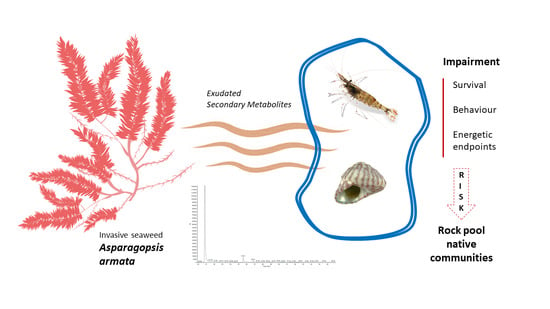
Impacts of the Invasive Seaweed Asparagopsis armata Exudate on Energetic Metabolism of Rock Pool Invertebrates
Abstract
:1. Introduction
2. Results
2.1. Survival
2.2. Behavioural Responses—Feeding Activity
2.3. Energy Metabolism-Related Biomarkers
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Test Organisms
5.2. Asparagopsis armata Collection and Preparation of Exudates
5.3. Exposure Setup
5.3.1. Survival
5.3.2. Sublethal Exposure for Biomarker Analysis
5.3.3. Sublethal Exposure for Feeding Inhibition Testing
5.4. Biomarkers Analysis
5.4.1. Tissue Preparation
5.4.2. Energy Reserves
5.4.3. Energy Metabolism-Related Enzymes
5.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, D.M.; Pyšek, P.; Carlton, J.T. A compendium of essential concepts and terminology in invasion ecology. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 409–420. [Google Scholar]
- Otero, M.; Cebrian, E.; Francour, P.; Galil, B.; Savini, D. Monitoring Marine Invasive Species in Mediterranean Marine Protected Areas (MPAs): A Strategy and Practical Guide for Managers; IUCN: Malaga, Spain, 2013; p. 136. [Google Scholar]
- McConnell, O.; Fenical, W. Halogen chemistry of the red alga Asparagopsis. Phytochemistry 1977, 16, 367–374. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Gaspar, H.; Botana, L.M.; Pedrosa, R. Marine Invasive Macroalgae: Turning a real threat into a major opportunity—The biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Burreson, B.J.; Moore, R.E.; Roller, P.P. Volatile Halogen Compounds in the Alga Asparagopsis taxiformis (Rhodophyta). J. Agric. Food Chem. 1976, 24, 856–861. [Google Scholar] [CrossRef]
- Paul, N.A.; Cole, L.; De Nys, R.; Steinberg, P.D. Ultrastructure of the gland cells of the red alga Asparagopsis armata (Bonnemaisoniaceae). J. Phycol. 2006, 42, 637–645. [Google Scholar] [CrossRef]
- Silva, C.O.; Lemos, M.F.L.; Gaspar, R.; Gonçalves, C.; Neto, J.M. The effects of the invasive seaweed Asparagopsis armata on native rock pool communities: Evidences from experimental exclusion. Preprints 2020, 2020100089. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien marine species in the Mediterranean—The 100 “worst invasives” and their impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- European Comission. EU Biodiversity Strategy for 2030, Bringing Nature Back into Our Lives; 2020 COM/2020/380 Final; European Comission: Brussels, Belgium, 2020. [Google Scholar]
- Blanco, A.; Lemos, M.F.L.; Pereira, L.; Gaspar, R.; Mouga, T.; Neto, J.M.; Troncoso, J.S.; Olabarria, C. Mapping Invasive Macroalgae in the Western Iberian Peninsula: A Methodological Guide; Olabarria, C., Blanco, A., Troncoso, J.S., Eds.; Servizo de Publicacións da Universidade de Vigo: Vigo, Spain, 2020; 73p, ISBN 978-84-8158-840-8. [Google Scholar]
- Maggs, C.A.; Stegenga, H. Red algal exotics on North Sea coasts. Helgol. Meeresunters. 1998, 52, 243–258. [Google Scholar] [CrossRef] [Green Version]
- Paul, N.A.; De Nys, R.; Steinberg, P.D. Seaweed-herbivore interactions at a small scale: Direct tests of feeding deterrence by filamentous algae. Mar. Ecol. Prog. Ser. 2006, 323, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cabecinhas, A.S.; Novais, S.C.; Santos, S.C.; Rodrigues, A.C.M.; Pestana, J.L.T.; Soares, A.M.V.M.; Lemos, M.F.L. Sensitivity of the sea snail Gibbula umbilicalis to mercury exposure--linking endpoints from different biological organization levels. Chemosphere 2015, 119, 490–497. [Google Scholar] [CrossRef]
- Azevedo-Pereira, H.M.V.S.; Lemos, M.F.L.; Soares, A.M.V.M. Behaviour and growth of Chironomus riparius Meigen (Diptera: Chironomidae) under Imidacloprid pulse and constant exposure scenarios. Water Air Soil Poll. 2011, 219, 215–224. [Google Scholar] [CrossRef]
- Dell’Omo, G. (Ed.) Behavioural Ecotoxicology 2002; John Wiley & Sons: Hoboken, NJ, USA, 2002; 492p. [Google Scholar]
- Fong, P.P.; Molnar, N. Antidepressants cause foot detachment from substrate in five species of marine snail. Mar. Environ. Res. 2013, 84, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Aderemi, A.O.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.; Hunter, C.; Pahl, O. Oxidative stress responses and cellular energy allocation changes in microalgae following exposure to widely used human antibiotics. Aquat. Toxicol. 2018, 203, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühnhold, H.; Kamyab, E.; Novais, S.; Indriana, L.; Kunzmann, A.; Slater, M.; Lemos, M.F.L. Thermal stress effects on energy resource allocation and oxygen consumption rate in the juvenile sea cucumber, Holothuria scabra (Jaeger, 1833). Aquaculture 2017, 467, 109–117. [Google Scholar] [CrossRef]
- Silva, C.S.E.; Novais, S.C.; Lemos, M.F.L.; Mendes, S.; Oliveira, A.P.; Gonçalves, E.J.; Faria, A.M. Effects of ocean acidification on the swimming ability, development and biochemical responses of sand smelt larvae. Sci. Total Environ. 2016, 56, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Peckol, P.; Putnam, A.B. Differential toxic effects of Ulva lactuca (Chlorophyta) on the herbivorous gastropods, Littorina littorea and L. obtusata (Mollusca). J. Phycol. 2017, 53, 361–367. [Google Scholar] [CrossRef]
- Orth, R.J.; Van Montfrans, J. Epiphyte-seagrass relationships with an emphasis on the role of micrograzing: A review. Aquat. Bot. 1984, 18, 43–69. [Google Scholar] [CrossRef]
- Southward, A.J. The Zonation of Plants and Animals on Rocky Sea Shores. Biol. Rev. 1958, 33, 137–177. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Novais, S.C.; Pimparel, I.; Granada, L.; Soares, A.M.; Barata, C.; Lemos, M.F.L. Fatty acid profile of the sea snail Gibbula umbilicalis as a biomarker for coastal metal pollution. Sci. Total Environ. 2017, 586, 542–550. [Google Scholar] [CrossRef]
- Silva, C.O.; Novais, S.C.; Alves, L.; Soares, A.M.V.M.; Barata, C.; Lemos, M.F.L. Linking cholinesterase inhibition with behavioural changes in the sea snail Gibbula umbilicalis: Effects of the organophosphate pesticide chlorpyrifos. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 225, 108570. [Google Scholar] [CrossRef]
- Amiard-Triquet, C.; Amiard, J.C.; Rainbow, P.S. Ecological Biomarkers: Indicators of Ecotoxicological Effects; CRC Press: Boca Raton, FL, USA, 2012; 464p. [Google Scholar]
- McConnell, O.J.; Hughes, P.A.; Targett, N.M.; Daley, J. Effects of secondary metabolites from marine algae on feeding by the sea urchin, Lytechinus variegatus. J. Chem. Ecol. 1982, 8, 1437–1453. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Iwase, Y.; Ina, K.; Fujita, D. Halogenated terpenes isolated from the red alga Plocamium leptophyllum as feedig inhibitors for marine herbivores. Nippon Suisan Gakkaishi 1991, 57, 743–746. [Google Scholar] [CrossRef] [Green Version]
- McLoughlin, N.; Yin, D.Q.; Maltby, L.; Wood, R.M.; Yu, H.X. Evaluation of sensitivity and specificity of two crustacean biochemical biomarkers. Environ. Toxicol. Chem. 2000, 19, 2085–2092. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Borell, E.M.; Foggo, A.; Coleman, R.A. Induced resistance in intertidal macroalgae modifies feeding behaviour of herbivorous snails. Oecologia 2004, 140, 328–334. [Google Scholar] [CrossRef]
- Paul, N.A.; De Nys, R.; Steinberg, P.D. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Lemos, M.F.L.; Soares, A.M.V.M.; Correia, A.; Esteves, A.C. Proteins in ecotoxicology—How, why and why not? Proteomics 2010, 10, 873–887. [Google Scholar] [CrossRef]
- Hay, M.E.; Fenical, W. Marine plant–herbivore interactions: The ecology of chemical defense. Ann. Rev. Ecol. Syst. 1988, 19, 111–145. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R. Marine chemical ecology. Nat. Prod. Rep. 2008, 25, 662–695. [Google Scholar] [CrossRef]
- Verslycke, T.; Roast, S.D.; Widdows, J.; Jones, M.B.; Janssen, C.R. Cellular energy allocation and scope for growth in the estuarine mysid Neomysis integer (Crustacea: Mysidacea) following chlorpyrifos exposure: A method comparison. J. Exp. Mar. Biol. Ecol. 2004, 306, 1–16. [Google Scholar] [CrossRef]
- Wu, R.S.S.; Lam, P.K.S. Glucose-6-phosphate dehydrogenase and lactate dehydroge-nase in the green-lipped mussel (Perna viridis): Possible biomarkers for hypoxia in the marine environment. Water Res. 1997, 31, 2797–2801. [Google Scholar] [CrossRef]
- Diamantino, T.C.; Almeida, E.; Soares, A.M.V.M.; Guilhermino, L. Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 2001, 45, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Smolders, R.; De Boeck, G.; Blust, R. Changes in cellular energy budget as ameasure of whole effluent toxicity in zebrafish (Danio rerio). Environ. Toxicol. Chem. 2003, 22, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Gschwend, P.M.; MacFarlane, J.K.; Newman, K.A. Volatile halogenated organic compounds released to seawater from temperate marine macroalgae. Science 1985, 227, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Bondu, S.; Cocquempot, B.; Deslandes, E.; Morin, P. Effects of salt and light stress on the release of volatile halogenated organic compounds by Solieria chordalis: A laboratory incubation study. Bot. Mar. 2008, 51, 485–492. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recover. 1997, 6, 43–55. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The missing biomarker link: Relationships between effects on the cellular energy allocation biomarker of toxicant-stressed Daphnia magna and corresponding population characteristics. Environ. Toxicol. Chem. 2003, 22, 1632–1641. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 2, 248–254. [Google Scholar] [CrossRef]
- Gnaiger, E. Calculation of energetic and biochemical equivalents of respiratory oxygen consumption. In Polarographic Oxygen Sensors. Aquatic and Physiological Applications; Gnaiger, E., Forstner, H., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1983; pp. 337–345. [Google Scholar]
- Vassault, A. (Ed.) Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1983; pp. 118–126. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.O.; Novais, S.C.; Soares, A.M.V.M.; Barata, C.; Lemos, M.F.L. Impacts of the Invasive Seaweed Asparagopsis armata Exudate on Energetic Metabolism of Rock Pool Invertebrates. Toxins 2021, 13, 15. https://doi.org/10.3390/toxins13010015
Silva CO, Novais SC, Soares AMVM, Barata C, Lemos MFL. Impacts of the Invasive Seaweed Asparagopsis armata Exudate on Energetic Metabolism of Rock Pool Invertebrates. Toxins. 2021; 13(1):15. https://doi.org/10.3390/toxins13010015
Chicago/Turabian StyleSilva, Carla O., Sara C. Novais, Amadeu M. V. M. Soares, Carlos Barata, and Marco F. L. Lemos. 2021. "Impacts of the Invasive Seaweed Asparagopsis armata Exudate on Energetic Metabolism of Rock Pool Invertebrates" Toxins 13, no. 1: 15. https://doi.org/10.3390/toxins13010015
APA StyleSilva, C. O., Novais, S. C., Soares, A. M. V. M., Barata, C., & Lemos, M. F. L. (2021). Impacts of the Invasive Seaweed Asparagopsis armata Exudate on Energetic Metabolism of Rock Pool Invertebrates. Toxins, 13(1), 15. https://doi.org/10.3390/toxins13010015