Microbiological and Toxicological Hazards in Sewage Treatment Plant Bioaerosol and Dust
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microclimatic Conditions
2.2. Dust Concentrations at Workstations
2.3. Microbial Contamination
2.4. Diversity of Microorganisms from the Sewage Plant Workstations
2.5. Endotoxin Concentrations
2.6. Secondary Metabolites
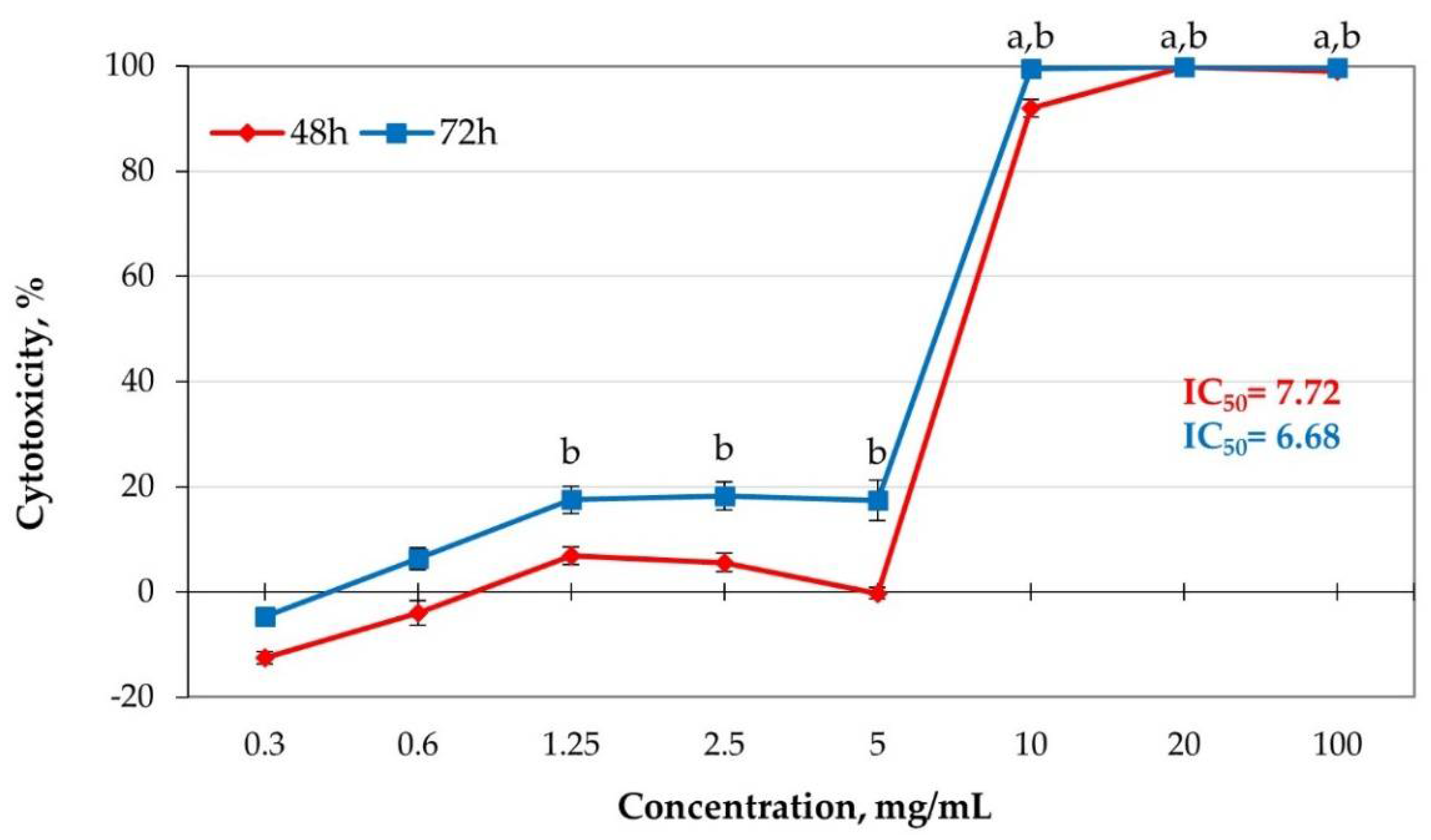
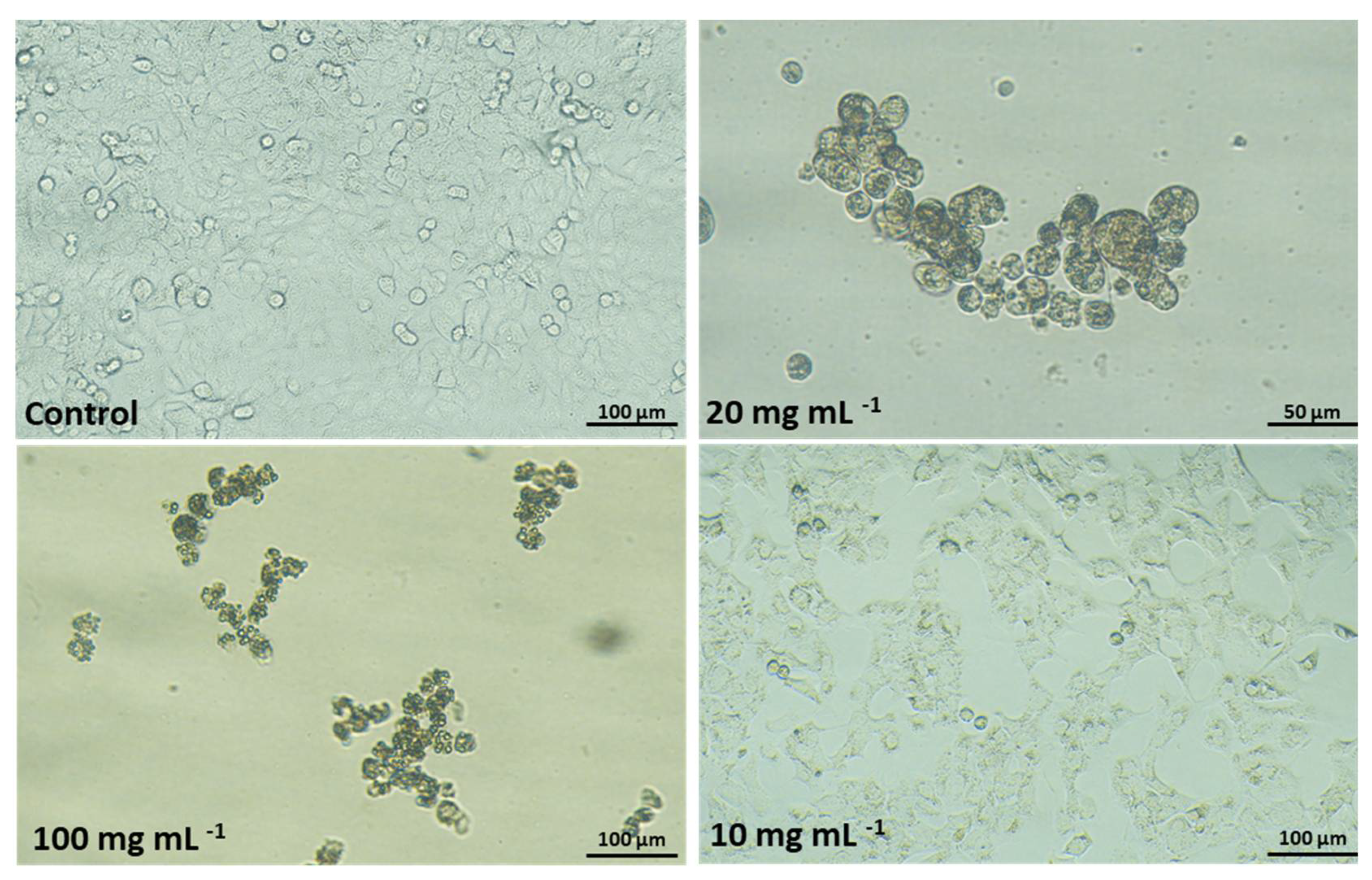
2.7. Cytotoxic Effects of Dust Samples
2.8. Targeted and Untargeted UPLC-HRMS Analysis
3. Conclusions
4. Materials and Methods
4.1. Workstations in the Tested Sewage Treatment Plant
4.2. Airborne Dust Concentration Measurement
4.3. Microbial Contamination Analysis
4.4. Assessment of Microbial Diversity by High-Throughput Sequencing
4.5. Analysis of Endotoxin in Dust Samples
4.6. Secondary Metabolites
4.7. Cell Culture and Cytotoxicity Testing
4.8. UHPLC-Q-ToF-UHRMS Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Ning, D.; Zhang, B.; Li, Y.; Zhang, P.; Shan, X.; Zhang, Q.; Brown, M.R.; Li, Z.; Van Nostrand, J.D.; et al. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat. Microbiol. 2019, 4, 1183–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Li, L.; Wang, Y.; Ma, J.; Li, P.; Han, C.; Liu, J. Composition, dispersion, and health risks of bioaerosols in wastewater treatment plants: A review. Front. Environ. Sci. Eng. 2020, 15, 38. [Google Scholar] [CrossRef]
- Central Statistical Office (GUS) Environmental Protection. 2019. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2019,1,20.html (accessed on 24 August 2021).
- European Environment Agency Urban Waste Water Directive Treatment Plants Data Viewer. Available online: https://www.eea.europa.eu/themes/water/european-waters/water-use-and-environmental-pressures/uwwtd/data-viewer-urban-wastewater-treatment-directive-1/urban-waste-water-directive-treatment (accessed on 24 August 2021).
- Michałkiewicz, M.; Kruszelnicka, I.; Widomska, M. The Variability of the Concentration of Bioaerosols Above the Chambers of Biological Wastewater Treatment. Ecol. Chem. Eng. 2018, 25, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Jiang, L.; Han, Y.; Liu, J.; Wang, X.; Yan, X.; Liu, J. Linking aerosol characteristics of size distributions, core potential pathogens and toxic metal(loid)s to wastewater treatment process. Environ. Pollut. 2020, 264, 114741. [Google Scholar] [CrossRef] [PubMed]
- Wlazło, A.; Pastuszka, J.S.; Łudzeń-Izbińska, B. Assessment of workers’ exposure to airborne bacteria at a small wastewater treatment plant. Med. Pr. 2002, 53, 109–114. [Google Scholar] [PubMed]
- Gotkowska-Płachta, A.; Filipkowska, Z.; Korzeniewska, E.; Janczukowicz, W.; Dixon, B.; Gołaś, I.; Szwalgin, D. Airborne Microorganisms Emitted from Wastewater Treatment Plant Treating Domestic Wastewater and Meat Processing Industry Wastes. CLEAN Soil Air Water 2013, 41, 429–436. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Zhang, X.; Xu, C.; Dong, L.; Yao, M. Bioaerosol emissions and detection of airborne antibiotic resistance genes from a wastewater treatment plant. Atmos. Environ. 2016, 124, 404–412. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Li, L.; Xu, G.; Liu, J.; Yang, K. Bacterial population and chemicals in bioaerosols from indoor environment: Sludge dewatering houses in nine municipal wastewater treatment plants. Sci. Total Environ. 2018, 618, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, K.; Yang, T.; Zhang, M.; Li, L. Bioaerosols emission and exposure risk of a wastewater treatment plant with A2O treatment process. Ecotoxicol. Environ. Saf. 2019, 169, 161–168. [Google Scholar] [CrossRef]
- Gaviria-Figueroa, A.; Preisner, E.C.; Hoque, S.; Feigley, C.E.; Norman, R.S. Emission and dispersal of antibiotic resistance genes through bioaerosols generated during the treatment of municipal sewage. Sci. Total Environ. 2019, 686, 402–412. [Google Scholar] [CrossRef]
- Dehghani, M.; Sorooshian, A.; Ghorbani, M.; Fazlzadeh, M.; Miri, M.; Badiee, P.; Parvizi, A.; Ansari, M.; Baghani, A.N.; Delikhoon, M. Seasonal Variation in Culturable Bioaerosols in a Wastewater Treatment Plant. Aerosol Air Qual. Res. 2018, 18, 2826–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, L.A.M.; Heederik, D.; Doekes, G.; Blom, C.; van Zweden, I.; Wouters, I.M. Exposure-response analysis of allergy and respiratory symptoms in endotoxin-exposed adults. Eur. Respir. J. 2008, 31, 1241–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewski, J.A.; Cyprowski, M.; Szymczak, W.; Gruchała, J. Health complaints from Workplace exposure to bioaerosols: A quwstionnaire stydy in sewage workers. Ann. Agric. Environ. Med. 2004, 11, 199–204. [Google Scholar]
- Thorn, J.; Kerekes, E. Health effects among employees in sewage treatment plants: A literature survey. Am. J. Ind. Med. 2001, 40, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewska, E. Emission of bacteria and fungi in the air from wastewater treatment plants—A review. Front. Biosci. 2011, S3, 159. [Google Scholar] [CrossRef] [Green Version]
- Szyłak-Szydłowski, M.; Kulig, A.; Miaśkiewicz-Pęska, E. Seasonal changes in the concentrations of airborne bacteria emitted from a large wastewater treatment plant. Int. Biodeterior. Biodegrad. 2016, 115, 11–16. [Google Scholar] [CrossRef]
- Skóra, J.; Matusiak, K.; Wojewódzki, P.; Nowak, A.; Sulyok, M.; Ligocka, A.; Okrasa, M.; Hermann, J.; Gutarowska, B. Evaluation of microbiological and chemical contaminants in poultry farms. Int. J. Environ. Res. Public Health 2016, 13, 192. [Google Scholar] [CrossRef]
- Gutarowska, B.; Szulc, J.; Nowak, A.; Otlewska, A.; Okrasa, M.; Jachowicz, A.; Majchrzycka, K. Dust at various workplaces-microbiological and toxicological threats. Int. J. Environ. Res. Public Health 2018, 15, 877. [Google Scholar] [CrossRef] [Green Version]
- Korzeniewska, E.; Filipkowska, Z.; Gotkowska-Płachta, A.; Janczukowicz, W.; Dixon, B.; Czułowska, M. Determination of emitted airborne microorganisms from a BIO-PAK wastewater treatment plant. Water Res. 2009, 43, 2841–2851. [Google Scholar] [CrossRef]
- Korzeniewska, E.; Filipkowska, Z.; Gotkowska-Płachta, A.; Janczukowicz, W.; Rutkowski, B. Bacteriological pollution of the atmospheric air at the municipal and dairy wastewater treatment plant area and in its surroundings. Arch. Environ. Prot. 2008, 34, 13–23. [Google Scholar]
- Filipkowska, Z.; Gotkowska-Płachta, A.; Korzeniewska, E. Grzyby pleśniowe oraz drożdże i grzyby drożdżoidalne w powietrzu atmosferycznym na terenie oczyszczalni ścieków z systemem stawów napowietrzanych i stabilizacyjnych. Woda-Sr.-Obsz. Wiej. 2008, 8, 69–82. [Google Scholar]
- Nierychlo, M.; McIlroy, S.J.; Kucheryavskiy, S.; Jiang, C.; Ziegler, A.S.; Kondrotaite, Z.; Stokholm-Bjerregaard, M.; Nielsen, P.H. Candidatus Amarolinea and Candidatus Microthrix Are Mainly Responsible for Filamentous Bulking in Danish Municipal Wastewater Treatment Plants. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Jon McIlroy, S.; Kristiansen, R.; Albertsen, M.; Michael Karst, S.; Rossetti, S.; Lund Nielsen, J.; Tandoi, V.; James Seviour, R.; Nielsen, P.H. Metabolic model for the filamentous ‘Candidatus Microthrix parvicella’ based on genomic and metagenomic analyses. ISME J. 2013, 7, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Pomorska, D.; Larsson, L.; Skórska, C.; Sitkowska, J.; Dutkiewicz, J. Levels of bacterial endotoxin in the samples of settled dust collected in animal houses. Bull. Vet. Inst. Pulawy 2009, 53, 37–41. [Google Scholar]
- Park, J.-H.; Szponar, B.; Larsson, L.; Gold, D.R.; Milton, D.K. Characterization of Lipopolysaccharides Present in Settled House Dust. Appl. Environ. Microbiol. 2004, 70, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melbostad, E.; Eduard, W.; Skogstad, A.; Sandven, P.; Lassen, J.; Søstrand, P.; Heldal, K. Exposure to bacterial aerosols and work-related symptoms in sewage workers. Am. J. Ind. Med. 1994, 25, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, S.; Nevalainen, A.; Kotimaa, M.; Liesivuori, J.; Martikainen, P.J. Relationship between bacterial counts and endotoxin concentrations in the air of wastewater treatment plants. Appl. Environ. Microbiol. 1992, 58, 3774–3776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, A.A.; Munford, R.S. Dephosphorylation of the lipid A moiety of Escherichia coli lipopolysaccharide by mouse macrophages. Infect. Immun. 1987, 55, 974–978. [Google Scholar] [CrossRef] [Green Version]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, J.; Weyer, F.; Tamura, H.; Nagaoka, I.; Motschmann, H. Low Endotoxin Recovery—Masking of Naturally Occuring Endotoxin. Int. J. Mol. Sci. 2019, 20, 838. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.; Guan, Z.; Raetz, C.R.H. An Undecaprenyl Phosphate-Aminoarabinose Flippase Required for Polymyxin Resistance in Escherichia coli. J. Biol. Chem. 2007, 282, 36077–36089. [Google Scholar] [CrossRef] [Green Version]
- Ławniczek-Wałczyk, A.; Górny, R.L. Endotoxins and β-glucans as markers of microbiological contamination—Characteristics, detection, and environmental exposure. Ann. Agric. Environ. Med. 2010, 17, 193–208. [Google Scholar]
- Degobbi, C.; Saldiva, P.H.N.; Rogers, C. Endotoxin as modifier of particulate matter toxicity: A review of the literature. Aerobiologia 2011, 27, 97–105. [Google Scholar] [CrossRef]
- Schlosser, O.; Robert, S.; Noyon, N. Airborne mycotoxins in waste recycling and recovery facilities: Occupational exposure and health risk assessment. Waste Manag. 2020, 105, 395–404. [Google Scholar] [CrossRef]
- Lu, C.-L.; Lin, H.-I.; Chen, B.-F.; Jow, G.-M. Beauvericin-induced cell apoptosis through the mitogen-activated protein kinase pathway in human nonsmall cell lung cancer A549 cells. J. Toxicol. Sci. 2016, 41, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-S.; Song, H.-H.; Jeong, J.-H.; Shin, C.-G.; Choi, S.-U.; Lee, C. Cytotoxicities of enniatins H, I, and MK1688 from Fusarium oxysporum KFCC 11363P. Toxicon 2008, 51, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Monographs on the Evaluation of Carcinogenic Risks to Humans: Agents Classified by the IARC Monographs; IARC: Lyon, France, 2012; Volume 1. [Google Scholar]
- Jia, C.; Batterman, S. A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air. Int. J. Environ. Res. Public Health 2010, 7, 2903–2939. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, E.; Kwon, J.-T.; Lee, D.-H.; Park, S.-Y.; Kim, H.-M.; Kim, P.; Choi, K. Ethylene glycol potentiated didecyldimethylammonium chloride toxicity in human bronchial epithelial cells. Mol. Cell. Toxicol. 2015, 11, 161–166. [Google Scholar] [CrossRef]
- ECRI. Disinfectant Concentrations, Contact Times, and Use Settings for EPA’s List of Products Effective against SARS-CoV-2, the Cause of COVID-19; ECRI: Plymouth Meeting, PA, USA, 2020. [Google Scholar]
- Heir, E.; Sundheim, G.; Holck, A.L. The qacG gene on plasmid pST94 confers resistance to quaternary ammonium compounds in staphylococci isolated from the food industry. J. Appl. Microbiol. 1999, 86, 378–388. [Google Scholar] [CrossRef]
- Sundheim, G.; Langsrud, S.; Heir, E.; Holck, A.L. Bacterial resistance to disinfectants containing quaternary ammonium compounds. Int. Biodeterior. Biodegrad. 1998, 41, 235–239. [Google Scholar] [CrossRef]
- Chapman, J.S. Disinfectant resistance mechanisms, cross-resistance, and co-resistance. Int. Biodeterior. Biodegrad. 2003, 51, 271–276. [Google Scholar] [CrossRef]
- Littlejohn, T. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 1992, 95, 259–265. [Google Scholar] [CrossRef]
- Lemaître, J.P.; Echchannaoui, K.; Michaut, G.; Divies, C.; Rousset, A. Plasmid-Mediated Resistance to Antimicrobial Agents among Listeriae. J. Food Prot. 1998, 61, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.; Pant, A.; Farombi, E. 4-Hydroxynonenal induces mitochondrial-mediated apoptosis and oxidative stress in SH-SY5Y human neuronal cells. Basic Clin. Pharmacol. Toxicol. 2012, 110, 441–448. [Google Scholar] [CrossRef]
- Gupta, R.C. Carbofuran toxicity. J. Toxicol. Environ. Health 1994, 43, 383–418. [Google Scholar] [CrossRef]
- Parrish, N.; Kuhajda, F.; Heine, H.; Bishai, W.; Dick, J. Antimycobacterial activity of cerulenin and its effects on lipid biosynthesis. J. Antimicrob. Chemother. 1999, 43, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Bañuelos, T.N.; Martínez-Hernández, I.; Hernández-Kelly, L.C.; Chi-Castañeda, D.; Vega, L.; Ortega, A. The neurotoxin diethyl dithiophosphate impairs glutamate transport in cultured Bergmann glia cells. Neurochem. Int. 2019, 123, 77–84. [Google Scholar] [CrossRef]
- Debieu, D.; Gall, C.; Gredt, M.; Bach, J.; Malosse, C.; Leroux, P. Ergosterol biosynthesis and its inhibition by fenpropimorph in Fusarium species. Phytochemistry 1992, 31, 1223–1233. [Google Scholar] [CrossRef]
- Sanctucci, K.; Shah, B. Association of Naphthalene with Acute Hemolytic Anemia. Acad. Emergy Med. 2000, 7, 42–47. [Google Scholar] [CrossRef]
- Abramson, S.N.; Radic, Z.; Manker, D.; Faulkner, D.J.; Taylor, P. Onchidal: A naturally occurring irreversible inhibitor of acetylcholinesterase with a novel mechanism of action. Mol. Pharmacol. 1989, 36, 349–354. [Google Scholar]
- Ferris, M.J.; Muyzer, G.; Ward, D.M.; Spring, O. Denaturing Gradient Gel Electrophoresis Profiles of 16S rRNA-Defined Populations Inhabiting a Hot Spring Microbial Mat Community. Appl. Environ. Microbiol. 1996, 62, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, E.A.; Berryman, D.E.; Murphy, E.R.; Carroll, R.K.; Joshua Busken, J.B. Reports analysis of mouse gut microbiomes via greater nucleotide diversity. Biotechniques 2019, 67, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szulc, J.; Okrasa, M.; Dybka-Stępień, K.; Sulyok, M.; Nowak, A.; Otlewska, A.; Szponar, B.; Majchrzycka, K. Assessment of microbiological indoor air quality in cattle breeding farms. Aerosol Air Qual. Res. 2020, 20, 1353–1373. [Google Scholar] [CrossRef] [Green Version]
- OECD Guidelines for the Testing of Chemicals, Section 4. Test No. 442D: In Vitro Skin Sensitisation Are-nrf2 Luciferase Test Method. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-tg442d-508.pdf (accessed on 24 August 2021).
- GESTIS: International Occupational Exposure Limit Values for Chemical Agents. Available online: https://limitvalue.ifa.dguv.de/ (accessed on 24 August 2021).
- MassBank of North America (MoNA). Available online: https://mona.fiehnlab.ucdavis.edu/ (accessed on 24 August 2021).
- National Institute of Standards and Technology Mass Spectrometry Data Center. Available online: https://chemdata.nist.gov/ (accessed on 24 August 2021).
- Hinkle, D.; Wiersma, W.; Jurs, S.G. Applied Statistics for Behavioral Sciences, 5th ed.; Houghton Mifflin Harcourt: Boston, MA, USA, 2003. [Google Scholar]



| No.* | Temperature (°C) | Relative Humidity (%) | Airflow Rate (m s−1) |
|---|---|---|---|
| 1 | M:17.6 b | M:71.0 a | M:0.87 d |
| SD:0.3 | SD:1.8 | SD:0.28 | |
| 2 | M:17.4 b | M:73.1 a | M:0.55 cd |
| SD:0.3 | SD:1.5 | SD:0.34 | |
| 3 | M:20.1 a | M:65.3 bd | M:0.39 bc |
| SD:1.0 | SD:3.2 | SD:0.11 | |
| 4 | M:21.5 a | M:57.7 c | M:0.03 a |
| SD:0.5 | SD:1.3 | SD:0.01 | |
| 5 | M:24.8 cd | M:71.1 ab | M:0.31 abc |
| SD:0.7 | SD:4.7 | SD:0.21 | |
| 6 | M:26.4 d | M:69.8 ab | M:0.37 abc |
| SD:2.7 | SD:5.1 | SD:0.10 | |
| EB | M:22.1 ac | M:58.2 cd | M:0.03 ab |
| SD:0.2 | SD:1.0 | SD:0.02 |
| No. | Dust Concentration Corresponding to Different Fractions, mg m−3 | ||||
|---|---|---|---|---|---|
| PM1 | PM2.5 | PM4 | PM10 | PMtotal | |
| 1 | M:0.040 a | M:0.040 a | M:0.041 b | M:0.042 c | M:0.044 a |
| SD:0.081 | SD:0.081 | SD:0.082 | SD:0.087 | SD:0.099 | |
| 2 | M:0.037 ac | M:0.038 ab | M:0.038 ab | M:0.040 abc | M:0.041 ab |
| SD:0.004 | SD:0.004 | SD:0.005 | SD:0.007 | SD:0.008 | |
| 3 | M:0.036 ac | M:0.037 ac | M:0.037 ab | M:0.038 ab | M:0.040 ab |
| SD:0.003 | SD:0.003 | SD:0.003 | SD:0.005 | SD:0.011 | |
| 4 | M:0.032 b | M:0.033 b | M:0.035 ac | M:0.041 abc | M:0.043 a |
| SD:0.006 | SD:0.006 | SD:0.006 | SD:0.009 | SD:0.013 | |
| 5 | M:0.044 b | M:0.044 b | M:0.045 c | M:0.047 d | M:0.047 b |
| SD:0.005 | SD:0.005 | SD:0.005 | SD:0.006 | SD:0.007 | |
| 6 | M:0.033 bc | M:0.034 bc | M:0.034 ac | M:0.037 ad | M:0.039 ab |
| SD:0.006 | SD:0.006 | SD:0.006 | SD:0.008 | SD:0.012 | |
| EB | M:0.030 a | M:0.030 a | M:0.031 b | M:0.034 bc | M:0.036 a |
| SD:0.006 | SD:0.006 | SD:0.007 | SD:0.010 | SD:0.017 | |
| No. | Microorganisms Count, CFU m−3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Actinomycetes | Mannitol-Positive Staphylococci | Enterobacteriaceae | Pseudomonas fluorescens | Haemolytic Staphylococci | Fungi | Xerophilic Fungi | |
| 1 | M:7.75 × 102 A | M:0.00 A | M:2.50 × 100 A | M:2.50 × 100 A | M:4.00 × 101 B | M:5.75 × 101 A | M:2.75 × 103 A | M:1.89 × 103 A |
| SD:5.74 × 101 | SD: 0.00 | SD:5.00 × 100 | SD:5.00 × 100 | SD:8.16 × 100 | SD:5.00 × 100 | SD:5.11 × 102 | SD:7.54 × 102 | |
| 2 | M:3.75 × 102 A | M:1.50 × 101 B | M:2.50 × 100 A | M:1.50 × 101 A | M:1.50 × 101 A | M:5.75 × 101 A | M:2.69 × 103 A | M:1.87 × 103 A |
| SD:1.49 × 102 | SD:1.00 × 101 | SD:5.00 × 100 | SD:1.00 × 101 | SD:1.00 × 101 | SD:1.71 × 101 | SD:5.36 × 102 | SD:3.74 × 102 | |
| 3 | M:3.05 × 102 A | M:0.00 A | M:7.50 × 100 A | M:1.50 × 101 A | M:7.50 × 100 A | M:5.00 × 101 A | M:2.13 × 103 A | M:1.13 × 103 A |
| SD:6.40 × 101 | SD:0.00 | SD:9.57 × 100 | SD:1.00 × 101 | SD:9.57 × 100 | SD:1.41 × 101 | SD:4.46 × 102 | SD:1.13 × 102 | |
| 4 | M:3.71 × 103 B | M:0.00 A | M:6.50 × 101 B | M:1.50 × 101 A | M:2.00 × 101 AB | M:2.10 × 102 A | M:1.91 × 103 A | M:1.55 × 103 A |
| SD:7.57 × 102 | SD:0.00 | SD:3.00 × 101 | SD:1.00 × 101 | SD:1.63 × 101 | SD:1.01 × 102 | SD:4.38 × 102 | SD:1.54 × 102 | |
| 5 | M:6.28 × 102 A | M:0.00 A | M:1.00 × 101 A | M:1.98 × 102 C | M:1.00 × 101 A | M:3.25 × 102 A | M:3.23 × 104 B | M:1.26 × 104 AB |
| SD:1.61 × 102 | SD:0.00 | SD:1.15 × 101 | SD:1.07 × 103 | SD:1.15 × 101 | SD:4.50 × 102 | SD:2.34 × 104 | SD:1.84 × 104 | |
| 6 | M:3.38 × 102 A | M:0.00 A | M:6.75 × 101 B | M:6.85 × 101 B | M:1.00 × 101 A | M:5.00 × 101 A | M:2.80 × 104 AB | M:2.39 × 104 B |
| SD:5.06 × 101 | SD:0.00 | SD:2.22 × 101 | SD:9.50 × 101 | SD:1.15 × 101 | SD:8.16 × 100 | SD:1.73 × 104 | SD:1.97 × 104 | |
| EB | M:2.15 × 102 A | M:1.50 × 101 B | M:2.50 × 100 A | M:1.00 × 101 A | M:0.00 A | M:1.50 × 101 A | M:1.02 × 104 AB | M:1.01 × 104 AB |
| SD:6.81 × 101 | SD:1.00 × 101 | SD:5.00 × 100 | SD:0.00 × 100 | SD:0.00 | SD:1.00 × 101 | SD:1.08 × 104 | SD:1.09 × 104 | |
| Metabolite | Concentration | |
|---|---|---|
| Air, ng m−3 | Dust, ng g−1 | |
| 3-Nitropropionic acid | 1.98 | 5.87 |
| Quinolactacin A | <LOD | 1.58 |
| Citreohybridinol | <LOD | 3.49 |
| Flavoglaucin | 1.54 | 1.65 |
| Pentoxifylline | <LOD | 0.90 |
| Beauvericin | <LOD | 0.24 |
| Enniatin A1 | <LOD | 0.58 |
| Enniatin B | <LOD | 2.32 |
| Enniatin B1 | <LOD | 1.21 |
| Lecanoric acid | <LOD | 129 |
| Usnic acid | <LOD | 14.0 |
| Prunasin | <LOD | 23.1 |
| Asperglaucide | <LOD | 2.30 |
| Asperphenamate | 0.46 | 7.37 |
| cyclo(L-Pro-L-Tyr) | <LOD | 18.1 |
| Emodin | <LOD | 2.59 |
| Compound | RT, min | Measured m/z | Mass Error, ppm | Molecular Formula | Ion Formula | Meas. Mode | mSigma | MS/MS |
|---|---|---|---|---|---|---|---|---|
| Didecyldimethylammonium chloride | 12.11 | 326.38 | 0.49 | C22H48ClN | [M−Cl]+ | +bbCID | 8.0 | yes |
| 4-Hydroxynonenal | 6.15 | 157.12 | 1.25 | C9H16O2 | [M+H]+ | +AutoMSMS | 5.6 | yes |
| Carbofuran | 29.92 | 222.11 | 0.94 | C12H15NO3 | [M+H]+ | +AutoMSMS | 12.1 | yes |
| Cerulenin | 0.08 | 224.13 | 0.96 | C12H17NO3 | [M+H]+ | +AutoMSMS | 12.8 | yes |
| Diethylphosphate | 5.78 | 155.05 | 0.91 | C4H11O4P | [M+H]+ | +AutoMSMS | 6.1 | yes |
| Fenpropimorph | 13.42 | 304.26 | 0.64 | C20H33NO | [M+H]+ | +AutoMSMS | 0.8 | yes |
| Naphthalene | 6.73 | 129.07 | 0.98 | C10H8 | [M+H]+ | +AutoMSMS | 6.3 | yes |
| Onchidal | 15.62 | 277.18 | 0.63 | C17H24O3 | [M+H]+ | +AutoMSMS | - | no |
| No. | Workstation Name | Description |
|---|---|---|
| 1 | Primary settlement tank inlet | Rectangular primary settlement tank (6 tanks ca. 4000 m3 volume each) featured with scrapers. The final stage of sewage mechanical treatment is carried out here. The sludge separated on the bottom is scraped to the hoppers from where it is removed to the fermentation chambers. |
| 2 | Activated sludge chamber inlet | Activated sludge rectangular chambers (7 chambers ca. 19,900 m3 volume each). Biological treatment of the sewage is carried out here. Organic and biogenic compounds (nitrogen, phosphorus) from the sewage are decomposed by the microorganisms in the activated sludge. The process varies, depending on a number of factors, including oxygen content, temperature, bacteria genus, supplied sewage characteristics and the adopted treatment method. |
| 3 | Aeration chambers | Oxygen (nitrification) zone composed of two piston flow chambers, equipped with a fine bubble aeration system. |
| 4 | Sludge thickening building | The sludge (primary and surplus) is thickened before being fed for further treatment. The primary sludge is gravitationally thickened in primary sludge hoppers and optionally in gravitational hoppers (3 hoppers 539 m3 volume each). The surplus sludge is thickened at sludge belt thickeners (5 thickeners 91.5 m3/h capacity each) using polyelectrolyte. The thickened sludge is stabilised through methane fermentation. |
| 5 | Screenings storage | The screenings obtained through mechanical treatment are deposited in a separated storage area. A coarse grate with 100 mm mesh size is used in the sewage treatment plant—it protects fine grates against large items supplied by combined sewers. Then the sewage is divided into 1 ÷ 4 lines in the main inlet chamber. Each line is handled by a set of two grates. Hook and slot grates work at two lines (6 mm clearance) and disc screen sets on the other two lines (55 mm clearance), with mills and lamellar grating. |
| 6 | Sludge lagoons | The wastewater from the grate room flows into four non-aerated sand traps. The sand collected at the bottom is scraped to the hoppers and pumped as a pulp into scrapers and then to the chamber scrubbers. The removed sand as a mineral (containing less than 3% of organic compounds) is deposited in sludge lagoons. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc, J.; Okrasa, M.; Majchrzycka, K.; Sulyok, M.; Nowak, A.; Ruman, T.; Nizioł, J.; Szponar, B.; Gutarowska, B. Microbiological and Toxicological Hazards in Sewage Treatment Plant Bioaerosol and Dust. Toxins 2021, 13, 691. https://doi.org/10.3390/toxins13100691
Szulc J, Okrasa M, Majchrzycka K, Sulyok M, Nowak A, Ruman T, Nizioł J, Szponar B, Gutarowska B. Microbiological and Toxicological Hazards in Sewage Treatment Plant Bioaerosol and Dust. Toxins. 2021; 13(10):691. https://doi.org/10.3390/toxins13100691
Chicago/Turabian StyleSzulc, Justyna, Małgorzata Okrasa, Katarzyna Majchrzycka, Michael Sulyok, Adriana Nowak, Tomasz Ruman, Joanna Nizioł, Bogumiła Szponar, and Beata Gutarowska. 2021. "Microbiological and Toxicological Hazards in Sewage Treatment Plant Bioaerosol and Dust" Toxins 13, no. 10: 691. https://doi.org/10.3390/toxins13100691
APA StyleSzulc, J., Okrasa, M., Majchrzycka, K., Sulyok, M., Nowak, A., Ruman, T., Nizioł, J., Szponar, B., & Gutarowska, B. (2021). Microbiological and Toxicological Hazards in Sewage Treatment Plant Bioaerosol and Dust. Toxins, 13(10), 691. https://doi.org/10.3390/toxins13100691







