Antifungal Activity of Biocontrol Agents In Vitro and Potential Application to Reduce Mycotoxins (Aflatoxin B1 and Ochratoxin A)
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Antifungal Activity of Biocontrol Strains
2.2. Identification of Antifungal Compounds in CFS
2.3. Antimycotoxigenic Activity of CFS
3. Conclusions
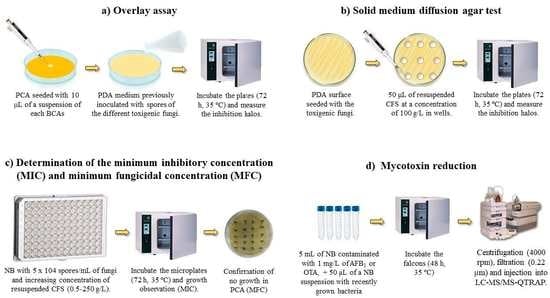
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Microbiological Culture
4.3. Process of Obtaining Cell-Free Supernatant (CFS)
4.4. Qualitative Antifungal Activity of Biocontrol Strains
4.5. Quantitative Antifungal Activity of Biocontrol Strains
4.6. Determination of Organic and Phenolic Acids in CFS
4.7. Antimycotoxigenic Activity of CFS
4.8. Determination of AFB1 and OTA by LC-MS/MS Spectrometry
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saladino, F.; Luz, C.; Manyes, L.; Franzón, M.F.; Meca, G. In Vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control. 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Jiang, Y.; Duan, X.; Qu, H.; Yang, B.; Chen, F.; Sivakumar, D. Natural Occurrence, Analysis, and Prevention of Mycotoxins in Fruits and their Processed Products. Crit. Rev. Food Sci. Nutr. 2013, 54, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Mañes, J.; Berrada, H. Exposure assessment approach through mycotox-in/creatinine ratio evaluation in urine by GC–MS/MS. Food Chem. Toxicol. 2014, 72, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Varsha, K.K.; Nampoothiri, K.M. Appraisal of lactic acid bacteria as protective cultures. Food Control. 2016, 69, 61–64. [Google Scholar] [CrossRef]
- IARC-International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. In A Review of Biological Agents for Human Carcinogens; IARC-International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Quiles, J.M.; Nazareth, T.D.M.; Luz, C.; Luciano, F.B.; Mañes, J.; Meca, G. Development of an antifungal and antimycotox-igenic device containing allyl isothiocyanate for silo fumigation. Toxins 2019, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Some traditional herbal medicines, some mycotoxins, naph-thalene and styrene. In Monograph on the Evaluation of Carcinogenic Risk to Humans; IARC: Lyon, France, 2002. [Google Scholar]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Vandghanooni, S.; Forouharmehr, A.; Eskandani, M.; Barzegari, A.; Kafil, V.; Kashanian, S.; Dolatabadi, J.E.N. Cytotoxicity and DNA Fragmentation Properties of Butylated Hydroxyanisole. DNA Cell Biol. 2013, 32, 98–103. [Google Scholar] [CrossRef]
- Izzo, L.; Luz, C.; Ritieni, A.; Mañes, J.; Meca, G. Whey fermented by using Lactobacillus plantarum strains: A promising approach to increase the shelf life of pita bread. J. Dairy Sci. 2020, 103, 5906–5915. [Google Scholar] [CrossRef]
- Nešić, K.; Habschied, K.; Mastanjević, K. Possibilities for the Biological Control of Mycotoxins in Food and Feed. Toxins 2021, 13, 198. [Google Scholar] [CrossRef]
- Varsha, K.K.; Priya, S.; Devendra, L.; Nampoothiri, K.M. Control of Spoilage Fungi by Protective Lactic Acid Bacteria Displaying Probiotic Properties. Appl. Biochem. Biotechnol. 2014, 172, 3402–3413. [Google Scholar] [CrossRef]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. The QuEChERS approach in a novel application for the identification of antifungal compounds produced by lactic acid bacteria cultures. Talanta 2014, 129, 364–373. [Google Scholar] [CrossRef]
- Luz, C.; D’Opazo, V.; Quiles, J.; Romano, R.; Mañes, J.; Meca, G. Biopreservation of tomatoes using fermented media by lactic acid bacteria. LWT 2020, 130, 109618. [Google Scholar] [CrossRef]
- Guimarães, A.; Santiago, A.; Teixeira, J.A.; Venâncio, A.; Abrunhosa, L. Antiaflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Omedi, J.O.; Huang, W.; Zheng, J. Effect of sourdough lactic acid bacteria fermentation on phenolic acid release and anti-fungal activity in pitaya fruit substrate. LWT Food Sci. Technol. 2019, 111, 309–317. [Google Scholar] [CrossRef]
- Nazareth, T.D.M.; Luz, C.; Torrijos, R.; Quiles, J.M.; Luciano, F.B.; Mañes, J.; Meca, G. Potential Application of Lactic Acid Bacteria to Reduce Aflatoxin B1 and Fumonisin B1 Occurrence on Corn Kernels and Corn Ears. Toxins 2019, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Calvo, H.; Marco, P.; Blanco, D.; Oria, R.; Venturini, M. Potential of a new strain of Bacillus amyloliquefaciens BUZ-14 as a biocontrol agent of postharvest fruit diseases. Food Microbiol. 2017, 63, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzello, C.G.; Cassone, A.; Coda, R.; Gobbetti, M. Antifungal activity of sourdough fermented wheat germ used as an in-gredient for bread making. Food Chem. 2011, 127, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Landete, J.M.; Rivas, B.D.L.; Muñoz, R. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 2008, 107, 1393–1398. [Google Scholar] [CrossRef] [Green Version]
- Dagnas, S.; Gauvry, E.; Onno, B.; Membré, J.-M.; Stéphane, G. Quantifying Effect of Lactic, Acetic, and Propionic Acids on Growth of Molds Isolated from Spoiled Bakery Products. J. Food Prot. 2015, 78, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal Activity of Phenyllactic Acid against Molds Isolated from Bakery Products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.; de Souza, M.; Badiale-Furlong, E. Antioxidant and antifungal activity of phenolic compounds and their relation to aflatoxin B1 occurrence in soybeans (Glycine max L.). J. Sci. Food Agric. 2019, 100, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cortes, T.; Pérez España, V.H.; López Pérez, P.A.; Rodríguez-Jimenes, G.D.C.; Robles-Olvera, V.J.; Aparicio Burgos, J.E.; Cuervo-Parra, J.A. Antifungal activity of vanilla juice and vanillin against Alternaria alternate. CyTA J. Food 2019, 17, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Topcu, A.; Bulat, T.; Wishah, R.; Boyaci, I. Detoxification of aflatoxin B1 and patulin by Enterococcus faecium strains. Int. J. Food Microbiol. 2010, 139, 202–205. [Google Scholar] [CrossRef]
- Farzaneh, M.; Shi, Z.Q.; Ghassempour, A.; Sedaghat, N.; Ahmadzadeh, M.; Mirabolfathy, M.; Javan-Nikkhah, M. Aflatox-in B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Control. 2012, 23, 100–106. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, L.; Ma, Q.; Li, X.; Shi, H.; Zhou, T.; Zhang, J.; Ji, C. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins. Food Chem. Toxicol. 2013, 59, 748–753. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.H.; Chung, S.H.; Kim, M. Ochratoxin A reduction ability of biocontrol agent Bacillus subtilis isolated from Korean traditional fermented food Kimchi. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, F.; Rastakhiz, N.; Iranmanesh, B.; Olia, S.S.S.J. Determination of Organic Acids in Fruit juices by UPLC. Int. J. Life Sci. 2015, 9, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Denardi-Souza, T.; Luz, C.; Mañes, J.; Badiale-Furlong, E.; Meca, G. Antifungal effect of phenolic extract of fermented rice bran with Rhizopus oryzae and its potential use in loaf bread shelf life extension. J. Sci. Food Agric. 2018, 98, 5011–5018. [Google Scholar] [CrossRef]
- Luz, C.; Carbonell, R.; Quiles, J.M.; Torrijos, R.; de Melo Nazareth, T.; Mañes, J.; Meca, G. Antifungal activity of peracetic ac-id against toxigenic fungal contaminants of maize and barley at the postharvest stage. LWT. Food Sci. Tech. 2021, 148, 111754. [Google Scholar] [CrossRef]

| % Inhibition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pathogen | F. sporotrichioides ITEM 12168 | F. graminearum ITEM 126 | F. proliferatum ITEM 12072 | F. poae ITEM 9151 | F. verticillioides ITEM 12043 | F. langsethiae ITEM 11031 | P. verrucosum CECT 2913 | B. cinerea CECT 20973 | A. flavus ITEM 8111 | A. alternata ITEM 8121 |
| Diameter control (cm) | 8.5 | 6.5 | 7.4 | 6.6 | 5 | 5.3 | 1.6 | 7.7 | Nd | 5.5 |
| Biocontrol agents | ||||||||||
| P. polymixa CECT 153 | Nd | Nd | Nd | 8.3 ± 0.2 | Nd | 8.1 | Nd | 10.2 ± 0.1 | Nd | Nd |
| P. polymixa CECT 155 | 45.2 ± 0.1 | 50.5 ± 0.1 | 48.2 ± 0.1 | 48.2 ± 0.1 | 40.3 ± 0.2 | 46.0 ± 0.2 | 19 ± 0.2 | 38.3 ± 0.1 | Nd | 34.3 ± 0.1 |
| B. thuringiensis CECT 197 | 40.1 ± 0.1 | 20.3 ± 0.2 | 20.2 ± 0.2 | 18.5 ± 0.2 | 18.2 ± 0.2 | 25.2 ± 0.3 | Nd | 18.2 ± 0.2 | Nd | 15.4 ± 0.2 |
| P. chibensis CECT 375 | 18.2 ± 0.2 | 17.4 ± 0.2 | 19.2 ± 0.2 | 16.2 ± 0.1 | 16.3 ± 0.1 | 25.1 ± 0.2 | Nd | 20.2 ± 0.1 | Nd | 19.3 ± 0.3 |
| P. alvei CECT 2 | 16.5 ± 0.1 | 15.4 ± 0.1 | 16.6 ± 0.1 | 16.7 ± 0.2 | 16.2 ± 0.2 | 19.4 ± 0.2 | Nd | 16.5 ± 0.4 | Nd | 13.8 ± 0.2 |
| B. licheniformis CECT 20 | 16.3 ± 0.2 | 17.5 ± 0.2 | 18.7 ± 0.2 | 16.9 ± 0.1 | Nd | 16.4 ± 0.1 | Nd | 16.2 ± 0.1 | Nd | 14.7 ± 0.1 |
| B. megaterium CECT 44 | 16.2 ± 0.1 | 19.2 ± 0.1 | 18.5 ± 0.1 | 11.4 ± 0.3 | Nd | 18.3 ± 0.2 | Nd | 20.2 ± 0.3 | Nd | 16.3 ± 0.3 |
| B. subtilis CECT 499 | 25.8 ± 0.3 | 20.2 ± 0.3 | 21.2 ± 0.3 | 25.2 ± 0.2 | Nd | 18.3 ± 0.1 | Nd | 21.5 ± 0.2 | Nd | 13.9 ± 0.2 |
| B. amyloliquefaciens CECT 493 | 25.3 ± 0.2 | 19.2 ± 0.2 | 22.8 ± 0.2 | 22.2 ± 0.2 | 20.2 ± 0.1 | 16.2 ± 0.2 | Nd | 33.2 ± 0.1 | Nd | 20.3 ± 0.2 |
| P. agglomerans CECT 850 | 47.4 ± 0.1 | 40.3 ± 0.1 | 37.6 ± 0.3 | 20.3 ± 0.1 | 21.2 ± 0.1 | 16.6 ± 0.1 | Nd | 25.3 ± 0.3 | Nd | 22.2 ± 0.1 |
| S. griseus CECT 3276 | 25.9 ± 0.3 | 19.4 ± 0.2 | 20.5 ± 0.2 | 22.2 ± 0.2 | Nd | Nd | Nd | 18.5 ± 0.2 | Nd | 14.2 ± 0.1 |
| S. calvus CECT 3271 | 19.5 ± 0.1 | 20.4 ± 0.3 | 26.4 ± 0.2 | 30.2 ± 0.2 | 20.2 ± 0.3 | Nd | Nd | 16.2 ± 0.1 | Nd | 11.5 ± 0.2 |
| P. syringae CECT 312 | 22.5 ± 0.2 | 35.3 ± 0.2 | 25.3 ± 0.1 | 21.3 ± 0.1 | 19.4 ± 0.2 | Nd | Nd | 16.2 ± 0.2 | Nd | 16.6 ± 0.3 |
| P. syringae CECT 4390 | 22.1 ± 0.2 | 21.2 ± 0.2 | Nd | Nd | 18.6 ± 0.1 | Nd | Nd | 16.3 ± 0.1 | Nd | 35.4 ± 0.1 |
| P. syringae CECT 4393 | 20.2 ± 0.2 | 20.1 ± 0.2 | Nd | Nd | 17.8 ± 0.1 | Nd | Nd | 16.1 ± 0.1 | Nd | 16.0 ± 0.2 |
| C. sake CECT 1044 | 31.2 ± 0.1 | 40.3 ± 0.1 | 48.2 ± 0.1 | 40.3 ± 0.2 | 20.3 ± 0.2 | 30.2 ± 0.1 | Nd | 19.2 ± 0.1 | Nd | 20.1 ± 0.2 |
| C. sake CECT 10034 | 45.2 ± 0.2 | 39.3 ± 0.1 | 45.2 ± 0.2 | 40.2 ± 0.1 | 39.2 ± 0.1 | 30.3 ± 0.1 | Nd | 33.3 ± 0.1 | Nd | 33.5 ± 0.1 |
| C. oleophila CECT 11891 | 45.1 ± 0.2 | 35.2 ± 0.2 | 45.4 ± 0.1 | 40.2 ± 0.2 | 39.2 ± 0.1 | 35.3 ± 0.2 | Nd | 20.4 ± 0.3 | Nd | 21.4 ± 0.2 |
| M. pulcherrima CECT 1691 | 45.2 ± 0.2 | 40.2 ± 0.1 | 50.4 ± 0.2 | 41.3 ± 0.2 | 40.3 ± 0.1 | 40.5 ± 0.1 | Nd | 19.2 ± 0.2 | Nd | 30.5 ± 0.1 |
| M. pulcherrima CECT 10408 | 45.0 ± 0.2 | 40.3 ± 0.2 | 50.1 ± 0.3 | 40.2 ± 0.1 | 39.2 ± 0.1 | 40.2 ± 0.2 | 20 ± 0.2 | 40.6 ± 0.4 | Nd | 20.3 ± 0.3 |
| Pathogen | F. sporotrichioides ITEM 12168 | F. graminearum ITEM 126 | F. proliferatum ITEM 12072 | F. poae ITEM 9151 | F. verticillioides ITEM 12043 | F. langsethiae ITEM 11031 | P. verrucosum CECT 2913 | B. cinerea CECT 20973 | A. flavus ITEM 8111 | A. alternata ITEM 8121 |
|---|---|---|---|---|---|---|---|---|---|---|
| Biocontrol Agents | ||||||||||
| P. polymixa CECT 153 | - | - | - | - | - | - | - | - | - | - |
| P. polymixa CECT 155 | - | - | - | - | - | - | - | - | - | - |
| B. thuringiensis CECT 197 | - | - | - | - | - | - | - | - | - | - |
| P. chibensis CECT 375 | - | +++ | - | +++ | +++ | +++ | +++ | - | +++ | ++ |
| P. alvei CECT 2 | - | - | - | - | - | - | - | - | - | - |
| B. licheniformis CECT 20 | - | - | - | - | + | - | - | - | - | - |
| B. megaterium CECT 44 | - | - | - | - | - | - | - | - | - | - |
| B. subtilis CECT 499 | - | - | - | - | - | - | - | - | - | - |
| B. amyloliquefaciens CECT 493 | - | +++ | - | +++ | - | - | - | - | - | + |
| P. agglomerans CECT 850 | ++ | + | ++ | + | + | ++ | +++ | +++ | - | +++ |
| S. griseus CECT 3276 | - | - | - | - | - | - | - | - | - | - |
| S. calvus CECT 3271 | - | - | - | - | - | - | - | - | - | - |
| P. syringae CECT 312 | - | - | - | - | - | - | - | - | - | - |
| P. syringae CECT 4390 | - | - | - | - | - | - | - | - | - | - |
| P. syringae CECT 4393 | - | - | - | - | - | - | - | - | - | - |
| C. sake CECT 1044 | - | - | - | - | - | - | - | - | - | - |
| C. sake CECT 10034 | - | - | - | - | - | - | - | - | - | - |
| C. oleophila CECT 11891 | - | - | - | - | - | - | - | - | - | - |
| M. pulcherrima CECT 1691 | - | - | - | - | - | - | - | - | - | - |
| M. pulcherrima CECT 10408 | - | - | - | - | - | - | - | - | - | - |
| Fungi | Biocontrol Agents | |||||
|---|---|---|---|---|---|---|
| P. chibensis CECT 375 | B. amyloliquefaciens CECT 493 | P. agglomerans CECT 850 | ||||
| MIC | MFC | MIC | MFC | MIC | MFC | |
| F. graminearum ITEM 126 | 125 | 250 | Nd | Nd | Nd | Nd |
| F. sporotrichioides ITEM 12168 | Nd | Nd | Nd | Nd | Nd | Nd |
| F. langsethiae ITEM 11031 | 125 | 250 | 125 | 250 | 125 | 250 |
| F. poae ITEM 9151 | 125 | 250 | Nd | Nd | 125 | 250 |
| F. verticillioides ITEM 12043 | 125 | 250 | Nd | Nd | 125 | Nd |
| P. verrucosum CECT 2913 | Nd | Nd | Nd | Nd | 125 | 250 |
| A. alternata ITEM 8121 | Nd | Nd | Nd | Nd | 125 | 250 |
| A. flavus ITEM 8111 | 125 | 250 | Nd | Nd | Nd | Nd |
| Biocontrol Agents | Organic Acids (mg/L) | |
|---|---|---|
| Lactic Acid | Acetic Acid | |
| P. polymixa CECT 153 | 2622 ± 0.12 | 1873 ± 0.15 |
| P. polymixa CECT 155 | 3372 ± 0.30 | 1271 ± 0.12 |
| B. thuringiensis CECT 197 | 3491 ± 0.05 | Nd |
| P. chibensis CECT 375 | 1676 ± 0.01 | Nd |
| P. alvei CECT 2 | 384 ± 0.07 | Nd |
| B. licheniformis CECT 20 | 288 ± 0.04 | Nd |
| B. megaterium CECT 44 | 2350 ± 0.04 | Nd |
| B. subtilis CECT 499 | 2553 ± 0.02 | Nd |
| B. amyloliquefaciens CECT 493 | 1251 ± 0.1 | Nd |
| P. agglomerans CECT 850 | 844 ± 0.1 | Nd |
| S. griseus CECT 3276 | 1327 ± 0.06 | Nd |
| S. calvus CECT 3271 | 1411 ± 0.08 | Nd |
| P. syringae CECT 312 | 788 ± 0.11 | 415 ± 0.02 |
| P. syringae CECT 4390 | 670 ± 0.06 | 203 ± 0.01 |
| P. syringae CECT 4393 | 913 ± 0.20 | 208 ± 0.11 |
| C. sake CECT 1044 | 469 ± 0.06 | Nd |
| C. sake CECT 10034 | 416 ± 0.06 | Nd |
| C. oleophila CECT 11891 | 129 ± 0.01 | Nd |
| M. pulcherrima CECT 1691 | 230 ± 0.01 | Nd |
| M. pulcherrima CECT 10408 | 1675 ± 0.03 | Nd |
| Phenolic Acids | Biocontrol Agents | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B. thuringensis CECT 197 | P. polymyxa CECT 153 | P. chibensis CECT 375 | B. amyloquefaciens CECT 493 | B. subtilis CECT 499 | P. alvei CECT 2 | B. licheniformis CECT 20 | B. megaterium CECT 44 | P. polymyxa CECT 155 | |
| Benzoic acid | 0.005 ± 0.03 | 0.060 ± 0.06 | 0.023 ± 0.01 | 0.004 ± 0.02 | 0.044 ± 0.01 | 0.067 ± 0.02 | 0.012 ± 0.02 | 0.047 ± 0.01 | 0.062 ± 0.01 |
| DL-3-Phenyllactic acid | 0.844 ± 0.06 | 0.184 ± 0.03 | Nd | Nd | Nd | 0.195 ± 0.01 | Nd | 0.058 ± 0.02 | 0.297 ± 0.02 |
| 1-2-Dihydroxybenzene | Nd | Nd | 0.040 ± 0.01 | 0.03 ± 0.02 | Nd | Nd | Nd | Nd | Nd |
| Hydroxicinnamic acid | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| P-Coumaric acid | 0.002 ± 0.01 | Nd | Nd | Nd | Nd | Nd | Nd | 0.030 ± 0.07 | Nd |
| 3-4-Dihydroxyhydrocinnamic | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.022 ± 0.06 |
| Protocatechuic acid | Nd | Nd | 0.008 ± 0.01 | Nd | Nd | Nd | Nd | Nd | Nd |
| Biocontrol Agents | % Reduction AFB1 | % Reduction OTA |
|---|---|---|
| P. polymixa CECT 153 | 11.19 ± 0.9 | 11.85 ± 4.0 |
| P. polymixa CECT 155 | 55.00 ± 3.1 | 18.78 ± 11.6 |
| B. thuringiensis CECT 197 | 12.71 ± 0.2 | 22.06 ± 3.7 |
| P. chibensis CECT 375 | 9.81 ± 4.0 | 7.33 ± 6.2 |
| P. alvei CECT 2 | 19.19 ± 0.9 | 22.80 ± 2.4 |
| B. licheniformis CECT 20 | 11.81 ± 7.9 | 26.50 ± 4.4 |
| B. megaterium CECT 44 | 28.87 ± 24.0 | 13.10 ± 3.5 |
| B. subtilis CECT 499 | 13.41 ± 6.7 | 13.67 ± 7.0 |
| B. amyloliquefaciens CECT 493 | 0.00 ± 0.0 | 10.30 ± 7.7 |
| P. agglomerans CECT 850 | 5.00 ± 5.0 | 7.26 ± 3.2 |
| S. griseus CECT 3276 | 3.51 ± 2.3 | 21.38 ± 2.2 |
| S. calvus CECT 3271 | 0.00 ± 0.0 | 10.96 ± 0.8 |
| P. syringae CECT 312 | 6.15 ± 0.13 | 16.06 ± 7.5 |
| P. syringae CECT 4390 | 28.09 ± 1.6 | 13.09 ± 3.8 |
| P. syringae CECT 4393 | 26.33 ± 0.7 | 4.73 ± 9.2 |
| C. sake CECT 1044 | 1.18 ± 2.2 | 7.33 ± 3.3 |
| C. sake CECT 10034 | 24.86 ± 5.9 | 10.07 ± 6.6 |
| C. oleophila CECT 11891 | 0.00 ± 0.0 | 18.69 ± 3.5 |
| M. pulcherrima CECT 1691 | 0.00 ± 0.0 | 7.19 ± 3.5 |
| M. pulcherrima CECT 10408 | 0.00 ± 0.0 | 8.41 ± 4.7 |
| Biocontrol Agent | CECT ID | Source of Isolation | Year | Country | First Description |
|---|---|---|---|---|---|
| P. polymixa | 153 | Water | 1981 | Unknown | Prazmowski, 1880 |
| P. polymixa | 155 | Decomposing plants and soil | 1974 | Unknown | Prazmowski, 1880 |
| B. thuringiensis | 197 | Ephestia kuhniella (Mediterranean flour moth) | 1980 | Unknown | Berliner, 1915 |
| P. chibensis | 375 | Unknown | 1979 | Unknown | Shida et al. 1997 |
| P. alvei | 2 | Foulbrood in bees | 1974 | Unknown | Cheshire and Cheyne 1885 |
| B. licheniformis | 20 | Unknown | 1984 | Unknown | Weigmann, 1898 |
| B. megaterium | 44 | Unknown | 1980 | Unknown | Bary, 1884 |
| B. subtilis | 499 | Unknown | 1982 | Unknown | Ehrenberg, 1835 |
| B. amyloliquefaciens | 493 | Bacterial amylase HT concentrate | 1981 | United States | Priest et al. 1987 |
| P. agglomerans | 850 | Knee laceration | 1987 | Unknown | Ewing and Fife, 1972 |
| S. griseus | 3276 | Soil | 1987 | United States | Krainsky, 1914 |
| S. calvus | 3271 | Soil | 1986 | India | Backus et al. 1957 |
| P. syringae | 312 | Nicotania tabacum | 1978 | Hungary | van Hall 1902 |
| P. syringae | 4390 | Phaseolus vulgaris | 1992 | Hungary | van Hall 1902 |
| P. syringae | 4393 | Lycopersicon esculentum | 1992 | United Kingdom | van Hall 1902 |
| C. sake | 1044 | Lambic beer | 1985 | Belgium | van Uden & H.R. Buckley 1983 |
| C. sake | 10034 | Feces of sheep | 1991 | Spain | van Uden & H.R. Buckley 1983 |
| C. oleophila | 11891 | Fruit of Olea europea (olive) | 2001 | Unknown | Montrocher, 1967 |
| M. pulcherrima | 1691 | Fruit of Phoenix dactylifera (date) | 1989 | Egypt | Pitt & M.W. Miller 1968 |
| M. pulcherrima | 10408 | Orange | 1991 | Spain | Pitt & M.W. Miller 1968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illueca, F.; Vila-Donat, P.; Calpe, J.; Luz, C.; Meca, G.; Quiles, J.M. Antifungal Activity of Biocontrol Agents In Vitro and Potential Application to Reduce Mycotoxins (Aflatoxin B1 and Ochratoxin A). Toxins 2021, 13, 752. https://doi.org/10.3390/toxins13110752
Illueca F, Vila-Donat P, Calpe J, Luz C, Meca G, Quiles JM. Antifungal Activity of Biocontrol Agents In Vitro and Potential Application to Reduce Mycotoxins (Aflatoxin B1 and Ochratoxin A). Toxins. 2021; 13(11):752. https://doi.org/10.3390/toxins13110752
Chicago/Turabian StyleIllueca, Francisco, Pilar Vila-Donat, Jorge Calpe, Carlos Luz, Giuseppe Meca, and Juan M. Quiles. 2021. "Antifungal Activity of Biocontrol Agents In Vitro and Potential Application to Reduce Mycotoxins (Aflatoxin B1 and Ochratoxin A)" Toxins 13, no. 11: 752. https://doi.org/10.3390/toxins13110752
APA StyleIllueca, F., Vila-Donat, P., Calpe, J., Luz, C., Meca, G., & Quiles, J. M. (2021). Antifungal Activity of Biocontrol Agents In Vitro and Potential Application to Reduce Mycotoxins (Aflatoxin B1 and Ochratoxin A). Toxins, 13(11), 752. https://doi.org/10.3390/toxins13110752