Abstract
The Senegalese cobra, Naja senegalensis, is a non-spitting cobra species newly erected from the Naja haje complex. Naja senegalensis causes neurotoxic envenomation in Western Africa but its venom properties remain underexplored. Applying a protein decomplexation proteomic approach, this study unveiled the unique complexity of the venom composition. Three-finger toxins constituted the major component, accounting for 75.91% of total venom proteins. Of these, cardiotoxin/cytotoxin (~53%) and alpha-neurotoxins (~23%) predominated in the venom proteome. Phospholipase A2, however, was not present in the venom, suggesting a unique snake venom phenotype found in this species. The venom, despite the absence of PLA2, is highly lethal with an intravenous LD50 of 0.39 µg/g in mice, consistent with the high abundance of alpha-neurotoxins (predominating long neurotoxins) in the venom. The hetero-specific VINS African Polyvalent Antivenom (VAPAV) was immunoreactive to the venom, implying conserved protein antigenicity in the venoms of N. senegalensis and N. haje. Furthermore, VAPAV was able to cross-neutralize the lethal effect of N. senegalensis venom but the potency was limited (0.59 mg venom completely neutralized per mL antivenom, or ~82 LD50 per ml of antivenom). The efficacy of antivenom should be further improved to optimize the treatment of cobra bite envenomation in Africa.
Keywords:
Naja (Uraeus) senegalensis; Naja haje complex; venomics; snakebite envenomation; immunoreactivity; antivenom neutralization Key Contribution:
The study unveiled the distinct venom proteome of Naja senegalensis (Mali; Western Africa), advancing our understanding of the pathophysiology of snakebite envenomation and the diversity of snake venom toxins. The venom toxicity and its cross-neutralization were also studied; providing insights into the treatment of cobra envenoming in the region.
1. Introduction
Each year, snakebite envenomation causes a death toll that surpasses 100,000, and approximately three times as many permanent disabilities and psychological complications in those survived [1]. Most of the victims are from impoverished and remote populations, typically those engaging in agricultural activities [2]. The exact morbidity and mortality of snakebite envenomation are, however, greatly underestimated due to the scarcity of reliable epidemiological data worldwide, in particular rural areas where health systems are suboptimal, and people have limited access to proper treatment. As such, in 2017 snakebite envenomation was reinstated as a priority neglected tropical disease by the World Health Organization [3]. To solve this long persistent global health crisis, key strategies were proposed to combat the various challenges faced [4]. One of the “pillar strategies” proposed is the provision of effective and safe treatment that entails the use of appropriate antivenom, for which its species-specific efficacy needs to be addressed.
Unfortunately, venomous snakes are highly diverse, with at least 200 species being medically important and widely distributed in different geographical areas [5]. In Africa and Asia, the cobras (genus: Naja) are probably the most commonly encountered and medically important venomous snakes. Systemic neurotoxicity and local tissue necrosis are the hallmark features of cobra envenomation, while the severity differs among species. There are approximately 25 extant cobra species (Naja spp.), and the venom composition of each species vary considerably in terms of the relative abundances and subtypes of toxins, although the three-finger toxins and phospholipases A2 are generally conserved [6,7,8,9]. Variations in the venom composition give rise to differential toxicity and clinically variable neutralization response to antivenom treatment. Thus, it is critical to understand the composition profile and toxicity neutralization of cobra venom according to the species and geographical origin, so that the pathophysiology of envenomation can be elucidated and treatment can be improved.
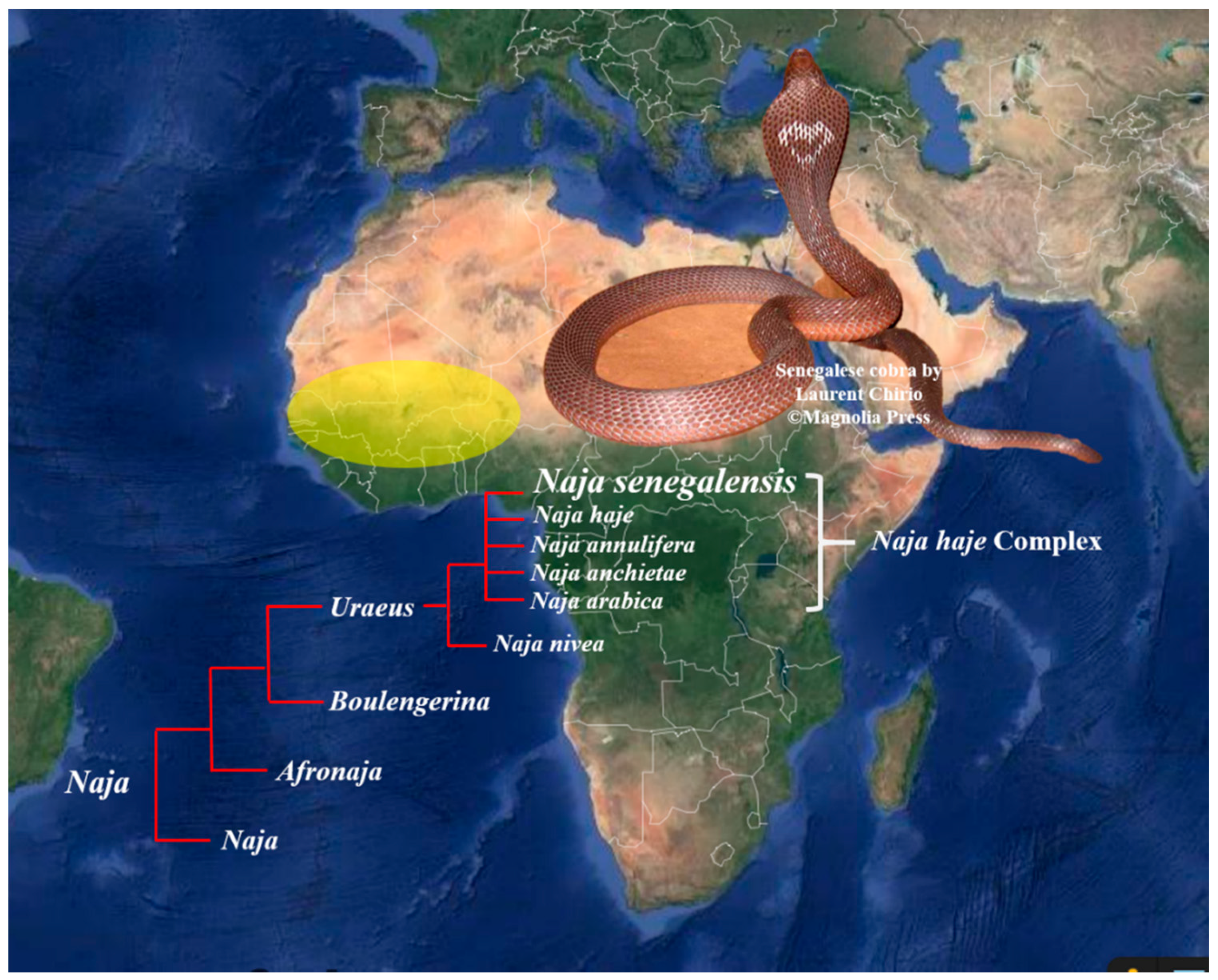
The present systematics places the diverse cobra species under four subgenera, i.e., Naja, Afronaja, Boulengerina, and Uraeus, with the latter three being African cobras. In the past two decades, the systematics of non-spitting African cobras, historically clustered under the Naja haje complex, has been extensively revised based on molecular markers (mitochondria DNA) and morphological characters, resulting in the elevation of several distinct species from within the complex. These relatively new species include Naja annulifera [10], Naja anchietae [11], Naja arabica, and N. senegalensis [12], which are assigned to the subgenus of Uraeus [13] along with Naja haje and Naja nivea. Among the six Uraeus cobra species, N. senegalensis (Senegalese cobra) is an endemic species distributed in Western Africa across Senegal, Gambia, Mali, Burkino Faso, Ghana, Benin, Guinea-Bissau, Nigeria, and the Ivory Coast [14,15] (Figure 1). The distinctive morphological characteristics (with a high number of scale rows around the neck) and genetic differences (unique mtDNA haplotypes) of N. senegalensis separated it from the other members of N. haje complex. An adult N. senegalensis may possess a considerably huge body size with a length up to 2.3 m. Adult snakes are dark-grayish brown dorsally and yellowish ventrally, with a dark collar around the neck, and occasionally bearing a heart-shaped nuchal mark on the hood (Figure 1) [12]. Clinically, envenomation by N. senegalensis has been reported to cause prominent neuromuscular paralysis with minimal local tissue damage [16], but little is known about the composition, pathophysiology, and neutralization of the venom of this new cobra species. Nonetheless, a recent study showed that the N. senegalensis venom was void of PLA2 activity [17], suggesting a venom phenotype that is unique to the species and perhaps the entire monophyletic clade of the Uraeus subgenus. This was further supported by the venomics of another non-spitting African cobra, N. annulifera (sub-Saharan snouted cobra), whose venom proteome is indeed deficient of PLA2 [18]. The presumed “loss” of PLA2 in N. senegalensis venom, and possible altered venom toxicity resulted therefrom, remain to be further validated. Hence, we sought to investigate the venom composition of N. senegalensis applying a protein decomplexing strategy of venomics, where chromatographic fractions of the venom were analyzed with nano-liquid chromatography-tandem mass spectrometry (nano-LCMS/MS) for protein identification and quantification [8]. Furthermore, the lethality of the venom, and the immunological binding activity, as well as the cross-neutralization efficacy of a hetero-specific antivenom marketed in Africa (VINS African Polyvalent Antivenom), were examined against Naja senegalensis venom. The findings provided insights into the pathophysiology and management of N. senegalensis envenomation in Western Africa.
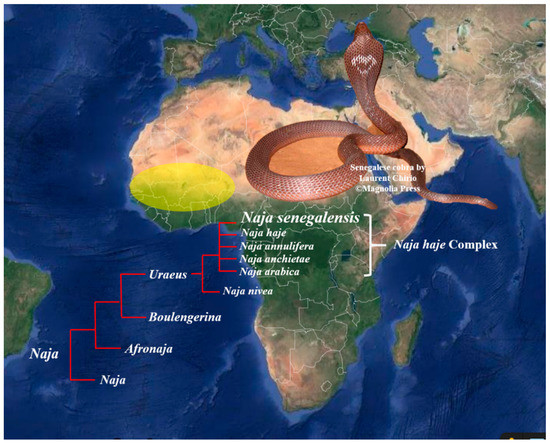
Figure 1.
Geographical distribution of Naja senegalensis in Western Africa, encompassing Senegal, Gambia, Mali, Burkino Faso, Ghana, Benin, Guinea-Bissau, Nigeria, and the Ivory Coast (yellow region) [5]. Insets: Naja senegalensis from the subgenus of Uraeus, elevated as a new species from the Naja haje complex. Note the distinct hood marking which is uncommon in African but Asiatic cobras. Snake photograph was courtesy of Laurent Chirio with copyright © Magnelia Press 2009 [12].
2. Results and Discussion
2.1. Chromatographic Separation of Naja Senegalensis Venom
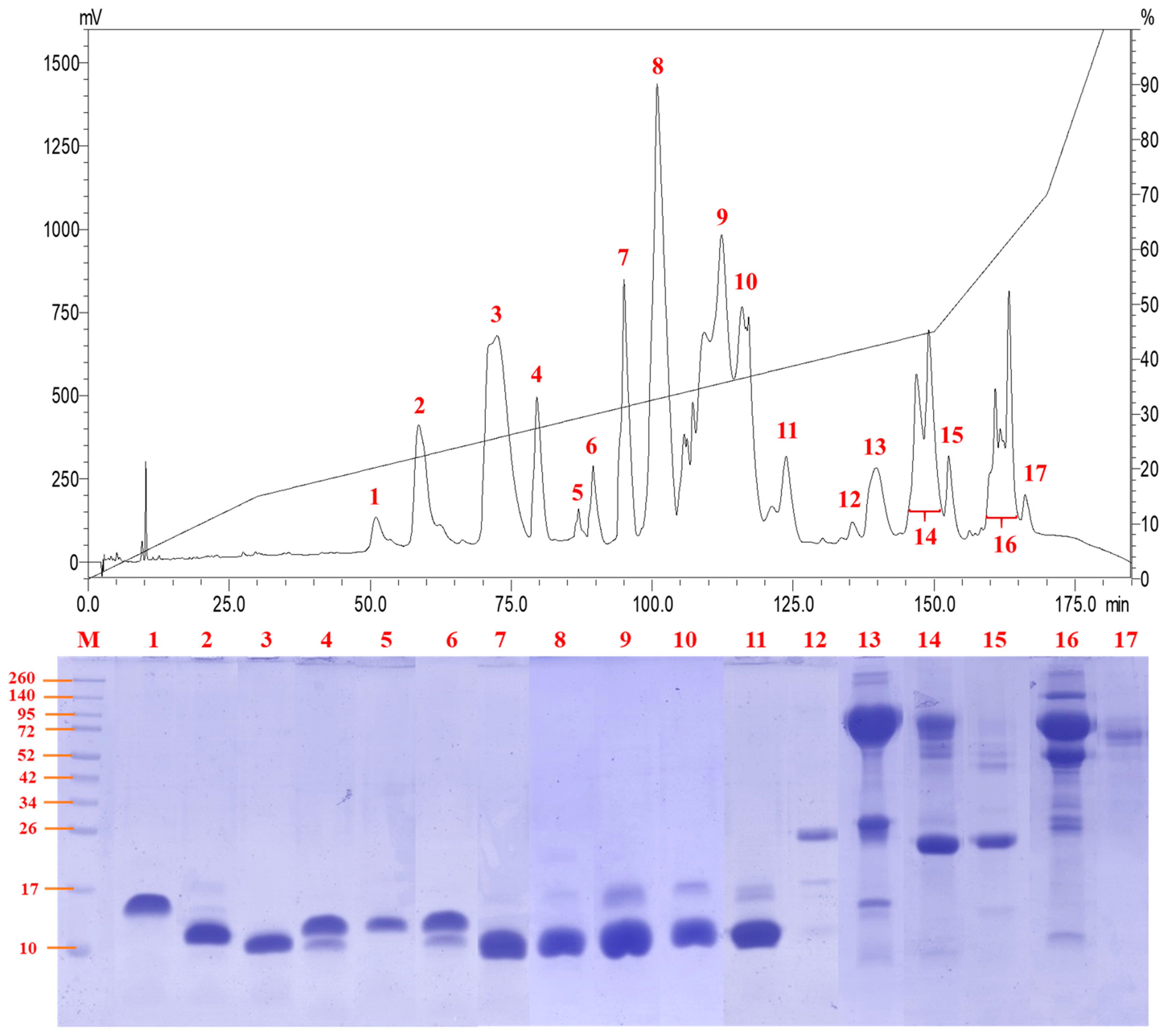
C18 reverse-phase HPLC resolved the venom proteins into 17 fractions of increasing hydrophobicity (Figure 2). SDS-PAGE of the fractions showed that the majority of the venom proteins were of low molecular weight (<20 kDa) under reducing conditions (Figure 2), although some proteins might exist as complexes in their native forms. Most of the low molecular weight proteins (fractions 1 to 11) were eluted between 50 and 125 min of RP-HPLC, and these made up approximately 80% of the total venom proteins. Proteins with middle (25–35 kDa) to high molecular weights (>40 kDa) were eluted through fractions 12 to 17 between 135 and 170 min of HPLC, together accounting for <20% of total venom proteins. The predominant presence of low molecular weight proteins in N. senegalensis venom is a typical venom phenotype of cobras irrespective of phylogeographical distribution, as observed in a number of Asiatic and African cobra venoms including Indian N. naja [19], Indonesian N. sputatrix [9], Philippine N. philippinensis [8], Thai N. kaouthia [20], and African N. annulifera [18] whose venoms were profiled under the same experimental conditions.
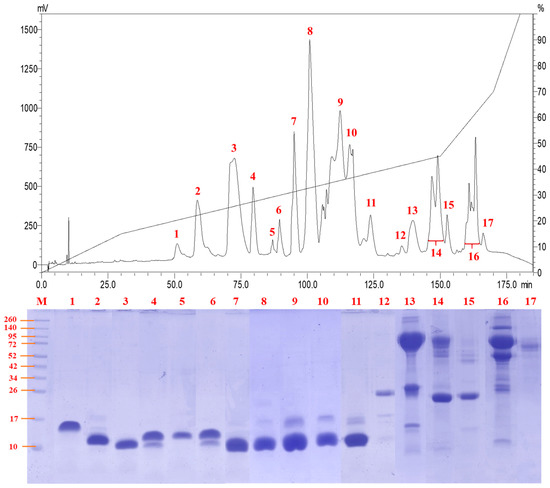
Figure 2.
Reverse-phase high-performance liquid chromatography of Naja senegalensis venom. Chromatogram of the venom fractionation (top panel) and separation of venom fractions using SDS-PAGE under reducing condition (bottom panel). M, protein ladder for estimating the molecular weight of sample proteins.
2.2. Venom Proteomes and Toxicity Correlation
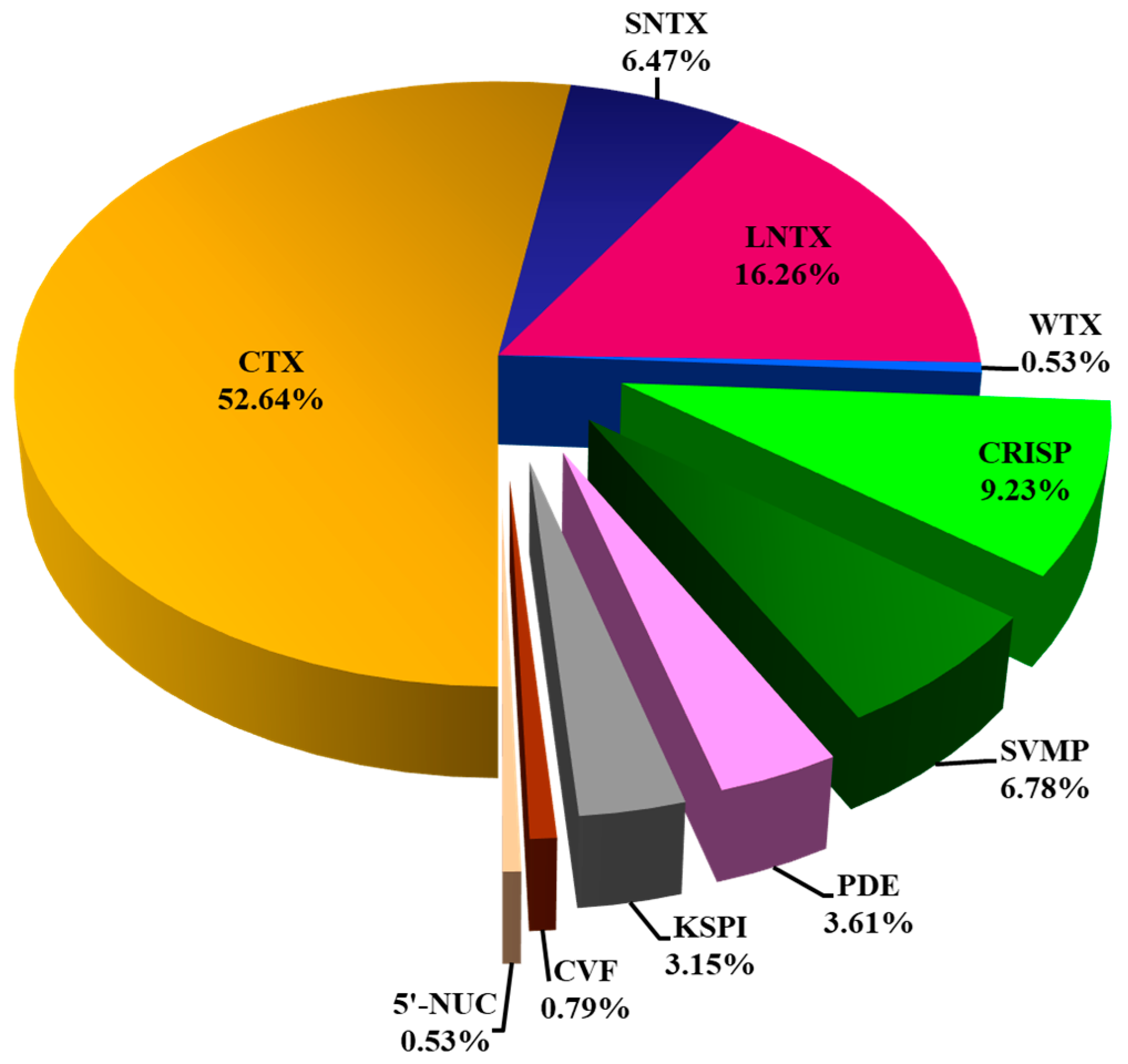
Nano-ESI-LCMS/MS analysis of the N. senegalensis venom fractions with QTOF mass spectrometry identified a total of 54 proteins (Table 1). Of these, 37 proteins were non-redundant distinct proteoforms categorized into 7 protein families (Figure 3). Details of protein identification from both mass spectrometry analyzers (matched sequences, peptide scores, mass-to-charge ratios, annotated accession codes, and corresponding species) were provided in Supplementary Files S1. All proteins identified were annotated to homologous proteins from closely related elapid species but not N. senegalensis, for there is a total lack of toxin database specific to this newly erected species. Regardless, the three-finger toxins (3FTX) superfamily constituted the most dominantly expressed proteins (with relative abundance >75%) in the venom proteome of N. senegalensis. Of note, the 3FTX were also the low molecular weight proteins eluted from the RP-HPLC of the venom (Figure 2), and within this protein family, the cytotoxins (also named as cardiotoxins, CTX) were predominated. Cysteine-rich secretory proteins (CRISP) were the second most abundant proteins in the venom, constituting about 9% of the total venom proteins. The remaining minor protein components in the venom included snake venom metalloproteinase protein (SVMP), phosphodiesterase (PDE), Kunitz-type serine protease inhibitor (KSPI), cobra venom factor (CVF), and 5′-nucleotidase (5′-NUC), altogether accounting for <20% of the total venom proteins.

Table 1.
Identification of proteins from reverse-phase HPLC fractions of Naja senegalensis venom through QTOF nano-ESI-LCMS/MS.
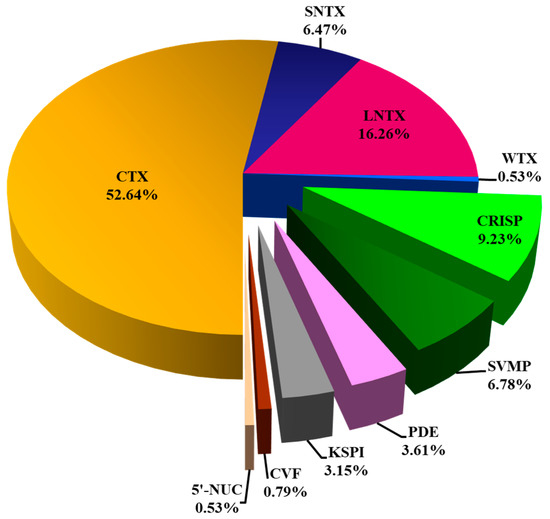
Figure 3.
Snake venom proteome of Naja senegalensis. Proteomic profiling was accomplished with QTOF nano-ESI-LCMS/MS of reverse-phase HPLC-fractionated venom proteins. All protein samples were subjected to in-solution tryptic digestion. Abbreviations: CTX, cytotoxin/cardiotoxin; SNTX, short neurotoxin; LNTX, long neurotoxin; WTX, weak neurotoxin; CRISP, cysteine-rich secretory protein; CVF, cobra venom factor; KSPI, Kunitz-type serine protease inhibitor; PDE, phosphodiesterase; SVMP, snake venom metalloproteinase; 5′-NUC, 5′-nucleotidase. CTX, SNTX, LNTX, and WTX constituted three-finger toxins (3FTX) superfamily.
Three-finger toxins (3FTX) of N. senegalensis venom were formed by the subfamilies/subgroups of short and long neurotoxins, weak neurotoxins, and cytotoxins. Of these, the cytotoxins or cardiotoxins (CTX) were the most diversely and abundantly expressed, comprising 14 distinct CTX proteoforms at 53% of the total venom proteins (Table 2). CTXs are commonly found in moderate to high abundances in cobra venoms [6,19]. Genomics and venom-gland transcriptomics of Asiatic cobras revealed that CTX multigenes are diversely and abundantly expressed while retaining high structural homology of the proteins [21,22,23]. The amount of CTX in the venom, however, can vary considerably amongst various cobra species, ranging from 20% to 86% of total venom proteins [6,7,8,9,19,24,25]. The vast dynamic range of CTX in a cobra venom presumably reflects the phenotypic adaptation of distinct species to a different ecological niche, although CTX generally serves a common function related to the cytotoxic and tissue-damaging effects. Cobras typically employ alpha-neurotoxins to paralyze prey (primary hunting strategy); hence, the abundantly expressed CTXs are likely needed for digestive and defensive (algesic) purposes [26,27]. In clinical envenomation, CTX is implicated in local tissue necrosis and venom ophthalmia [28,29], but it is unlikely the direct lethal factor of the venom since its lethal effect is much weaker compared to alpha-neurotoxins (intravenous median lethal dose, LD50 of CTX > 1.0 µg/g, c.f. LD50 of alpha-NTX < 0.2 µg/g, tested in mice) [30,31]. The cytotoxic activities of CTX are, nevertheless, pharmacologically diverse. Depending on the dose of CTX, the cell death could be caused by apoptosis or necrosis [32] mediated through various mechanisms, e.g., mitochondrial fragmentation (CTX binding to cardiolipin) and membrane pore formation (CTX interacting electrostatically with anionic lipids) [33,34]. Earlier studies also showed that some cobra CTXs could elicit cardiotoxicity through disrupting calcium regulation in cardiac muscle cells, thus prolonging membrane depolarization, resulting in systolic heart arrest [35,36]. Cardiotoxicity, however, has not been well recognized in clinical envenomation and death caused by most cobra bites, where respiratory failure following systemic paralysis is the main cause of death. The tissue-damaging effect of cobra CTX, however, is variable across different species, with cytotoxic potential that appears to rise with the hood-displaying behavior and aposematic coloration to warn off predators or aggressors [27]. The high abundance of CTX in N. senegalensis venom suggests its important role in the pathophysiology of envenomation, at least in tissue-damaging activities. Unfortunately, clinical data of envenomation caused by N. senegalensis remains scarce to allow further interpretation of the medical relevance of its CTX.

Table 2.
Overview of protein families and non-redundant proteins identified in Naja senegalensis venom proteome.
The alpha-neurotoxins (SNTX and LNTX) constituted the second major group of 3FTX proteins in the N. senegalensis venom (Table 2). These are post-synaptic neurotoxins that bind and block nicotinic acetylcholine receptors at the neuromuscular junction, leading to systemic paralysis, respiratory failure, and death [37]. A clear correlation exists between the abundance of alpha-neurotoxins and the lethal potency of cobra venoms [8], and the high abundance of alpha-neurotoxins in N. senegalensis venom (up to ~23% of total venom proteins) indeed correlated well with its neurotoxic activity and potent lethality (i.v. LD50 = 0.39 µg/g), as observed in this study (Table 3). The finding is also consistent with the prominent neurotoxic effect reported in clinical envenomation caused by the Senegalese cobra [16]. Furthermore, the quantitative composition of alpha-neurotoxins in N. senegalensis venom (current study) appeared to be higher than that reported in other African cobra species (α-neurotoxins < 20% of total venom proteins), whose venom LD50 were correspondingly higher (0.53–1.42 µg/g) [6,18,25,38], supporting that alpha-NTX is the principal toxin that drives the lethality of the cobra venom. In brief, alpha-neurotoxins consist of Type I (short neurotoxins, SNTX) and Type II (long neurotoxins, LNTX), both of which are highly neurotoxic with LD50 values well established between 0.05–0.2 µg/g for cobras [8,9,30,31,39,40]. The SNTX and LNTX, however, differ in the length of the amino acid sequence, the number of disulfide bonds, the type of binding receptor, and affinity [41,42]. We previously reported that in cobra venoms, the relative abundance ratio of LNTX to SNTX can be strikingly variable among different cobra venoms. The subproteomic variation in LNTX and SNTX composition has an impact on the toxicity of venom and its neutralization by antivenom [30,31]. In the present work, the abundance of long neurotoxins (LNTX) in N. senegalensis venom was much higher than that of short neurotoxins (SNTX), implying that the neurotoxic activity (and hence the lethality) of the venom is mediated primarily by LNTX. The similar phenotypic predilection for a LNTX-predominating venom is observed in most neurotoxic Asiatic cobras, including the Thai N. kaouthia (LNTX:SNTX ~ 4:1) [20], Thai N. siamensis (LNTX:SNTX ~ 4:1) [7], Malaysian N. sumatrana (LNTX:SNTX ~ 4:1) [43], and Pakistani N. naja venom (LNTX:SNTX ~ 3–5:1) [19,44]. On the other hand, SNTX-predominating cobra venoms were found mainly in species inhabiting the eastern part of Asia, including the Taiwanese N. atra (SNTX:LNTX ~ 10:1) [45], Javan N. sputatrix (SNTX: LNTX ~ 16:1) [9], and the Philippine N. philippinensis which represents the most exclusive case of extreme bias to SNTX expression (SNTX:LNTX ~ 45:1) [8]. Although SNTX and LNTX are essentially both post-synaptic acting neurotoxins, recent studies indicated that these toxins are not necessarily equipotent, as SNTX may produce a more reversible blockage of nicotinic acetylcholine receptor (nAChR) compared to LNTX [46,47,48]. The present proteomic finding thus supports that the antivenom production for use in Western Africa should be tailored to reverse the toxicity of the alpha-neurotoxins, in particular the LNTX in N. senegalensis venom.

Table 3.
Lethality and antivenom neutralization of venoms from the African non-spitting cobras (genus: Uraeus).
In addition to CTX and α-NTX, the N. senegalensis venom also contained minute weak neurotoxins (WTX, 0.5% of total venom proteins). WTX interacts with muscle and neuronal nAChR at low affinity, and has toxicity that is 50 to 100 times lower than α-NTX (WTX LD50 = 5–80 µg/g c.f. α-NTX LD50 = 0.05–0.2 µg/g) [49,50]. As such, these toxins which were minimally expressed in the proteome, likely do not play a major role in the pathophysiology of envenomation, although their biological significance in the evolution of cobra venom remains to be elucidated.
2.3. Other Protein Constituents
Other components present in the N. senegalensis venom proteome were proteins with higher molecular weights (>25 kDa). Of these, cysteine-rich secretory protein (CRISP) shows a broad range of biological activities, e.g., increasing vascular permeability and promoting inflammatory response which may be implicated in the pathogenesis of envenomation [51,52,53]. Snake venom metalloproteinase (SVMP) are multi-domain, zinc-dependent enzymatic toxins abundantly present in viperid and crotalid venoms, and contribute to hemotoxic envenomation [54,55]. SVMPs, typically the P-III subtype, have been discovered from most cobra venoms (as in the current study) but the amount is usually very low and the function is unlikely related to hemotoxicity. A Kunitz-type serine protease inhibitor was also detected in the current study. KSPIs contribute to neurotoxic activities of krait and mamba venoms [56,57,58]; however, they are not known to implicate in neurotoxic envenomation caused by cobras.
The presence of phosphodiesterase (PDE), 5′-nucleotidase (5′-NUC), and cobra venom factor (CVF) in the N. senegalensis venom suggested their involvement in facilitating the spread of venom toxins. The concomitant hydrolysis of ATP and ADP by PDE and 5′-NUC, respectively, generates adenosine, a potent vasodilator that increases local vascular permeability and thus toxin diffusion further from the bite site [59]. CVF, a complement-activating protein in cobra venom, is structurally and functionally highly homologous to complement component C3. Its presence, although minute in the venom, is believed to also facilitate the distribution of venom toxins via vasodilation [60].
2.4. The Lack of Phospholipase A2 (PLA2) in Naja Senegalensis Venom
Recent proteomic and enzymatic studies demonstrated that amongst cobras, species of the Uraeus subgenus appeared to have venoms that lack secretory PLA2 [17,18]. The phenomenon is, again, revealed in the present work tested on N. senegalensis venom. We fractionated the venom through C18 RP-HPLC as per the decomplexing venomic protocol [61], but the subsequent QTOF tandem mass spectrometry analysis did not detect any PLA2 which was expected to co-elute, typically, with the hydrophobic CTX [8,9,19]. The finding is consistent with the negligible PLA2 enzymatic activity in the venoms of N. senegalensis and representative species of the Uraeus subgenus (N. haje, N. annulifera, and N. nivea), contrary to the other Asiatic and African cobra species (Naja, Afronaja and Boulengerina subgenera) which have evolved venom phenotype characterized by abundantly expressed PLA2 [17]. It has been shown that positive selection has a dominant role on elapid (cobra) PLA2, in which the PLA2 multigene evolved from an ancestral non-toxic PLA2 through repeated gene duplication, followed by functional divergence [62]. The resultant increases in genomic (multigene) and phenotypic (venom composition) complexity are deemed essential to allow the cobras to adapt to ecological niche shifts and new prey types. Our present finding, however, suggests that the PLA2 gene in at least one cobra species underwent purifying selection instead. Based on the observed phenomenon, we speculate a possible scenario where post-speciation niche shifts subjected the PLA2 multigene to the birth-and-death and “selective sieve” processes of gene duplication, divergence, and loss, in a way similar to the evolution of the elapid three-finger toxins as reported previously [63,64]. Depending on the functionality of the PLA2, genes that are no longer effective in subduing new prey species or deterring predators were lost as in pseudogenization—a more common fate for a duplicated gene actually. Collectively, the event contributes to shaping the diversity of the venom in its present form. The observation warrants further genetic analysis, and we anticipate genomic and venom-gland transcriptomic studies to further shed light on the evolution of snake venom PLA2 in N. senegalensis, and related cobra species from the Uraeus subgenus.
2.5. Immunoreactivity and Neutralization of Antivenom
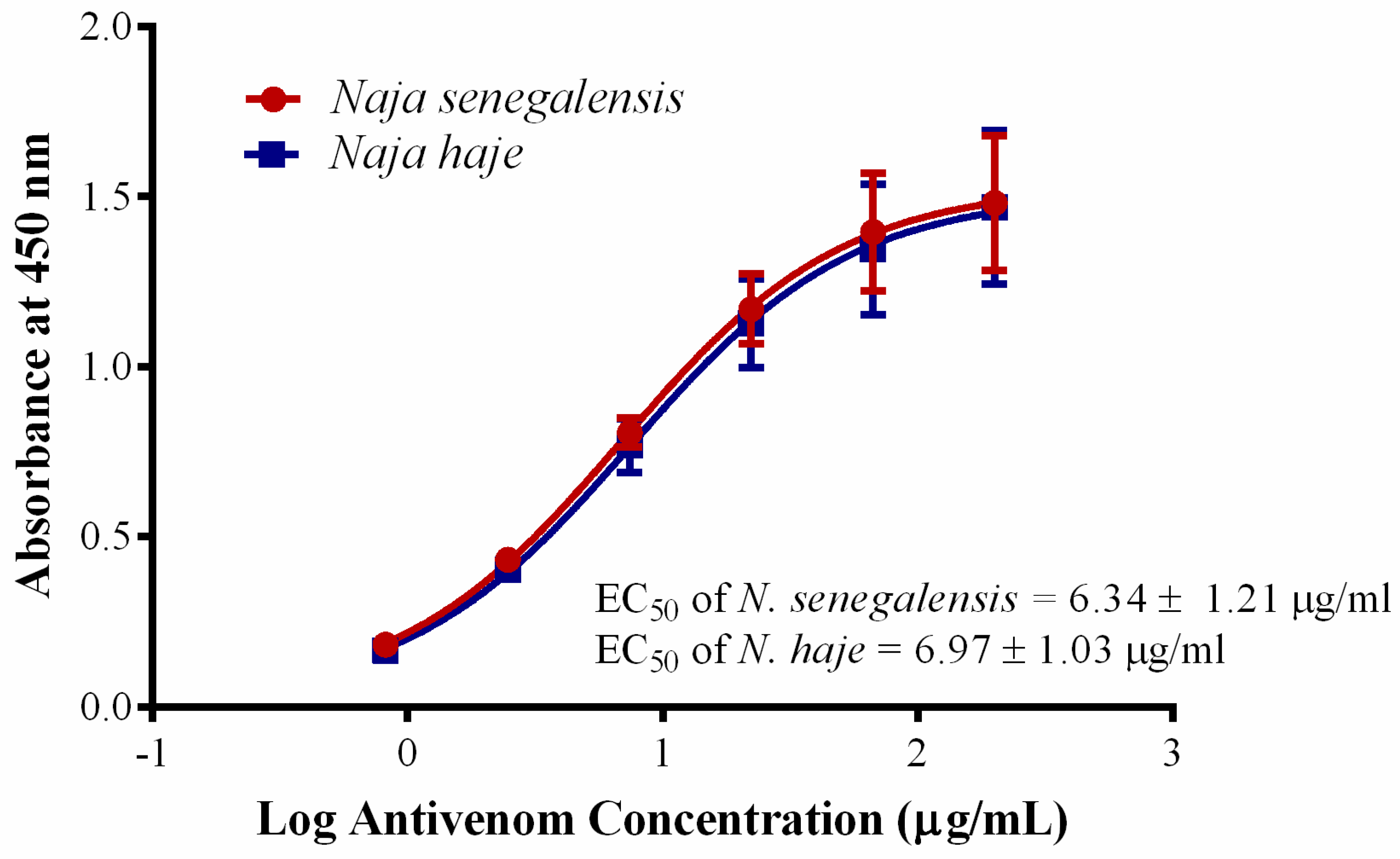
Cobras are common causes of snakebite envenomation in Sub-Saharan Africa, causing two distinctive toxic manifestations. The African non-spitting cobras (N. haje, N. nivea, N. senegalensis, N. annulifera) and the forest cobra (N. melanoleuca) cause systemic neurotoxic envenomation, while the African spitting cobras (N. nigricollis, N. katiensis, N. mossambica, N. pallida, N. ashei) cause predominantly local cytotoxic envenomation (tissue necrosis) [65]. Due to the limited local capacity for antivenom production in Africa, currently, various antivenom products are imported and marketed in Sub-Saharan Africa; these include SAIMR polyvalent antivenom produce from South Africa, Antivipmyn-Africa from Mexico, VACSERA from Egypt, EchiTAb-Plus-ICP from Costa Rica, VINS Pan Africa antivenom and ASNA antivenom from India, and Inoserp Pan Africa from Mexico with limited information regarding their clinical effectiveness [66]. Considering that N. senegalensis is a highly neurotoxic species (venom LD50 = 0.39 µg/g, current study) and widely distributed in Western Africa, an effective antivenom is undoubtedly the definitive treatment needed to treat the envenomation caused by this cobra. Unfortunately, up to now, there is still no species-specific antivenom available to treat its envenomation. Hetero-specific polyvalent antivenom products, e.g., VAPAV are available for envenomation caused by N. haje but the cross-neutralization efficacy against N. senegalensis venom has not been examined. In this study, VAPAV showed dose-dependent immunological cross-reactivity toward N. senegalensis venom (Figure 4), with immunological binding activity (indicated by half-maximal effective concentration, EC50 = 6.34 ± 1.21 µg/mL) that was comparable to its binding to the homologous N. haje venom (EC50 = 6.97 ± 1.03 µg/mL) (p > 0.05). The finding revealed conserved protein antigenicity in the venoms of N. senegalensis and N. haje, which are phylogenetically related species within the Uraeus genus. The cross-reactivity suggested cross-neutralization capability of VAPAV, which we subsequently evaluated with the WHO-recommended antivenom efficacy test (in vivo neutralization test) [67,68]. Our finding showed that the hetero-specific VAPAV was able to cross-neutralize the lethality of Senegalese cobra venom moderately, with a median effective dose (ED50) of 60.81 µL against 5 × LD50 challenge dose of the venom. In terms of neutralizing potency, each milliliter of VAPAV was able to completely neutralize 0.59 mg of the venom. The protein concentration of VAPAV was 130.68 mg/mL (by bicinchoninic acid assay).
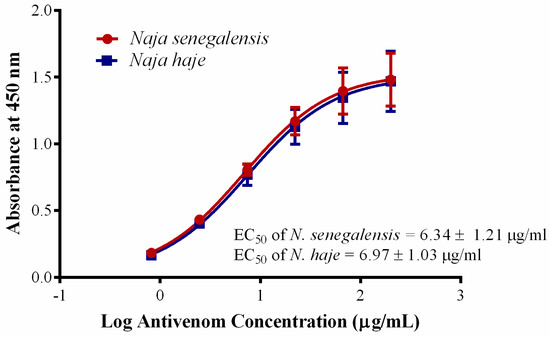
Figure 4.
Immunological binding activity of VINS African Polyvalent Antivenom (VAPAV) toward N. senegalensis and N. haje venoms. The immunological binding activity was represented as half the maximum concentration (EC50). Values were means ± S.E.M. of triplicates.
In this study, the neutralization of VAPAV against the Senegalese cobra venom was moderately effective. Its neutralization potency was determined as 0.59 mg/mL, which falls within the range of potency reported for a few African antivenom products tested in vivo: 0.31–1.20 mg venom neutralized per mL of antivenom, in mice against African cobra venoms [18,66,69,70,71] (Table 3). However, the neutralization potency (mg/mL) has a limitation when compared between different antivenom products against different venoms, as venom toxicity varies among different cobra species. Therefore, the protective efficacy (R, defined as the number of LD50 neutralized per mL antivenom at which 50% of mice survived) was included as an indicator for comparative purpose. The protective efficacy (R) of VAPAV against N. senegalensis venom was 82.2 LD50/mL antivenom, indicating a relatively high efficacy when compared amongst other African antivenoms against the venoms of Uraeus species (R = 9.8–112.3 LD50/mL). In general, the neutralization potency of these commonly used African antivenoms rarely exceeded 1 mg venom/mL antivenom; this poses a challenge in the clinical dosing of antivenom as an adult cobra can easily inject a large quantity of venom beyond 50–100 mg. In the case of N. senegalensis, assuming there is an initial systemic bioavailability of 50 mg venom, a starting dose of at least 10 vials of the African antivenom (such as VAPAV) would therefore be required to initiate neutralization. Clinically, the doses often need to be escalated or repeated over time when the residual venom is slowly absorbed from the subcutaneous depot (bite site) while the antivenom is continuously metabolized or eliminated from the body. Furthermore, from the clinical perspective, it is impossible to overlook the cost issue and adverse effects (fatal hypersensitivity reactions) associated with high doses of antivenom. Based on an antivenom costing to Kenyan hospitals, antivenoms produced by non-Africa-based manufacturers (typically from India) were indeed marketed at costs considerably lower than the Africa-based products like SAIMR or Fav Afrique antivenoms—for instance, in 2016 VAPAV was marketed at USD 47.90 per vial c.f. SAIMR at USD 315 per vial [71], but the cost of using multiple doses of VAPAV remains prohibitive in the face of extreme poverty in those countries. The finding of the cross-neutralization indicated that the antivenom resources (poly-specific antivenom with an indication for N. haje or the Egyptian cobra bite) may be shared in the region for the treatment of envenomation caused by Senegalese cobra, without the need to introduce a new antivenom product into the market. However, the main challenge, i.e., how to increase the potency of the antivenom while ensuring its safety and affordability, remains unresolved.
There are several suggestions to revolutionize antivenom production, e.g., the development of recombinant antivenom [73], development of elapid antivenom by immunizing horses with recombinant consensus short-chain α-neurotoxins [74], and re-formulation of toxin-targeting, pan-species antivenom [75,76]. These new initiatives are, however, challenging in terms of practicality owing to the enormous time and cost needed in R&D, various regulatory constraints, limited market size, and low-profit margin of the product. The problem could be circumvented by repurposing and making use of existing hetero-specific antivenoms which have been shown to confer an effective cross-neutralization effect when a species-specific antivenom is not available [71,72,77]. Besides, the use of small toxin inhibitors such as varespladip, a phospholipase A2 inhibitor has been advocated as an adjunct treatment in snakebite envenomation, as it showed a promising effect in attenuating neurotoxicity caused by elapid snake venoms [78,79]. The inhibitor varespladip, however, likely has no use in snakebite envenomation caused by most cobra species whose venom PLA2 are not the principal toxins [30,31,80], or, snakes with atypical venoms that contain no PLA2, such as the Senegalese cobra (present study). Antivenom thus remains the mainstream and the most realistic treatment of cobra bites, though there is a need to optimize its supply and distribution as well as judicious clinical use in terms of product selection, dosing, and administration.
3. Conclusions
This is the first report on the quantitative venom proteomics of N. senegalensis, a distinct non-spitting cobra species with medical importance in Western Africa. Cytotoxins/cardiotoxins (CTX) and alpha-neurotoxins from the three-finger toxins family (3FTX) constituted the major proteins of the venom. The long α-neurotoxins were more abundant compared to short α-neurotoxins (LNTX:SNTX = 3:1), suggesting that LNTX plays a more prominent role in the potent neurotoxicity of the venom as reflected by its low LD50. The study also uncovered an unusual venom phenotype that is characterized by the distinct lack of PLA2, implying that the PLA2 multigene in this species and probably the whole lineage of the Uraeus subgenus underwent purifying evolution in which they were pseudogenized in adaptation to new dietary shifts and changing ecological niche. The study further revealed well-conserved venom antigenicity in N. senegalensis and the phylogenetically related Naja haje, as the African polyvalent antivenom, VAPAV was able to immunorecognize the N. senegalensis venom and cross-neutralize its in vivo toxicity albeit the potency was low. The findings provide insights into the use of regional antivenom available in Africa, and potentially the improvement of snakebite management in Western Africa where the Senegalese cobra contributes to the disease burden of snakebite envenomation.
4. Materials and Methods
4.1. Consumables and Reagents
Ammonium bicarbonate, dithiothreitol (DTT), and iodoacetamide (IAA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). MS grade trypsin protease, Spectra™ Multicolor Broad Range Protein Ladder (10 to 260 kDa, catalog number: 26634), and HPLC grade solvents were purchased from Thermo Scientific™ Pierce™ (Rockford, IL, USA). Acetonitrile (HPLC grade), LiChrospher® WP 300 C18 RP-HPLC column, and Millipore ZipTip® C18 Pipette Tips were obtained from Merck (Kenilworth, NJ, USA). Other chemicals and solvents used were of analytical grade and purchased from Sigma-Aldrich (USA).
4.2. Venom and Antivenom
The venoms of Naja senegalensis and Naja haje (used in immunoreactivity study) were supplied by Latoxan (Valence, France) in lyophilized form and stored at −20 °C until use. The antivenom used was VINS African polyvalent antivenom manufactured by VINS Bioproducts Limited (VAPAV, batch no.: 07AS1604; expired date: February 2020). VAPAV is a purified F(ab’)2 obtained from the horse serum hyperimmunized against the venoms of Naja melanoleuca, N. nigricollis, N. haje, D. polylepis, D. viridis, D. jamesoni, B. gabonica, B. arietans, E. leucogaster, and E. ocellatus. The antivenom was in lyophilized form and reconstituted in 10 mL of saline water and each mL can neutralize 20 LD50 of N. melanoleuca and N. nigricollis venoms; and 25 LD50 of N. haje, D. polylepis, D. viridis, D. jamesoni, B. gabonica, B. arietans, E. leucogaster, and E. ocellatus venoms. The antivenom was used before the expiration date.
4.3. Animals and Ethics Statement
Mice used in this study were of albino ICR strain (20–25 g) supplied by the Animal Experimental Unit from the University of Malaya. The protocol for animal experimentation was carried out based on the Council for International Organizations of Medical Sciences (CIOMS) guidelines [81] and was approved by the Institutional Animal Care and Use Committee of the University of Malaya (Ethics clearance number: 2014-09-11/PHAR/R/TCH, approved 9 November 2014).
4.4. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
Three milligrams of N. senegalensis venom was reconstituted in 200 µL ultrapure water and the impurities were removed by centrifugation prior subjected to C18 reverse-phase high-performance liquid chromatography (HPLC). The LiChrospher® WP 300 C18 column (5 µm particle size) column was pre-equilibrated with 0.1% TFA in water (Eluent A) and the sample was eluted with 0.1% TFA in acetonitrile (Eluent B) using a linear gradient of 5% B for 10 min, 5–15% B over 20 min, 15–45% B over 120 min and 45–70% B over 20 min. The flow rate was set at 1 mL/min. Absorbance was monitored at 215 nm and the resulting peaks were collected, lyophilized, and kept at −20 °C prior to use.
4.5. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The fractions collected from RP-HPLC was reconstituted in ultrapure water and separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing condition at 100 V for 2 h. Thermo Scientific Spectra™ Multicolor Broad Range Protein Ladder (Thermo Scientific™ Pierce™, Rockford, IL, USA) containing 10 prestained proteins ranging from 10 kDa to 260 kDa was used for molecular mass calibration. Gel was stained with Coomassie Brilliant Blue R-250 and the bands were scanned using ImageScanner III (GE Healthcare, Uppsala, Sweden).
4.6. Venom Fractions in-Solution (FIS) Tryptic Digestion and Agilent Q-TOF Mass Spectrometry
The protein fractions were reduced with dithiothreitol (DTT), alkylated with iodoacetamide (IAA), and digested with MS grade trypsin (PierceTM) before desalting with C18 ZipTip® Pipette Tips. The tryptic peptides were then reconstituted in 7 µL of 0.1% formic acid in water and subjected to nano-electrospray ionization liquid chromatography-tandem mass spectrometry (ESI-LCMS/MS) on Agilent 1200 HPLC-Chip/MS Interface (Agilent Technologies, Santa Clara, CA, USA), coupled with Agilent 6550 Accurate-Mass Q-TOF LC/MS system (Agilent Technologies, Santa Clara, CA, USA). Samples were loaded in a 75 µm × 150 mm analytical column packed with Zorbax C18 (Pore size: 300 Å, 160 nL trapping column, 5 µm particles). The sample loading volume was 1 µL and separated with 0.1% formic acid in acetonitrile using a linear flow gradient of 5–50% B for 11 min, 50–70% B for 4 min, and 70% B for 3 min. The flow rate was set to 0.4 µL/min. The drying gas flow was 11 L/min, drying gas temperature was 290 °C, fragmentor voltage was 175 V and the capillary voltage was set to 1800 V. In the positive ionization mode, spectra were acquired in an MS/MS mode with an MS scan range of 200–3000 m/z and MS/MS scan range of 50–3200 m/z. Precursor ion charge stage was set as double and above with the reference ions of 1221.9906 m/z (z = 1) and 299.2944 (z = 1). Data were extracted with an MH+ mass range between 50 and 3200 Da. The MS/MS data were processed with Agilent Spectrum Mill MS Proteomics Workbench software packages to provide protein and peptide identifications. Carbamidomethyl cysteine (C) was set as a fixed modification and oxidized methionine as a variable modification. The raw data was blasted against a non-redundant NCBI database of Serpentes (taxid: 8570) merged with an in-house transcript database as previously described [8]. Protein identification was validated with the following filters: protein score > 20, peptide score > 10 and score peak intensity (SPI) > 70%. Identified proteins were filtered at < 1% false discovery rate (FDR).
4.7. Estimation of Protein Relative Abundance
In venom fraction in-solution (FIS) profiling, the protein abundance ratio of a venom protein in its chromatographic fraction was estimated by dividing the mean spectral intensity (MSI) of its peptides by the total spectral intensity of all proteins detected in the fraction. The relative protein abundance expressed as the percentage of total venom proteins was determined by multiplying the relative spectral intensity of the protein and the chromatographic peak area under the curve (AUC) as follows:
4.8. Immunological Binding Activity Study of Antivenom
The immunological binding activity of the VINS African polyvalent antivenom (VAPAV) toward N. senegalensis venom was examined using an indirect enzyme-linked immunosorbent assay (ELISA) according to the method as described previously [19]. The homologous N. haje venom was used as a positive reference. In short, 10 ng/100 µL per well of venom antigen was pre-coated in a 96 well ELISA plate and incubated overnight at 4 °C. The plate was then flicked dry and washed three times with phosphate-buffered saline with 0.5% Tween®20 (PBST). Various dilutions of antivenom were added to each antigen-coated well and incubated for an hour at room temperature. After washing the plate three times with PBST, 100 µL of appropriately diluted horseradish peroxidase-conjugated antihorse-IgG (Jackson ImmunoResearch Inc., West Grove, PA, USA) in PBST (1:10,000) was added to the well and incubated for another hour at room temperature. The excess unbound antibody-enzyme conjugate was removed by rinsing three times with PBST. A hundred microliters of freshly prepared substrate solution (0.5 mg/mL o-phenylenediamine and 0.003% hydrogen peroxide in 0.1 M citrate-phosphate buffer, pH 5.0) was added to each well. The plate was left in dark for 30 min at room temperature and the reaction was terminated by adding 50 µL of 12.5% sulfuric acid. The venom-antivenom complexes were monitored at 450 nm using an ELISA reader (SUNRISE-TECAN Type Touch Screen F039300, Tecan, Männedorf, Switzerland). All experiments were performed in triplicate. The half-maximal dose (EC50) of the venom-antivenom binding reaction was determined from the OD values through non-linear regression analysis using Prism software version 6.0 (Graphpad Software Inc., San Diego, CA, USA)).
4.9. Determination of Median Lethal Dose (LD50) and Median Effective Dose (ED50)
The LD50 of the N. senegalensis venom was determined in a murine model. Various dilutions of venom in normal saline were injected intravenously into the caudal veins of ICR mice (n = 4 for each dose). The survival ratio was recorded after 24 h. Neutralization of the venom by the homologous antivenom was determined by preincubating a challenge dose (5 × LD50) of the venom with various dilutions of the antivenom at 37 °C for 30 min. The mixture was then injected intravenously into the caudal vein of mice (n = 4 for each dosage). Probit analysis method [82] was used to calculate the median lethal dose (LD50) and median effective dose (ED50) using the BioStat 2009 analysis software (AnalystSoft Inc., Vancouver, Canada). The potency (P) of antivenom neutralization was calculated according to Morais et al. [83]. For comparison, the neutralization potency was further normalized to normalized potency (n-P) as described previously [18,84].
4.10. Data Availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium3 via the iProX partner repository [85] with the dataset identifier PXD014239.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/13/1/60/s1, Table S1: Mass spectrometry analysis of in-solution tryptic digested peptides from RP-HPLC fractions of Naja senegalensis venom.
Author Contributions
Conceptualization, C.H.T., and K.Y.T.; Data curation, C.H.T.; Formal analysis, K.Y.W., and K.Y.T.; Funding acquisition, K.Y.T., and C.H.T.; Investigation, K.Y.W., C.H.T., and K.Y.T.; Methodology, K.Y.W., K.Y.T., N.H.T., and C.H.T.; Project supervision, C.H.T., K.Y.T., and N.H.T.; Resources, C.H.T., K.Y.T., and N.H.T.; Validation, K.Y.T., C.H.T., and K.Y.T.; Writing—original draft, K.Y.W.; Writing—review & editing: C.H.T., K.Y.W., K.Y.T., and N.H.T. All authors have read and agreed to the published version of the manuscript.
Funding
Research funding from the University of Malaya, Malaysia (BKS003-2020).
Institutional Review Board Statement
The protocol for animal experimentation was carried out based on the Council for International Organizations of Medical Sciences (CIOMS) guidelines and was approved by the Institutional Animal Care and Use Committee of the University of Malaya (Ethics clearance number: 2014-09-11/PHAR/R/TCH, approved 09-11-2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef]
- WHO. Venomous Snakes and Antivenoms Search Interface. Available online: http://apps.who.int/bloodproducts/snakeantivenoms/database/ (accessed on 2 April 2020).
- Petras, D.; Sanz, L.; Segura, Á.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.G.; et al. Snake Venomics of African Spitting Cobras: Toxin Composition and Assessment of Congeneric Cross-Reactivity of the Pan-African EchiTAb-Plus-ICP Antivenom by Antivenomics and Neutralization Approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Liu, C.-C.; You, C.-H.; Wang, P.-J.; Yu, J.-S.; Huang, G.-J.; Liu, C.-H.; Hsieh, W.-C.; Lin, C.-C. Analysis of the efficacy of Taiwanese freeze-dried neurotoxic antivenom against Naja kaouthia, Naja siamensis and Ophiophagus hannah through proteomics and animal model approaches. PLoS Negl. Trop. Dis. 2017, 11, e0006138. [Google Scholar] [CrossRef]
- Tan, C.H.; Wong, K.Y.; Chong, H.P.; Tan, N.H.; Tan, K.Y. Proteomic insights into short neurotoxin-driven, highly neurotoxic venom of Philippine cobra (Naja philippinensis) and toxicity correlation of cobra envenomation in Asia. J. Proteom. 2019, 206, 103418. [Google Scholar] [CrossRef]
- Tan, N.H.; Wong, K.Y.; Tan, C.H. Venomics of Naja sputatrix, the Javan spitting cobra: A short neurotoxin-driven venom needing improved antivenom neutralization. J. Proteom. 2017, 157, 18–32. [Google Scholar] [CrossRef]
- Broadley, D.G. The snouted cobra, Naja annulifera, a valid species in southern Africa. J. Herpetol. Assoc. Afr. 1995, 44, 26–32. [Google Scholar] [CrossRef]
- Broadley, D.G.; Wüster, W. A review of the southern African ‘non-spitting’ cobras (Serpentes: Elapidae: Naja). Afr. J. Herpetol. 2004, 53, 101–122. [Google Scholar] [CrossRef]
- Trape, J.-F.; Chirio, L.; Broadley, N.G.; Wüster, W. Phylogeography and systematic revision of the Egyptian cobra (Serpentes: Elapidae: Naja haje) species complex, with a description of a new species from West Africa. Zootaxa 2009, 2236, 1–25. [Google Scholar] [CrossRef]
- Wallach, V.; Wüster, W.; Broadley, D. In Praise of Subgenera: Taxonomic Status of Cobras of the Genus Naja Laurenti (Serpentes: Elapidae). Zootaxa 2009, 2236, 26–36. [Google Scholar] [CrossRef]
- Trape, J.-F.; Mané, Y. The snakes of Mali. Bonn Zool. Bull. 2017, 66, 107–133. [Google Scholar]
- Trape, J.-F.; Mané, Y. The snakes of Niger. Amphib. Reptile Conserv. 2015, 9, 39–55. [Google Scholar]
- WHO. Guidelines for the Prevention and Clinical Management of Snakebite in Africa; World Health Organ: Brazzaville, Congo, 2010. [Google Scholar]
- Tan, C.H.; Wong, K.Y.; Tan, N.H.; Ng, T.S.; Tan, K.Y. Distinctive Distribution of Secretory Phospholipases A2 in the Venoms of Afro-Asian Cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus). Toxins 2019, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Wong, K.Y.; Tan, N.H.; Tan, C.H. Quantitative proteomics of Naja annulifera (sub-Saharan snouted cobra) venom and neutralization activities of two antivenoms in Africa. Int. J. Biol. Macromol. 2020, 158, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Elucidating the biogeographical variation of the venom of Naja naja (spectacled cobra) from Pakistan through a venom-decomplexing proteomic study. J. Proteom. 2018, 175, 156–173. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Suryamohan, K.; Krishnankutty, S.P.; Guillory, J.; Jevit, M.; Schröder, M.S.; Wu, M.; Kuriakose, B.; Mathew, O.K.; Perumal, R.C.; Koludarov, I.; et al. The Indian cobra reference genome and transcriptome enables comprehensive identification of venom toxins. Nat. Genet. 2020, 52, 106–117. [Google Scholar] [CrossRef]
- Chong, H.P.; Tan, K.Y.; Tan, N.H.; Tan, C.H. Exploring the Diversity and Novelty of Toxin Genes in Naja sumatrana, the Equatorial Spitting Cobra from Malaysia through De Novo Venom-Gland Transcriptomics. Toxins 2019, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Chanhome, L.; Tan, N.H. Comparative venom gland transcriptomics of Naja kaouthia (monocled cobra) from Malaysia and Thailand: Elucidating geographical venom variation and insights into sequence novelty. PeerJ 2017, 5, e3142. [Google Scholar] [CrossRef] [PubMed]
- Senji Laxme, R.R.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the ‘big four’: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef] [PubMed]
- Malih, I.; Ahmad Rusmili, M.R.; Tee, T.Y.; Saile, R.; Ghalim, N.; Othman, I. Proteomic analysis of Moroccan cobra Naja haje legionis venom using tandem mass spectrometry. J. Proteom. 2014, 96, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Tan, C.H. Cytotoxicity of Snake Venoms and Toxins: Mechanisms and Applications; Nova Science Publishers: New York, NY, USA, 2016. [Google Scholar]
- Panagides, N.; Jackson, T.; Ikonomopoulou, M.; Arbuckle, K.; Pretzler, R.; Yang, D.; Ali, S.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the Cobra Got Its Flesh-Eating Venom: Cytotoxicity as a Defensive Innovation and Its Co-Evolution with Hooding, Aposematic Marking, and Spitting. Toxins 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Huang, Y.K.; Chen, Y.W.; Chen, M.H.; Tu, A.T.; Chen, Y.C. Venom Ophthalmia and Ocular Complications Caused by Snake Venom. Toxins 2020, 12, 576. [Google Scholar] [CrossRef]
- Sharma, V.K.; Baranwal, V.K. Snake venom ophthalmia. Med. J. Armed Forces India 2015, 71, S197–S198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, K.Y.; Tan, C.H.; Tan, N.H. Venom and Purified Toxins of the Spectacled Cobra (Naja naja) from Pakistan: Insights into Toxicity and Antivenom Neutralization. Am. J. Trop. Med. Hyg. 2016, 94, 1392–1399. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Neutralization of the Principal Toxins from the Venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into Toxin-Specific Neutralization by Two Different Antivenoms. Toxins 2016, 8, 86. [Google Scholar] [CrossRef]
- Chong, H.P.; Tan, K.Y.; Tan, C.H. Cytotoxicity of Snake Venoms and Cytotoxins From Two Southeast Asian Cobras (Naja sumatrana, Naja kaouthia): Exploration of Anticancer Potential, Selectivity, and Cell Death Mechanism. Front. Mol. Biosci. 2020, 7, 7. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wu, W.-G. Amphiphilic β-sheet cobra cardiotoxin targets mitochondria and disrupts its network. FEBS Lett. 2005, 579, 3169–3174. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Chen, Z.; Shrivastava, I.H.; Gasanoff, E.S.; Dagda, R.K. Naja mossambica mossambica Cobra Cardiotoxin Targets Mitochondria to Disrupt Mitochondrial Membrane Structure and Function. Toxins 2019, 11, 152. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.C.; Chiu, T.H.; Chiu, P.J.S.; Tseng, T.C.; Lee, S.Y. Pharmacological properties of cardiotoxin isolated from Formosan cobra venom. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1968, 259, 360–374. [Google Scholar] [CrossRef]
- Kwan, C.; Kwan, T.; Huang, S. Effect of calcium on the vascular contraction induced by cobra venom cardiotoxin. Clin. Exp. Pharmacol. Physiol. 2002, 29, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Alpha neurotoxins. Toxicon 2013, 66, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutierrez, J.M. Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteom. 2017, 150, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Wong, K.Y.; Tan, K.Y.; Tan, N.H. Venom proteome of the yellow-lipped sea krait, Laticauda colubrina from Bali: Insights into subvenomic diversity, venom antigenicity and cross-neutralization by antivenom. J. Proteom. 2017, 166, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, K.Y.; Ng, T.S.; Sim, S.M.; Tan, N.H. Venom Proteome of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia: Toxicity Correlation, Immunoprofiling and Cross-Neutralization by Sea Snake Antivenom. Toxins 2019, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.; Awal, W.; Niranjan, N.R. Snake α-Neurotoxins and the Nicotinic Acetylcholine Receptor. In Snake Venoms; Gopalakrishnakone, P., Inagaki, H., Mukherjee, A.K., Rahmy, T.R., Vogel, C.-W., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 215–252. [Google Scholar]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Yap, M.K.; Fung, S.Y.; Tan, K.Y.; Tan, N.H. Proteomic characterization of venom of the medically important Southeast Asian Naja sumatrana (Equatorial spitting cobra). Acta Trop. 2014, 133, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.H.H.B.; McCleary, R.J.R.; Salafutdinov, I.; Alam, F.; Shah, H.S.; Bibi, S.; Ali, A.; Khalid, S.; Hasan, S.M.F.; Sabatier, J.-M.; et al. Proteomics study of Southern Punjab Pakistani cobra (Naja naja: Formerly Naja naja karachiensis) venom. Toxicol. Environ. Chem. 2019, 101, 91–116. [Google Scholar] [CrossRef]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Silva, A.; Cristofori-Armstrong, B.; Rash, L.D.; Hodgson, W.C.; Isbister, G.K. Defining the role of post-synaptic alpha-neurotoxins in paralysis due to snake envenoming in humans. Cell Mol. Life Sci. 2018, 75, 4465–4478. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Tan, N.H. Geographical venom variations of the Southeast Asian monocled cobra (Naja kaouthia): Venom-induced neuromuscular depression and antivenom neutralization. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2016, 185-186, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Huynh, T.M.; Isbister, G.K.; Hodgson, W.C. Isolation and pharmacological characterization of α-Elapitoxin-Na1a, a novel short-chain postsynaptic neurotoxin from the venom of the Chinese Cobra (Naja atra). Biochem. Pharmacol. 2020, 181, 114059. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N.; Kukhtina, V.V.; Kryukova, E.V.; Chiodini, F.; Bertrand, D.; Methfessel, C.; Tsetlin, V.I. “Weak toxin” from Naja kaouthia is a nontoxic antagonist of alpha 7 and muscle-type nicotinic acetylcholine receptors. J. Biol. Chem. 2001, 276, 15810–15815. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, F.H.H. Snake venom toxins the primary structure of protein S4C11: A neurotoxin homologue from the venom of forest cobra (Naja melanoleuca). Biochim. Biophys. Acta Protein Struct. 1975, 400, 310–321. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef]
- Heyborne, W.H.; Mackessy, S.P. Cysteine-rich secretory proteins in reptile venoms. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 325–336. [Google Scholar]
- Tadokoro, T.; Modahl, C.M.; Maenaka, K.; Aoki-Shioi, N. Cysteine-Rich Secretory Proteins (CRISPs) From Venomous Snakes: An Overview of the Functional Diversity in A Large and Underappreciated Superfamily. Toxins 2020, 12, 175. [Google Scholar] [CrossRef]
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic snake venoms: Their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef]
- Rowan, E.G. What does β-bungarotoxin do at the neuromuscular junction? Toxicon 2001, 39, 107–118. [Google Scholar] [CrossRef]
- Chu, C.C.; Chu, S.T.; Chen, S.W.; Chen, Y.H. The non-phospholipase A2 subunit of beta-bungarotoxin plays an important role in the phospholipase A2-independent neurotoxic effect: Characterization of three isotoxins with a common phospholipase A2 subunit. Biochem. J. 1994, 303 Pt 1, 171–176. [Google Scholar] [CrossRef]
- Owen, D.G.; Hall, A.; Stephens, G.; Stow, J.; Robertson, B. The relative potencies of dendrotoxins as blockers of the cloned voltage-gated K+ channel, mKv1.1 (MK-1), when stably expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997, 120, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Dhananjaya, B.L.; D’Souza, C.J. The pharmacological role of nucleotidases in snake venoms. Cell Biochem. Funct. 2010, 28, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.W.; Fritzinger, D.C. Cobra venom factor: Structure, function, and humanization for therapeutic complement depletion. Toxicon 2010, 56, 1198–1222. [Google Scholar] [CrossRef]
- Tan, C.; Tan, K.Y.; Tan, N. A Protein Decomplexation Strategy in Snake Venom Proteomics: Methods and Protocols; Humana Press: New York, NY, USA, 2019; Volume 1871, pp. 83–92. [Google Scholar]
- Lynch, V.J. Inventing an arsenal: Adaptive evolution and neofunctionalization of snake venom phospholipase A(2) genes. BMC Evol. Biol. 2007, 7, 2. [Google Scholar] [CrossRef]
- Fry, B.G.; Wuster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef]
- Malhotra, A. Mutation, Duplication, and More in the Evolution of Venomous Animals and Their Toxins. In Evolution of Venomous Animals and Their Toxins; Malhotra, A., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 33–45. [Google Scholar] [CrossRef]
- Benjamin, J.M.; Abo, B.N.; Brandehoff, N. Review Article: Snake Envenomation in Africa. Curr. Trop. Med. Rep. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Ainsworth, S.; Menzies, S.K.; Casewell, N.R.; Harrison, R.A. An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: Inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management. PLoS Negl. Trop. Dis. 2020, 14, e0008579. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Solano, G.; Pla, D.; Herrera, M.; Segura, A.; Vargas, M.; Villalta, M.; Sanchez, A.; Sanz, L.; Lomonte, B.; et al. Preclinical Evaluation of the Efficacy of Antivenoms for Snakebite Envenoming: State-of-the-Art and Challenges Ahead. Toxins 2017, 9, 163. [Google Scholar] [CrossRef]
- Warrell, D.A.; Gutierrez, J.M.; Calvete, J.J.; Williams, D. New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J. Med. Res. 2013, 138, 38–59. [Google Scholar] [PubMed]
- Ramos-Cerrillo, B.; de Roodt, A.R.; Chippaux, J.P.; Olguín, L.; Casasola, A.; Guzmán, G.; Paniagua-Solís, J.; Alagón, A.; Stock, R.P. Characterization of a new polyvalent antivenom (Antivipmyn Africa) against African vipers and elapids. Toxicon 2008, 52, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, G.; Casewell, N.R.; Pla, D.; Quesada-Bernat, S.; Logan, R.A.E.; Bolton, F.M.S.; Wagstaff, S.C.; Gutiérrez, J.M.; Calvete, J.J.; Harrison, R.A. Defining the pathogenic threat of envenoming by South African shield-nosed and coral snakes (genus Aspidelaps), and revealing the likely efficacy of available antivenom. J. Proteom. 2019, 198, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Oluoch, G.O.; Ainsworth, S.; Alsolaiss, J.; Bolton, F.; Arias, A.-S.; Gutiérrez, J.-M.; Rowley, P.; Kalya, S.; Ozwara, H.; et al. Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Negl. Trop. Dis. 2017, 11, e0005969. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Segura, A.; Vargas, M.; Herrera, M.; Villalta, M.; Estrada, R.; Wu, F.; Litschka-Koen, T.; Perry, M.A.; Alape-Giron, A.; et al. Expanding the neutralization scope of the EchiTAb-plus-ICP antivenom to include venoms of elapids from Southern Africa. Toxicon 2017, 125, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Laustsen, A.H. Recent Advances in Next Generation Snakebite Antivenoms. Trop. Med. Infect. Dis. 2018, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, G.; Olvera, F.; Archundia, I.G.; Lomonte, B.; Alagón, A.; Corzo, G. Horse immunization with short-chain consensus α-neurotoxin generates antibodies against broad spectrum of elapid venomous species. Nat. Commun. 2019, 10, 3642. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K.; Tan, K.Y.; Eursakun, S.; Tan, C.H.; Simsiriwong, P.; Pamornsakda, T.; Wiriyarat, W.; Klinpayom, C.; Tan, N.H. A Simple and Novel Strategy for the Production of a Pan-specific Antiserum against Elapid Snakes of Asia. PLoS Negl. Trop. Dis. 2016, 10, e0004565. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K.; Tan, K.Y.; Pruksaphon, K.; Klinpayom, C.; Gutiérrez, J.M.; Quraishi, N.H.; Tan, C.H. A pan-specific antiserum produced by a novel immunization strategy shows a high spectrum of neutralization against neurotoxic snake venoms. Sci. Rep. 2020, 10, 11261. [Google Scholar] [CrossRef]
- Tan, C.H.; Palasuberniam, P.; Blanco, F.B.; Tan, K.Y. Immunoreactivity and neutralization capacity of Philippine cobra antivenom against Naja philippinensis and Naja samarensis venoms. Trans. R. Soc. Trop. Med. Hyg. 2020, 115, 78–84. [Google Scholar] [CrossRef]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lewin, M.R.; Williams, D.J.; Lomonte, B. Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms. Toxins 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Dayananda, K.S.; Sharath, B.K.; Gopinath, S.M. Purification of non toxic acidic phospholipase a2 from indian cobra (Naja naja) venom. Int. J. Pharma Bio Sci. 2013, 4, 408–415. [Google Scholar]
- Howard-Jones, N. A CIOMS ethical code for animal experimentation. WHO Chron. 1985, 39, 51–56. [Google Scholar] [PubMed]
- Finney, D.J. Probit Analysis, 2nd ed.; Cambridge University Press: New York, NY, USA, 1952; Volume 41, p. 627. [Google Scholar] [CrossRef]
- Morais, V.; Ifran, S.; Berasain, P.; Massaldi, H. Antivenoms: Potency or median effective dose, which to use? J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 191–193. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, N.H.; Tan, C.H. Venom proteomics and antivenom neutralization for the Chinese eastern Russell’s viper, Daboia siamensis from Guangxi and Taiwan. Sci. Rep. 2018, 8, 8545. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).