Protein-Bound Uremic Toxins Lowering Effect of Sevelamer in Pre-Dialysis Chronic Kidney Disease Patients with Hyperphosphatemia: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Results
2.1. Patients Characteristics
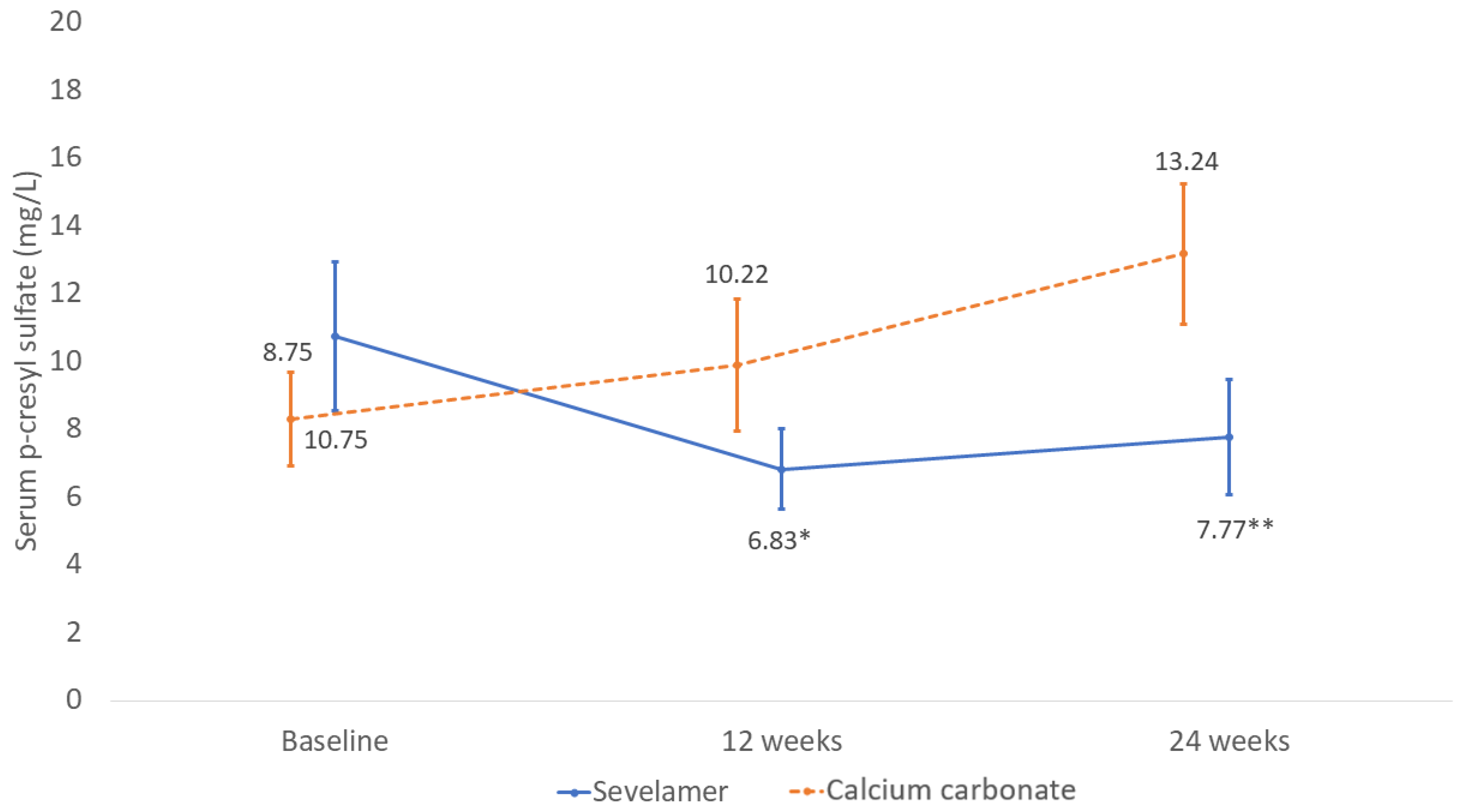
2.2. Changes in Serum P-cresyl Sulfate Concentration
2.3. Changes in Serum Indoxyl Sulfate Concentration
2.4. Changes in Serum Calcium Phosphate, PTH, and FGF 23
2.5. Effects on Renal Function and Proteinuria
2.6. Effects on Low-Density Lipoprotein (LDL) Cholesterol
2.7. Effects on Inflammatory Markers (hs-CRP Levels)
2.8. Effects on Vascular Stiffness and Peripheral Arterial Disease
2.9. Follow-Up Data
2.10. Adverse Events
3. Discussion
4. Conclusions
5. Methods
5.1. Study Population
5.2. Study Design and Procedures
5.3. Data Collection
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic Kidney Disease and Mortality Risk: A Systematic Review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [Green Version]
- Major, R.W.; Cheng, M.R.I.; Grant, R.A.; Shantikumar, S.; Xu, G.; Oozeerally, I.; Brunskill, N.J.; Gray, L.J. Cardiovascular disease risk factors in chronic kidney disease: A systematic review and meta-analysi. PLoS ONE 2018, 13, e0192895. [Google Scholar] [CrossRef]
- Silver, J.; Rodriguez, M.; Slatopolsky, E. FGF23 and pth-double agents at the heart of CKD. Nephrol. Dial. Transplant. 2012, 27, 1715–1720. [Google Scholar] [CrossRef]
- Hsu, H.J.; Wu, M.S. Fibroblast growth factor 23: A possible cause of left ventricular hypertrophy in hemodialysis patients. Am. J. Med. Sci. 2009, 337, 116–122. [Google Scholar] [CrossRef]
- Mirza, M.A.; Larsson, A.; Lind, L.; Larsson, T.E. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 2009, 205, 385–390. [Google Scholar] [CrossRef]
- Mendoza, J.M.; Isakova, T.; Cai, X.; Bayes, L.Y.; Faul, C.; Scialla, J.; Lash, J.P.; Chen, J.; He, J.; Navaneethan, S.; et al. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. 2017, 91, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Lin, C.J.; Wu, V.; Wu, P.C.; Wu, C.J. Meta-analysis of the associations of p-cresyl sulfate (pCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef] [PubMed]
- Takkavatakarn, K.; Wuttiputinun, T.; Phannajit, J.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Protein-bound uremic toxin lowering strategies in chronic kidney disease: A systematic review and meta-analysis. J. Nephrol. 2021. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Thermote, F.; Lameire, N.; Vanholder, R. Sevelamer hydrochloride (renagel®) adsorbs the uremic compound indoxyl sulfate. J. Am. Soc. Nephrol 2004, 15, 505. [Google Scholar]
- Lin, C.-J.; Pan, C.-F.; Chuang, C.-K.; Liu, H.-L.; Huang, S.-F.; Chen, H.-H.; Wu, C.-J. Effects of Sevelamer Hydrochloride on Uremic Toxins Serum Indoxyl Sulfate and P-Cresyl Sulfate in Hemodialysis Patients. J. Clin. Med. Res. 2017, 9, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Riccio, E.; Sabbatini, M.; Bruzzese, D.; Grumetto, L.; Marchetiello, C.; Amicone, M.; Andreucci, M.; Guida, B.; Passaretti, D.; Russo, G.; et al. Plasma p-cresol lowering effect of sevelamer in non-dialysis CKD patients: Evidence from a randomized controlled trial. Clin. Exp. Nephrol. 2018, 22, 529–538. [Google Scholar] [CrossRef]
- Bennis, Y.; Cluet, Y.; Titeca-Beauport, D.; El Esper, N.; Ureña, P.; Bodeau, S.; Combe, C.; Dussol, B.; Fouque, D.; Choukroun, G.; et al. The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Toxins 2019, 11, 279. [Google Scholar] [CrossRef] [Green Version]
- Rukavina Mikusic, N.L.; Kouyoumdzian, N.M.; Choi, M.R. Gut microbiota and chronic kidney disease: Evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflug. Arch. 2020, 472, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Suki, W.N.; Moore, L.W. Phosphorus Regulation in Chronic Kidney Disease. Methodist DeBakey Cardiovasc. J. 2016, 12, 6–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, V.M.; Schlieper, G.; Heussen, N.; Holzmann, S.; Busch, B.; Evenepoel, P.; Vanholder, R.; Meijers, B.; Meert, N.; Fassbender, W.J.; et al. Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: A prospective study. Nephrol. Dial. Transplant. 2010, 25, 2672–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervloet, M. Renal and extrarenal effects of fibroblast growth factor 23. Nat. Rev. Nephrol. 2019, 15, 109–120. [Google Scholar] [CrossRef]
- Haring, R.; Enserro, D.; Xanthakis, V.; Mitchell, G.F.; Benjamin, E.J.; Hamburg, N.M.; Sullivan, L.; Nauck, M.; Wallaschofski, H.; Vasan, R.S. Plasma Fibroblast Growth Factor 23: Clinical Correlates and Association with Cardiovascular Disease and Mortality in the Framingham Heart Study. J. Am. Heart Assoc. 2016, 5, e003486. [Google Scholar] [CrossRef] [Green Version]
- Takkavatakarn, K.; Wuttiputhanun, T.; Phannajit, J.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Effectiveness of fibroblast growth factor 23 lowering modalities in chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2021. [Google Scholar] [CrossRef]
- Sun, P.P.; Perianayagam, M.C.; Jaber, B.L. Endotoxin-binding affinity of sevelamer: A potential novel anti-inflammatory mecha-nism. Kidney Int. Suppl. 2009, 114, S20–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, C.W.; Harrison, L.E.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.C.; John, S.G.; Sigrist, M.K.; Burton, J.; Hothi, D.; Korsheed, S.; et al. Circulating Endotoxemia: A Novel Factor in Systemic Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 6, 133–141. [Google Scholar] [CrossRef]
- Perianayagam, M.C.; Jaber, B.L. Endotoxin-Binding Affinity of Sevelamer Hydrochloride. Am. J. Nephrol. 2008, 28, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Stinghen, A.; Gonçalves, S.; Bucharles, S.; Branco, F.; Gruber, B.; Hauser, A.; Pecoits-Filho, R. Sevelamer Decreases Systemic Inflammation in Parallel to a Reduction in Endotoxemia. Blood Purif. 2010, 29, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef] [Green Version]
- Gross, P.; Massy, Z.A.; Henaut, L.; Boudot, C.; Cagnard, J.; March, C.; Kamel, S.; Drueke, T.B.; Six, I. Para-cresyl sulfate acutely impairs vascular reactivity and induces vascular remodeling. J. Cell. Physiol. 2015, 230, 2927–2935. [Google Scholar] [CrossRef]
- Han, H.; Chen, Y.; Zhu, Z.; Su, X.; Ni, J.; Du, R.; Zhang, R.; Jin, W. p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front. Med. 2016, 10, 320–329. [Google Scholar] [CrossRef]
- Meijers, B.K.I.; Van Kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The Uremic Retention Solute p-Cresyl Sulfate and Markers of Endothelial Damage. Am. J. Kidney Dis. 2009, 54, 891–901. [Google Scholar] [CrossRef]
- Raggi, P.; Bommer, J.; Chertow, G.M. Valvular calcification in hemodialysis patients randomized to calcium-based phosphorus binders or sevelamer. J. Heart Valve Dis. 2004, 13, 134–141. [Google Scholar] [PubMed]
- Chertow, G.M.; Raggi, P.; McCarthy, J.T.; Schulman, G.; Silberzweig, J.; Kuhlik, A.; Goodman, W.G.; Boulay, A.; Burke, S.K.; Toto, R.D. The effects of sevelamer and calcium acetate on proxies of atherosclerotic and arteriosclerotic vascular disease in hemodialysis patients. Am. J. Nephrol. 2003, 23, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and Interpretation of the Ankle-Brachial Index: A Scientific Statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamamoto, T.; Tsuda, S.; Maruyama, M.; Shirai, K. The Background of Calculating CAVI: Lesson from the Discrepancy Between CAVI and CAVI0. Vasc. Health Risk Manag. 2020, 16, 193–201. [Google Scholar] [CrossRef]


| Parameters | Sevelamer (n = 20) | Calcium Carbonate (n = 20) | p-Value |
|---|---|---|---|
| Age (year) | 59.8 ± 2.95 | 52.05 ± 3.49 | 0.07 |
| BMI (kg/m2) | 26.08 ± 1.17 | 26.94 ± 1.33 | 0.92 |
| Cause of CKD (%) | 0.40 | ||
| Diabetic nephropathy Hypertensive nephropathy | 12 (60%) 1 (5%) | 8 (40%) 4 (20%) | |
| Chronic glomerulonephritis ADPKD | 1 (5%) 1 (5%) | 2 (10%) 1 (5%) | |
| Unknown | 5 (25%) | 5 (25%) | |
| BUN (8–20 mg/dL) | 69.1 ± 3.66 | 68.43 ± 4.60 | 0.91 |
| Creatinine (0.6–1.2 mg/dL) | 6.51 ± 0.69 | 6.03 ± 0.62 | 0.68 |
| eGFR (mL/min/1.73 m2) | 9.79 ± 1.50 | 11.08 ± 2.09 | 0.61 |
| CKD stage | 0.34 | ||
| 4 (eGFR 15–30 mL/min/1.73 m2) | 3 (15%) | 1 (5%) | |
| 5 (eGFR < 15 mL/min/1.73 m2) | 17 (85%) | 19 (95%) | |
| Proteinuria (0–0.15 g/day) | 2.12 ± 0.06 | 2.45 ±0.05 | 0.58 |
| Urine sodium (mEq/day) | 136.13 ± 4.42 | 167.02 ± 8.01 | 0.48 |
| Dietary protein intake (g/kg/day) | 0.72 ± 0.06 | 0.70 ± 0.05 | 0.90 |
| Calcium (8.6–10.2 mg/dL) | 8.96 ± 0.18 | 8.88 ± 0.20 | 0.83 |
| Phosphate (3–4.5 mg/dL) | 5.50 ± 0.17 | 5.39 ± 0.10 | 0.62 |
| iPTH (15–65 pg/mL) | 336.5 ± 42.22 | 303.78 ± 58.27 | 0.64 |
| FGF23 (pg/mL) | 47.19 (91.08) | 61.50 (83.73) | 0.64 |
| 25 OH vitamin D (20–40 ng/mL) | 25.85 ± 2.61 | 26.68 ± 4.49 | 0.87 |
| LDL cholesterol (mg/dL) | 112.89 ± 13.18 | 89 ± 7.95 | 0.15 |
| HDL cholesterol (mg/dL) | 40.97 ± 3.24 | 42.68 ± 3.39 | 0.72 |
| Albumin (3.4–5.4 g/dL) | 3.94 ± 0.09 | 3.87 ± 0.08 | 0.67 |
| Hemoglobin (12–15.5 g/dL) | 9.76 ± 0.24 | 9.44 ± 0.27 | 0.38 |
| Hs-CRP (0–3 mg/L) | 0.81 (0.96) | 0.8 (2.75) | 0.59 |
| ABI (1–1.4) | 1.1 ± 0.02 | 1.09 ± 0.03 | 0.52 |
| CAVI (<8) | 8.36 ± 0.27 | 7.47 ±0.37 | 0.08 |
| LVEF (≥50%) | 62.48 ± 3.84 | 65.52 ± 2.35 | 0.54 |
| LVMI (≤115 g/m2 for male and ≤95 g/m2 for female) | 123.19 ± 9.94 | 124.24 ± 10.18 | 0.94 |
| LVH (%) | 17 (85%) | 16 (80%) | 0.81 |
| Serum p-cresyl sulfate (mg/L) | 10.75 ± 2.20 | 8.75 ± 1.26 | 0.45 |
| Serum indoxyl sulfate (mg/L) | 17.97 ± 2.55 | 16.16 ± 2.49 | 0.62 |
| Sevelamer | Calcium Carbonate | p-Value (between Groups) | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Baseline | 12 weeks | 24 weeks | Baseline | 12 weeks | 24 weeks | |
| CKD-MBD parameters | |||||||
| Calcium (mg/dL) | 8.96 ± 0.18 | 8.89 ± 0.17 | 8.65 ± 0.28 | 8.88 ± 0.20 | 9 ± 0.27 | 8.85 ± 0.40 | 0.42 |
| Phosphate (mg/dL) | 5.50 ± 0.17 | 4.85 ± 0.22 | 5.4 ± 0.44 | 5.39 ± 0.10 | 5.14 ± 0.23 | 6.05 ± 0.70 | 0.36 |
| iPTH (pg/mL) | 336.5 ± 42.22 | 319.85 ± 45.83 | 357.3 ± 72.99 | 303.78 ± 58.27 | 317.72 ± 60.27 | 339.33 ± 79.02 | 0.75 |
| FGF23 (pg/mL) | 47.19 (91.08) | 53.18 (230.28) | 57.20 (122.27) | 61.50 (83.73) | 180.63 (212.11 *) | 106.07 (208.4 *) | 0.01 |
| Lipid profiles | |||||||
| LDL cholesterol (mg/dL) | 112.89 ± 13.18 | 72.55 ± 7.28 * | 72.37 ± 12.74 * | 89 ± 7.95 | 83.44 ± 4.33 | 77.91 ± 7.81 | 0.04 |
| HDL cholesterol (mg/dL) | 40.97 ± 3.24 | 42.58 ± 3.70 | 45.21 ± 4.48 | 42.68 ± 3.39 | 44.67 ± 3.17 | 41.1 ± 4.05 | 0.58 |
| Nutrition parameters | |||||||
| Albumin (g/dL) | 3.94 ± 0.44 | 4.68 ± 0.81 | 3.85 ± 0.15 | 3.87 ± 0.35 | 3.98 ± 0.09 | 3.89 ± 0.12 | 0.91 |
| Dietary protein intake (g/kg/day) | 0.72 ± 0.06 | 0.68 ± 0.07 | 0.71 ±0.04 | 0.70 ± 0.05 | 0.72 ±0.04 | 0.68±0.04 | 0.39 |
| Renal parameters | |||||||
| BUN (mg/dL) | 69.1 ± 3.66 | 73 ± 5.02 | 75.93 ± 6.64 | 68.43 ± 4.60 | 82.55 ± 6.22 | 91.41 ± 6.84 | 0.21 |
| Creatinine (mg/dL) | 6.51 ± 0.69 | 6.95 ± 3.22 | 8.89 ± 1.65 | 6.03 ± 0.62 | 7.89 ± 4.13 | 10.52 ± 1.90 | 0.26 |
| eGFR (mL/min/1.73 m2) | 9.79 ± 1.50 | 8.55 ± 1.12 * | 7.73 ± 1.04 * | 11.08 ± 2.09 | 9.58 ± 2.42 * | 6.25 ± 0.88 * | 0.99 |
| Proteinuria (g/day) | 2.12 ± 0.06 | 2.34 ± 0.62 | 2.56 ± 1.39 | 2.45 ±0.05 | 2.61 ± 1.02 | 2.68 ± 1.63 | 0.78 |
| Anemia and inflammatory markers | |||||||
| Hemoglobin (g/dL) | 9.76 ± 0.24 | 9.55 ± 0.27 | 9.28 ± 0.22 | 9.44 ± 0.27 | 9.34 ± 0.31 | 8.84 ± 0.52 | 0.56 |
| Hs-CRP (mg/L) | 0.81 (0.96) | 0.98 (2.37) | 1.03 (5.95) | 0.8 (2.75) | 0.96 (1.89) | 2.23 (3.55) | 0.64 |
| Vascular stiffness parameters | |||||||
| ABI (1–1.4) | 1.1 ± 0.02 | N/A | 1.1 ± 0.02 | 1.09 ± 0.03 | N/A | 1.1 ± 0.03 | 0.57 |
| CAVI (<8) | 8.36 ± 0.27 | N/A | 8.25 ± 0.31 | 7.47 ± 0.37 | N/A | 7.54 ± 0.37 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takkavatakarn, K.; Puapatanakul, P.; Phannajit, J.; Sukkumme, W.; Chariyavilaskul, P.; Sitticharoenchai, P.; Leelahavanichkul, A.; Katavetin, P.; Praditpornsilpa, K.; Eiam-Ong, S.; et al. Protein-Bound Uremic Toxins Lowering Effect of Sevelamer in Pre-Dialysis Chronic Kidney Disease Patients with Hyperphosphatemia: A Randomized Controlled Trial. Toxins 2021, 13, 688. https://doi.org/10.3390/toxins13100688
Takkavatakarn K, Puapatanakul P, Phannajit J, Sukkumme W, Chariyavilaskul P, Sitticharoenchai P, Leelahavanichkul A, Katavetin P, Praditpornsilpa K, Eiam-Ong S, et al. Protein-Bound Uremic Toxins Lowering Effect of Sevelamer in Pre-Dialysis Chronic Kidney Disease Patients with Hyperphosphatemia: A Randomized Controlled Trial. Toxins. 2021; 13(10):688. https://doi.org/10.3390/toxins13100688
Chicago/Turabian StyleTakkavatakarn, Kullaya, Pongpratch Puapatanakul, Jeerath Phannajit, Warumphon Sukkumme, Pajaree Chariyavilaskul, Patita Sitticharoenchai, Asada Leelahavanichkul, Pisut Katavetin, Kearkiat Praditpornsilpa, Somchai Eiam-Ong, and et al. 2021. "Protein-Bound Uremic Toxins Lowering Effect of Sevelamer in Pre-Dialysis Chronic Kidney Disease Patients with Hyperphosphatemia: A Randomized Controlled Trial" Toxins 13, no. 10: 688. https://doi.org/10.3390/toxins13100688
APA StyleTakkavatakarn, K., Puapatanakul, P., Phannajit, J., Sukkumme, W., Chariyavilaskul, P., Sitticharoenchai, P., Leelahavanichkul, A., Katavetin, P., Praditpornsilpa, K., Eiam-Ong, S., & Susantitaphong, P. (2021). Protein-Bound Uremic Toxins Lowering Effect of Sevelamer in Pre-Dialysis Chronic Kidney Disease Patients with Hyperphosphatemia: A Randomized Controlled Trial. Toxins, 13(10), 688. https://doi.org/10.3390/toxins13100688






