.19 ± 0.47 b | 5.51 ± 0.28 b | 6.53 ± 0.61 c |
| Regression Equation | Regression | p | The Maximum Allowable Doses of OTA (µg/kg) |
|---|---|---|---|
| Growth performance | |||
| YPWG = −0.0794x + 449.56 Ymin = 386.53 | 0.9996 | <0.01 | 803.53 µg/kg |
| YSGR = −0.0003x + 2.8965 Ymin = 2.63 | 0.9975 | <0.01 | 874.33 µg/kg |
| PI | |||
| YROS = 0.014x + 94.995 Ymin = 101.67 | 0.8516 | <0.05 | 476.78 µg/kg |
| YMDA = 0.0048x + 16.022 Ymin = 17.96 | 0.8043 | <0.05 | 404.79 µg/kg |
| MI | |||
| YROS = 0.0103x + 96.430 Ymin = 102.71 | 0.9370 | <0.01 | 609.42 µg/kg |
| YMDA = 0.0057x + 14.00 Ymin = 16.68 | 0.8521 | <0.05 | 469.49 µg/kg |
| DI | |||
| YROS = 0.0075x + 100.39 Ymin = 103.32 | 0.7946 | <0.05 | 390.67 µg/kg |
| YMDA = 0.0053x + 15.531 Ymin = 17.57 | 0.7812 | <0.05 | 384.53 µg/kg |
| Ingredients | % | Nutrient Content | % |
|---|---|---|---|
| Fish meal | 7.30 | Crude protein d | 32.22 |
| Casein | 23.50 | Crude lipid d | 4.86 |
| Gelatin | 6.83 | n-3 e | 1.04 |
| α-starch | 24.00 | n-6 e | 0.96 |
| Rice flour | 13.764 | Available phosphorus f | 0.84 |
| Fish oil | 1.44 | ||
| Soybean oil | 1.81 | ||
| Cellulose | 5.00 | ||
| Ca(H2PO4)2 | 3.13 | ||
| Vitamin premix a | 1.00 | ||
| Mineral premix b | 2.00 | ||
| Choline chloride (50%) | 1.00 | ||
| Ethoxyquin (30%) | 0.050 | ||
| Trp (99.2%) | 0.031 | ||
| Thr (98.5%) | 0.145 | ||
| OTA premix c | 9.00 |
| Target Gene | Primer Sequence, Forward (5′→3′) | Primer Sequence, Reverse (5′→3′) | Temperature (°C) | Accession Number |
|---|---|---|---|---|
| ZO-1 | CGGTGTCTTCGTAGTCGG | CAGTTGGTTTGGGTTTCAG | 59.4 | KJ000055 |
| Occludin | TATCTGTATCACTACTGCGTCG | CATTCACCCAATCCTCCA | 59.4 | KF193855 |
| ZO-2b | TACAGCGGGACTCTAAAATGG | TCACACGGTCGTTCTCAAAG | 60.3 | KM112095 |
| Claudin-b | GAGGGAATCTGGATGAGC | ATGGCAATGATGGTGAGA | 57.0 | KF193860 |
| JAM-A | ACTGTGAGGTGCTTGGAA | CTGTTGTGACTGAAGAAGGA | 61.4 | KY780630 |
| Claudin-c | GAGGGAATCTGGATGAGC | CTGTTATGAAAGCGGCAC | 59.4 | KF193859 |
| RhoA | GCAGGACAAGAGGACTATG | GTGTTCATCATTCCGTAGGT | 63.3 | MN661351 |
| Claudin-f | GCTGGAGTTGCCTGTCTTATTC | ACCAATCTCCCTCTTTTGTGTC | 57.1 | KM112097 |
| Claudin-3c | ATCACTCGGGACTTCTA | CAGCAAACCCAATGTAG | 57.0 | KF193858 |
| ROCK | AGTCCAAGTCTGCTGCTA | CCTCTCCTTCTGCTTCATC | 63.3 | KY780630 |
| Claudin-7a | ACTTACCAGGGACTGTGGATGT | CACTATCATCAAAGCACGGGT | 59.3 | KT625604 |
| Claudin-7b | CTAACTGTGGTGGTGATGAC | AACAATGCTACAAAGGGCTG | 59.3 | KT445866 |
| Claudin-11 | TCTCAACTGCTCTGTATCACTGC | TTTCTGGTTCACTTCCGAGG | 62.3 | KT445867 |
| Nectin | GCCAGTGACCAAGATGAC | ACAGTGCCATTCGGATTG | 61.4 | MN661350 |
| Claudin-12 | CCCTGAAGTGCCCACAA | GCGTATGTCACGGGAGAA | 55.4 | KF998571 |
| Claudin-15a | TGCTTTATTTCTTGGCTTTC | CTCGTACAGGGTTGAGGTG | 59.0 | KF193857 |
| Claudin-15b | AGTGTTCTAAGATAGGAGGGGAG | AGCCCTTCTCCGATTTCAT | 62.3 | KT757304 |
| Afadin | CCTGTGCTCACACTACTG | GTCGTTGCCTGGACTATG | 61.4 | MN661352 |
| E-cadherin | GACTGTAACGCTGAAGAGA | CTGTGGAGAGGAGATGTTC | 61.4 | MN661354 |
| α-catenin | GCAATCTTCTCTCCTTTATCC | ACTTGTGAACTCCAGCAAT | 61.4 | HQ338751 |
| β-catenin | GTCTGCTTGCCATCTTCA | CAGGTTGTGTAGAGTCGTAA | 64.5 | MN661349 |
| MLCK | GAAGGTCAGGGCATCTCA | GGGTCGGGCTTATCTACT | 53.0 | KM279719 |
| NMII | AGCCAACTCGTCAATGTC | CCTTGGAATACTTCTCTGTCT | 61.4 | MN661353 |
| β-actin | GGCTGTGCTGTCCCTGTA | GGGCATAACCCTCGTAGAT | 61.4 | M25013 |
| Growth Indicators | Formulas |
|---|---|
| PWG | 100 × [FBW (g/fish) − IBW (g/fish)]/IBW (g/fish) |
| SGR | 100 × [In (mean final weight − In (mean initial weight)]/days |
| FE | [FBW (g/fish) − IBW (g/fish)]/FI (g/fish) |
| ISI | 100 × [wet intestine weight (g)/wet body weight (g)] |
| ILI | 100 × [intestine length (cm)/total body length (cm)] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wu, P.; Jiang, W.-D.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q.; Feng, L. Effects of Dietary Ochratoxin A on Growth Performance and Intestinal Apical Junctional Complex of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins 2021, 13, 11. https://doi.org/10.3390/toxins13010011
Liu X, Wu P, Jiang W-D, Liu Y, Jiang J, Kuang S-Y, Tang L, Zhou X-Q, Feng L. Effects of Dietary Ochratoxin A on Growth Performance and Intestinal Apical Junctional Complex of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins. 2021; 13(1):11. https://doi.org/10.3390/toxins13010011
Chicago/Turabian StyleLiu, Xin, Pei Wu, Wei-Dan Jiang, Yang Liu, Jun Jiang, Sheng-Yao Kuang, Ling Tang, Xiao-Qiu Zhou, and Lin Feng. 2021. "Effects of Dietary Ochratoxin A on Growth Performance and Intestinal Apical Junctional Complex of Juvenile Grass Carp (Ctenopharyngodon idella)" Toxins 13, no. 1: 11. https://doi.org/10.3390/toxins13010011
APA StyleLiu, X., Wu, P., Jiang, W.-D., Liu, Y., Jiang, J., Kuang, S.-Y., Tang, L., Zhou, X.-Q., & Feng, L. (2021). Effects of Dietary Ochratoxin A on Growth Performance and Intestinal Apical Junctional Complex of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins, 13(1), 11. https://doi.org/10.3390/toxins13010011







