In Vitro Assay of Translation Inhibition by Trichothecenes Using a Commercially Available System
Abstract
:1. Introduction
2. Results
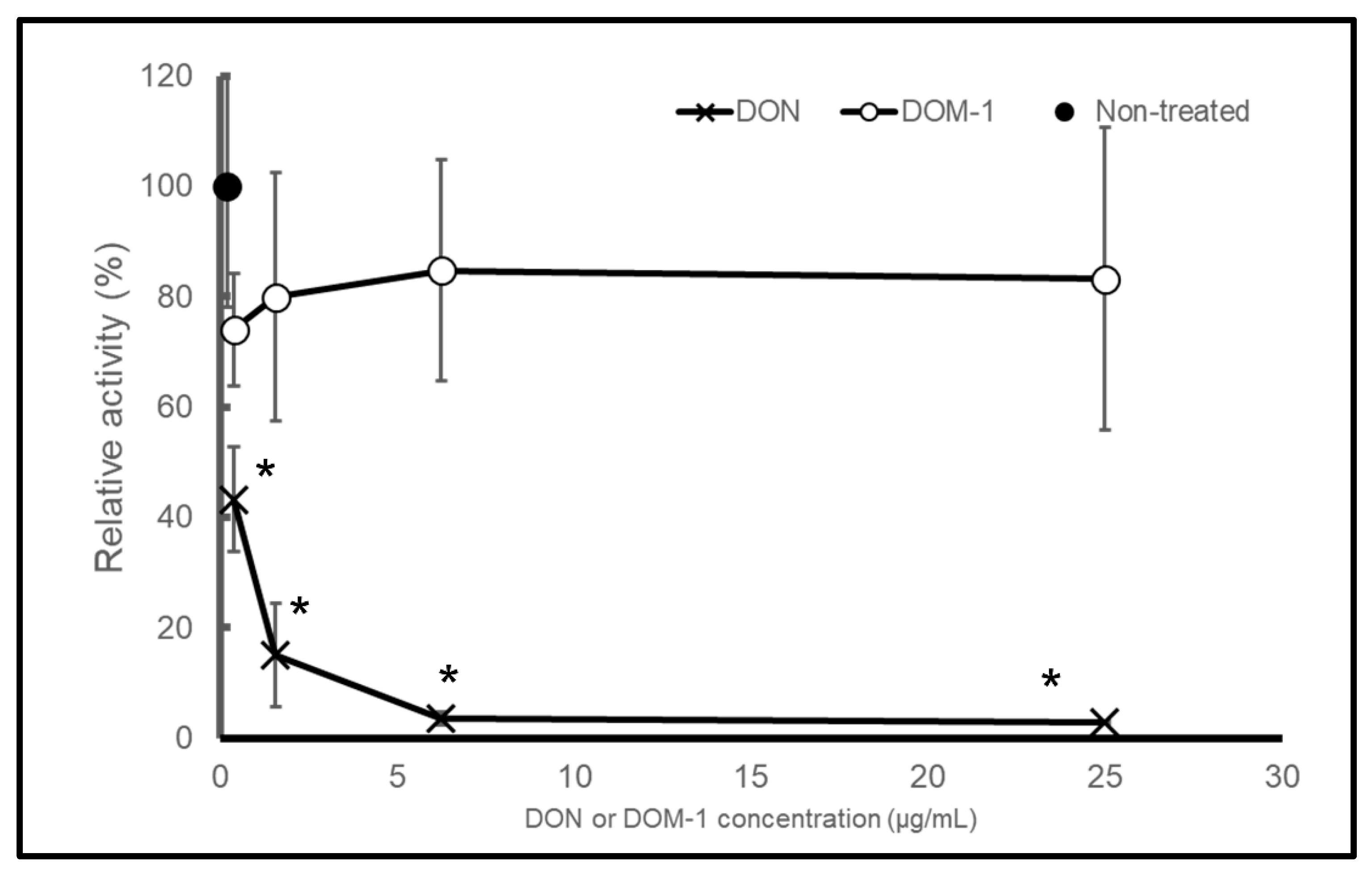
2.1. DON, but Not DOM-1, Inhibits Protein Synthesis in a Cell-Free In Vitro System
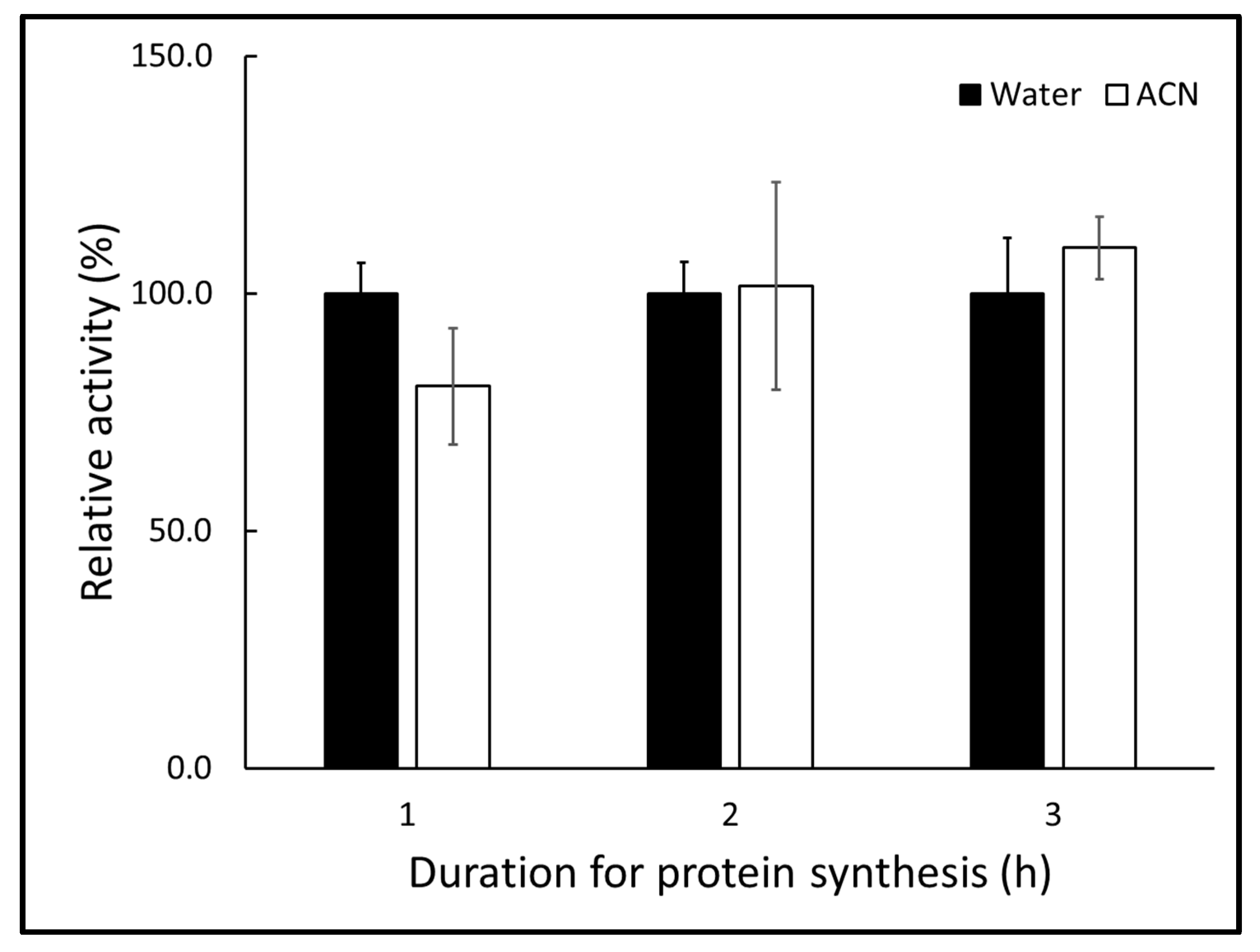
2.2. Acetonitrile as a Solvent Has No Preventative Effect on Protein Synthesis
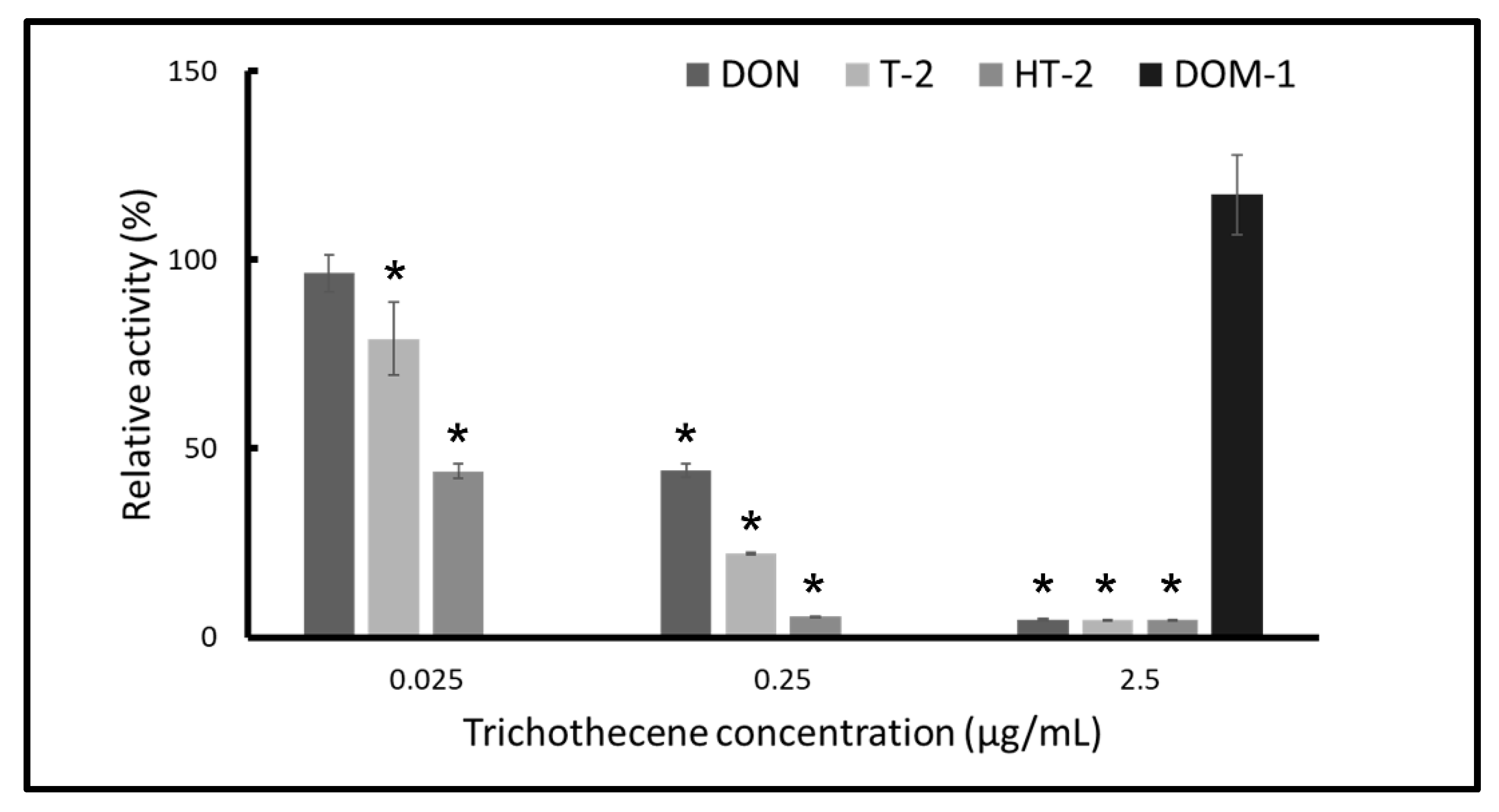
2.3. T-2 and HT-2 Toxins Show Stronger Inhibition than that of DON
2.4. Effect on the Transcription Step in the Presence of DON
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Cell-Free In Vitro Transcription and Translation
5.3. β-Galactosidase Assay
5.4. RNA and cDNA Preparation and qPCR
5.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; McCormick, S.P.; Kim, H.-S.; Cardoza, R.E.; Stanley, A.M.; Lindo, L.; Kelly, A.; Brown, D.W.; Lee, T.; Vaughan, M.M.; et al. Evolution of Structural Diversity of Trichothecenes, a Family of Toxins Produced by Plant Pathogenic and Entomopathogenic Fungi. PLoS Pathog. 2018, 14, e1006946. [Google Scholar] [CrossRef] [Green Version]
- Paszkiewicz, M.; Tyma, M.; Ligęza-Żuber, M.; Włóka, E.; Boguś, M.I.; Stepnowski, P. Trichothecenes Production by Entomopathogenic Fungus Conidiobolus Coronatus. Adv. Toxicol. Toxic Eff. 2016, 1, 007–014. [Google Scholar] [CrossRef] [Green Version]
- Marasas, W.F.O.; Nelson, P.E.; Toussoun, T.A. Toxigenic Fusarium Species. Identity and Mycotoxicology; Pennsylvania State University: Centre County, PA, USA, 1984. [Google Scholar]
- Yoshizawa, T. Human and Animal Intoxication Episodes Caused by Trichothecene Mycotoxins. Mycotoxins 2003, 53, 113–118. [Google Scholar] [CrossRef]
- Yoshizawa, T. Red-Mold Diseases and Natural Occurrence in Japan. In Trichothecenes: Chemical, Biological, and Toxicological Aspects; Developments in Food Science; Ueno, Y., Ed.; Elsevier Science Limited: Amsterdam, The Netherlands, 1983; Volume 4, pp. 195–209. [Google Scholar]
- Li, F.Q.; Luo, X.Y.; Yoshizawa, T. Mycotoxins (trichothecenes, Zearalenone and Fumonisins) in Cereals Associated with Human Red-Mold Intoxications Stored since 1989 and 1991 in China. Nat. Toxins 1999, 7, 93–97. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Abraham, N.; Li, X.-Z.; Kimber, M.; Zhou, T. The Ribosome-Binding Mode of Trichothecene Mycotoxins Rationalizes Their Structure—Activity Relationships. Int. J. Mol. Sci. 2021, 22, 1604. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhou, H.-R.; Pestka, J.J. Targets and Intracellular Signaling Mechanisms for Deoxynivalenol-Induced Ribosomal RNA Cleavage. Toxicol. Sci. 2012, 127, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garreau de Loubresse, N.; Prokhorova, I.; Holtkamp, W.; Rodnina, M.V.; Yusupova, G.; Yusupov, M. Structural Basis for the Inhibition of the Eukaryotic Ribosome. Nature 2014, 513, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.L.; Wannemacher, R.W., Jr. Structure-Function Relationships of 12,13-Epoxytrichothecene Mycotoxins in Cell Culture: Comparison to Whole Animal Lethality. Toxicon 1986, 24, 985–994. [Google Scholar] [CrossRef]
- McCormick, S.P. Microbial Detoxification of Mycotoxins. J. Chem. Ecol. 2013, 39, 907–918. [Google Scholar] [CrossRef]
- Sundstøl Eriksen, G.; Pettersson, H.; Lundh, T. Comparative Cytotoxicity of Deoxynivalenol, Nivalenol, Their Acetylated Derivatives and de-Epoxy Metabolites. Food Chem. Toxicol. 2004, 42, 619–624. [Google Scholar] [CrossRef]
- Mayer, E.; Novak, B.; Springler, A.; Schwartz-Zimmermann, H.E.; Nagl, V.; Reisinger, N.; Hessenberger, S.; Schatzmayr, G. Effects of Deoxynivalenol (DON) and Its Microbial Biotransformation Product Deepoxy-Deoxynivalenol (DOM-1) on a Trout, Pig, Mouse, and Human Cell Line. Mycotoxin Res. 2017, 33, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Boguś, M.I.; Wrońska, A.K.; Kaczmarek, A.; Boguś-Sobocińska, M. In Vitro Screening of 65 Mycotoxins for Insecticidal Potential. PLoS ONE 2021, 16, e0248772. [Google Scholar] [CrossRef]
- Robbana-Barnat, S.; Lafarge-Frayssinet, C.; Cohen, H.; Neish, G.A.; Frayssinet, C. Immunosuppressive Properties of Deoxynivalenol. Toxicology 1988, 48, 155–166. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Takeda, H.; Toshinori, O.H.I. Structure of a Novel Metabolite from Deoxynivalenol, a Trichothecene Mycotoxin, in Animals. Agric. Biol. Chem. 1983, 47, 2133–2135. [Google Scholar] [CrossRef]
- Fuchs, E.; Binder, E.M.; Heidler, D.; Krska, R. Structural Characterization of Metabolites after the Microbial Degradation of Type A Trichothecenes by the Bacterial Strain BBSH 797. Food Addit. Contam. 2002, 19, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Rotter, B.A.; Thompson, B.K.; Clarkin, S.; Owen, T.C. Rapid Colorimetric Bioassay for Screening of Fusarium Mycotoxins. Nat. Toxins 1993, 1, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.P.; Helaszek, C.; Buck, W.B.; Rood, H.D., Jr.; Haschek, W.M. The Role of Intestinal Microflora in the Metabolism of Trichothecene Mycotoxins. Food Chem. Toxicol. 1988, 26, 823–829. [Google Scholar] [CrossRef]
- Swanson, S.P.; Rood, H.D.; Behrens, J.C.; Sanders, P.E. Preparation and Characterization of the Deepoxy Trichothecenes: Deepoxy HT-2, Deepoxy T-2 Triol, Deepoxy T-2 Tetraol, Deepoxy 15-Monoacetoxyscirpenol, and Deepoxy Scirpentriol. Appl. Environ. Microbiol. 1987, 53, 2821–2826. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, T.; Morooka, N. Studies on the Toxic Substances in the Infected Cereals (III) Acute Toxicities of New Trichothecene Mycotoxins: Deoxynivalenol and Its Monoacetate. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1974, 15, 261–269_1. [Google Scholar] [CrossRef] [Green Version]
- Forsell, J.H.; Jensen, R.; Tai, J.H.; Witt, M.; Lin, W.S.; Pestka, J.J. Comparison of Acute Toxicities of Deoxynivalenol (vomitoxin) and 15-Acetyldeoxynivalenol in the B6C3F1 Mouse. Food Chem. Toxicol. 1987, 25, 155–162. [Google Scholar] [CrossRef]
- Zielonka, Ł.; Wiśniewska, M.; Gajecka, M.; Obremski, K.; Gajecki, M. Influence of Low Doses of Deoxynivalenol on Histopathology of Selected Organs of Pigs. Pol. J. Vet. Sci. 2009, 12, 89–95. [Google Scholar] [PubMed]
- Nakagawa, H. Research on Mycotoxin Glucosides (masked Mycotoxins). JSM Mycotoxins 2016, 66, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked Mycotoxins: Determination of a Deoxynivalenol Glucoside in Artificially and Naturally Contaminated Wheat by Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef]
- Lancova, K.; Hajslova, J.; Kostelanska, M.; Kohoutkova, J.; Nedelnik, J.; Moravcova, H.; Vanova, M. Fate of Trichothecene Mycotoxins during the Processing: Milling and Baking. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 650–659. [Google Scholar] [CrossRef]
- Lautraite, S.; Parent-Massin, D.; Rio, B.; Hoellinger, H. In Vitro Toxicity Induced by Deoxynivalenol (DON) on Human and Rat Granulomonocytic Progenitors. Cell Biol. Toxicol. 1997, 13, 175–183. [Google Scholar] [CrossRef]
- Thuvander, A.; Wikman, C.; Gadhasson, I. In Vitro Exposure of Human Lymphocytes to Trichothecenes: Individual Variation in Sensitivity and Effects of Combined Exposure on Lymphocyte Function. Food Chem. Toxicol. 1999, 37, 639–648. [Google Scholar] [CrossRef]
- Sugiyama, K.-I.; Muroi, M.; Kinoshita, M.; Hamada, O.; Minai, Y.; Sugita-Konishi, Y.; Kamata, Y.; Tanamoto, K.-I. NF-κB Activation via MyD88-Dependent Toll-like Receptor Signaling Is Inhibited by Trichothecene Mycotoxin Deoxynivalenol. J. Toxicol. Sci. 2016, 41, 273–279. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyotome, T.; Kamei, K. In Vitro Assay of Translation Inhibition by Trichothecenes Using a Commercially Available System. Toxins 2021, 13, 696. https://doi.org/10.3390/toxins13100696
Toyotome T, Kamei K. In Vitro Assay of Translation Inhibition by Trichothecenes Using a Commercially Available System. Toxins. 2021; 13(10):696. https://doi.org/10.3390/toxins13100696
Chicago/Turabian StyleToyotome, Takahito, and Katsuhiko Kamei. 2021. "In Vitro Assay of Translation Inhibition by Trichothecenes Using a Commercially Available System" Toxins 13, no. 10: 696. https://doi.org/10.3390/toxins13100696
APA StyleToyotome, T., & Kamei, K. (2021). In Vitro Assay of Translation Inhibition by Trichothecenes Using a Commercially Available System. Toxins, 13(10), 696. https://doi.org/10.3390/toxins13100696





