Comparison of the Scorpionism Caused by Centruroides margaritatus, Tityus pachyurus and Tityus n. sp. aff. metuendus Scorpion Venoms in Colombia
Abstract
:1. Introduction
2. Results
2.1. Lethal Dose 50 (LD50)
2.2. Clinical In Vivo Effects of Envenomation
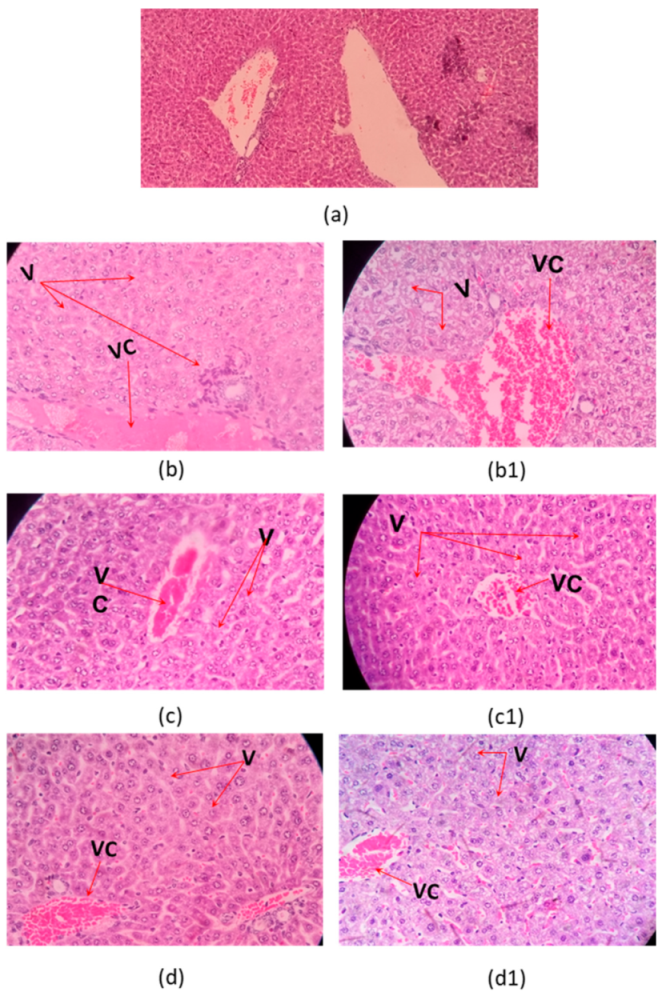
2.3. Histophatological Effect
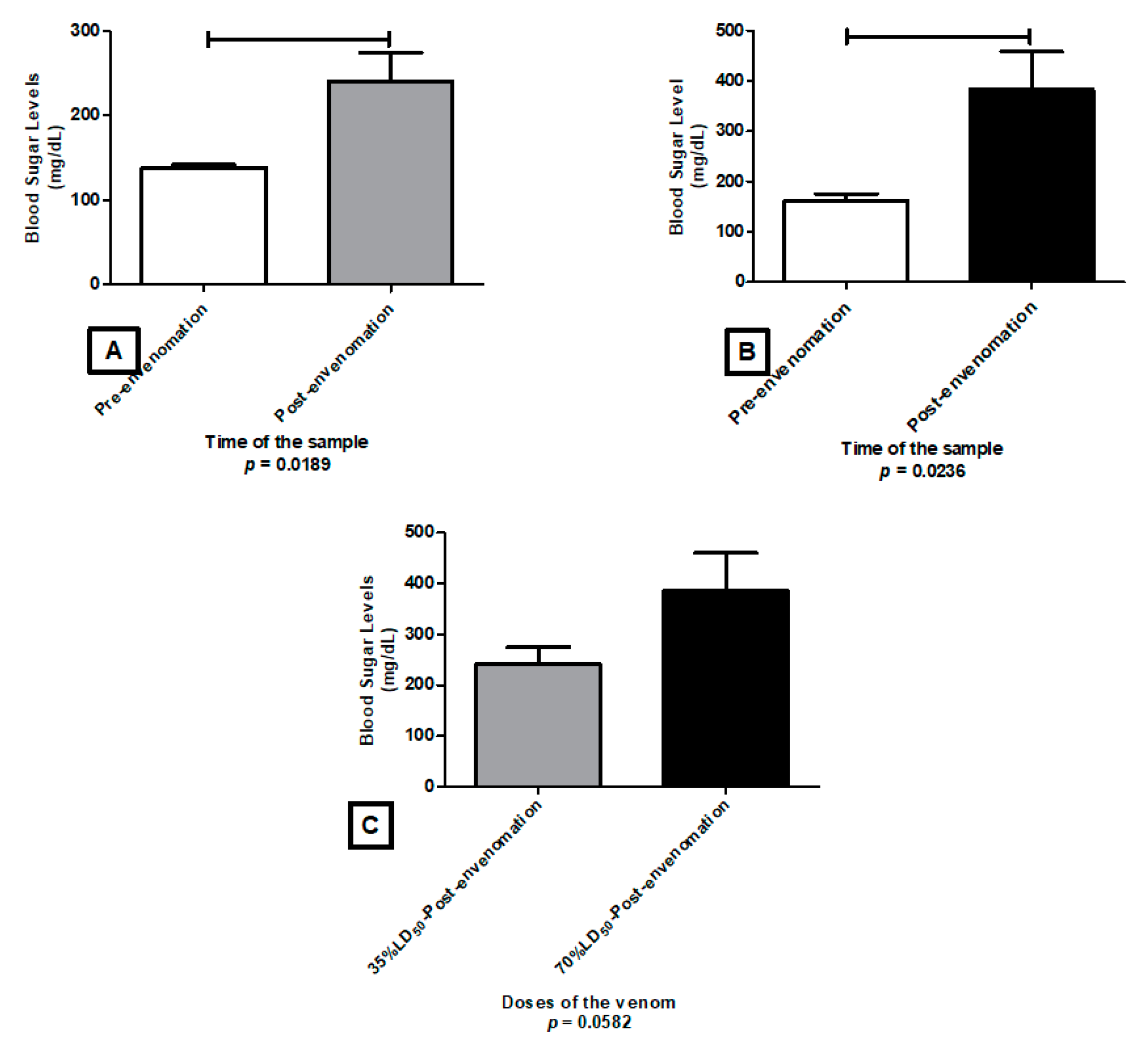
2.4. Glycemia Level
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Mice
5.2. Scorpions
5.3. Determination of the Lethal Dose 50 (LD50)
5.4. Histopathological Study
5.5. Glycemia Level
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chippaux, J.-P.P.; Goyffon, M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008, 107, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Khattabi, A.; Soulaymani-Bencheikh, R.; Achour, S.; Salmi, L.-R.R. Classification of clinical consequences of scorpion stings: Consensus development. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 364–369. [Google Scholar] [CrossRef]
- Guerrero-Vargas, J.A.; Rodriguez-Buitrago, J.; Ayerbe, S.; Florez-Daza, E.; Beltran-Vidal, J.T. Scorpionism and Dangerous Species of Colombia. In Scorpion Venoms; Gopalakrishnakone, P., Possani, L.D., Schwartz, E.F., Rodriguez de la Vega, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 245–272. ISBN 9400764030. [Google Scholar]
- Rodríguez-Vargas, A. Casuística de Accidentes por Arácnidos Línea Nacional de Toxicología; Instituto Nacional de Salud INS: Bogotá, Colombia, 2015.
- Borges, A.; De Sousa, L.; Espinoza, J.; Santos, R.G.; Kalapothakis, E.; Valadares, D.; Chávez-Olórtegui, C. Characterization of Tityus scorpion venoms using synaptosome binding assays and reactivity towards Venezuelan and Brazilian antivenoms. Toxicon 2008, 51, 66–79. [Google Scholar] [CrossRef]
- Borges, A.; Tsushima, R.G.; Backx, P.H. Antibodies against Tityus discrepans venom do not abolish the effect of Tityus serrulatus venom on the rat sodium and potassium channels. Toxicon 1999, 37, 867–881. [Google Scholar] [CrossRef]
- Carmo, A.O.; Chatzaki, M.; Horta, C.C.R.; Magalhães, B.F.; Oliveira-Mendes, B.B.R.; Chávez-Olórtegui, C.; Kalapothakis, E. Evolution of alternative methodologies of scorpion antivenoms production. Toxicon 2015, 97, 64–74. [Google Scholar] [CrossRef]
- Estrada-Gómez, S.; Gomez-Rave, L.; Vargas-Muñoz, L.J.; van der Meijden, A. Characterizing the biological and biochemical profile of six different scorpion venoms from the Buthidae and Scorpionidae family. Toxicon 2017, 130, 104–115. [Google Scholar] [CrossRef]
- Estrada-Gómez, S.; Vargas-Muñoz, L.J.; Saldarriaga-Córdoba, M.; Quintana Castillo, J.C. Venom from Opisthacanthus elatus scorpion of Colombia, could be more hemolytic and less neurotoxic than thought. Acta Trop. 2016, 153, 70–78. [Google Scholar] [CrossRef]
- Pessini, A.C.; Takao, T.T.; Cavalheiro, E.C.; Vichnewski, W.; Sampaio, S.V.; Giglio, J.R.; Arantes, E.C. A hyaluronidase from Tityus serrulatus scorpion venom: Isolation, characterization and inhibition by flavonoids. Toxicon 2001, 39, 1495–1504. [Google Scholar] [CrossRef]
- Horta, C.C.R.; de Magalhães, B.F.; Oliveira-Mendes, B.B.R.; Carmo, A.O.; Duarte, C.G.; Felicori, L.F.; Machado-de-Ávila, R.A.; Chávez-Olórtegui, C.; Kalapothakis, E. Molecular, Immunological, and Biological Characterization of Tityus serrulatus Venom Hyaluronidase: New Insights into Its Role in Envenomation. PLoS Negl. Trop. Dis. 2014, 8, e2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Armas, L.F.; Sarmiento, D.L.; Flórez-Daza, E.; Luna Sarmiento, D. Composición del género Centruroides Marx, 1980 (Scorpiones: Buthidae) en Colombia, con la descripción de una nueva especie. Boletín Soc. Entomológica Aragon. 2012, 50, 105–114. [Google Scholar]
- Perafán, C.; Sabogal-González, A.; Moreno-González, J.A.; García-Rincón, A.; Luna-Sarmiento, D.; Romero-Ortíz, C.; Flórez-Daza, E. Diagnóstico del estado actual de la fauna de arácnidos y de su gestión en Colombia. Memorias 40 Congr. Colomb. Entomol. SOCOLEN 2013, 308–335. [Google Scholar]
- Flórez-Daza, E. Escorpiones de la Familia Buthidae (Chelicerata: Scorpiones) de Colombia. Biota Colomb. 2001, 2, 25–30. [Google Scholar]
- Florez, E.; Sanchez, H. La Diversidad de Aracnidos en Colombia. Aproximación inicial. In Colombia Diversidad Biótica I; Rangel, O., Ed.; Universidad Nacional de Colombia—Instituto de Ciencias Naturales: Bogotá, Colombia, 1995; pp. 327–372. [Google Scholar]
- Rein, J.O. The Scorpion Files. Available online: http://www.ntnu.no/ub/scorpion-files/index.php (accessed on 1 December 2020).
- Lourenço, W.R. The evolution and distribution of noxious species of scorpions (Arachnida: Scorpiones). J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Ayerbe, S.G.; Buitrago, J.R. Accidente Aracnidico. In Guias Para el Manejo de Urgencias Toxicológicas; Correa, L.F., Berdejo, J.P., Mora, V.H., Sánchez Alarcón, D.M., Gutiérrez De Salazar, M., Eds.; Ministerio de Protección Social: Bogotá, Colombia, 2008; pp. 290–293. [Google Scholar]
- Gómez, J.P.; Quintana, J.C.; Arbeláez, P.; Fernández, J.; Silva, J.F.; Barona, J.; Gutiérrez, J.C.; Díaz, A.; Otero, R. Picaduras por escorpión Tityus asthenes en Mutatá, Colombia: Aspectos epidemiológicos, clínicos y toxinológicos. Biomédica 2010, 30, 126–139. [Google Scholar] [CrossRef] [Green Version]
- D’Suze, G.; Salazar, V.; Díaz, P.; Sevcik, C.; Azpurua, H.; Bracho, N. Histophatological changes and inflammatory response induced by Tityus discrepans scorpion venom in rams. Toxicon 2004, 44, 851–860. [Google Scholar] [CrossRef]
- Pineda, D.; Flórez, E. Picaduras de escorpión. In Accidente por Animales Venenoso; Instituto Nacional de Salud de Colombia: Bogotá, Colombia, 2002; p. 194. [Google Scholar]
- Otero, R.; Navio, E.; Céspedes, F.A.; Núñez, M.J.; Lozano, L.; Moscoso, E.R.; Matallana, C.; Arsuza, N.B.; García, J.; Fernández, D.; et al. Scorpion envenoming in two regions of Colombia: Clinical, epidemiological and therapeutic aspects. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 742–750. [Google Scholar] [CrossRef]
- Cesaretli, Y.; Ozkan, O. Scorpion stings in Turkey: Epidemiological and Clinical aspects between the years 1995 and 2004. Rev. Inst. Med. Trop. 2010, 52, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Barona, J.; Otero, R.; Núñez, V. Aspectos toxinológicos e inmunoquímicos del veneno del escorpión Tityus pachyurus Pocock de Colombia: Capacidad neutralizante de antivenenos producidos en Latinoamérica. Biomédica 2004, 24, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, L.M.; Rodríguez Buitrago, J.R. Cardiovascular dysfunction and pulmonary edema secondary to severe envenoming by Tityus pachyurus sting. Case report. Toxicon 2012, 60, 603–606. [Google Scholar] [CrossRef]
- Guerrero-Vargas, J.A.; Ayarbe, S.; Rada-Mendoza, M.; Vélez, P.; D’Suze, G. Estandarización de la extracción de veneno del escorpión Centruroides margaritatus (Scorpionida: Buthidae), del municipio del Patía, determinación de la DL-50. In Proceedings of the XXX Congreso Nacional de la Sociedad de Entomología, Cali, Colombia, 17–19 June 2003; p. 66. [Google Scholar]
- Morales-Duque, H. Determinación de la Actividad Neurotóxica y Antimicrobiana del Veneno del Escorpión Tityus sp. (Buthidæ); Del Município de Popayán: Popayán, Colombia, 2011.
- Morales Duque, H. Caracterização de Novos Peptidos Bloqueadores de Cananis Para K+ Isolados da Peçonha do Escopião Tityus sp.; Universidade de Brasilia: Brasilia, Brazil, 2013. [Google Scholar]
- Ayerbe, S.; Rodríguez, J.R. Accidente Escorpiónico. In Guias para el Manejo de Urgencias Toxinológicas; Correa, L.F., Berdejo, J.P., Mora, V.H., Sánchez, D.M., Gutiérrez, M., Eds.; Ministerio de la Proteccion Social: Bogota, Colombia, 2008; p. 294. [Google Scholar]
- De Girolami, U.; Anthony, D.; Frosch, M. El Sitema Nervioso Central. In Patologia Estructural y funcional; Cotran, R., Kumar, V., Robins, S., Eds.; Mc Graw-Hill Interamericana: Mexico City, México, 2000; pp. 1339–1424. [Google Scholar]
- Schoen, F. El Corazón. In Patologia Estructural y Funcional; Cotran, R., Kumar, V., Robins, S., Eds.; Mc Graw-Hill Interamericana: Mexico City, México, 2000; pp. 571–629. [Google Scholar]
- Valderrama, R. Envenenamiento por picadura de escorpiones. Prim. Simp. Colomb. Toxinología toxinas y envenenamiento por Anim. plantas Microorg. 1998, 169–178. [Google Scholar]
- Konzik, L. Pulmón. In Pastología Estructural y Funcional; Cotran, R., Kumar, V., Robins, S., Eds.; Mc Graw-Hill Interamericana: Mexico City, México, 2000; pp. 727–788. [Google Scholar]
- Clot-Faybesse, O.; Guieu, R.; Rochat, H.; Devaux, C. Toxicity during early development of the mouse nervous system of a scorpion neurotoxin active on sodium channels. Life Sci. 1999, 66, 185–192. [Google Scholar] [CrossRef]
- Nunan, E.A.; Moraes, M.F.D.; Cardoso, V.N.; Moraes-Santos, T. Effect of age on body distribution of Tityustoxin from Tityus serrulatus scorpion venom in rats. Life Sci. 2003, 73, 319–325. [Google Scholar] [CrossRef]
- Nencioni, A.L.A.; Lourenço, G.A.; Lebrun, I.; Florio, J.C.; Dorce, V.A.C. Central effects of Tityus serrulatus and Tityus bahiensis scorpion venoms after intraperitoneal injection in rats. Neurosci. Lett. 2009, 463, 234–238. [Google Scholar] [CrossRef]
- Cupo, P.; Azevedo-Marques, M.M.; Hering, S.E. Escorpionismo. In Animais Peçonhentos No Brasil. Biologia, Clínica e Terapêutica dos Acidentes; Cardoso, J.L.C., França, F.O.S., Wen, F.H., Málaque, C.M.S., Haddad, V., Jr., Eds.; Sarver; FAPESP: São Paulo, Brazil, 2003; pp. 198–208. [Google Scholar]
- Yatani, A.; Kirsch, G.E.; Possani, L.D.; Brown, A.M. Effects of New World scorpion toxins on single-channel and whole cell cardiac sodium currents. Am. J. Physiol. Hear. Circ. Physiol. 1988, 254, 443–451. [Google Scholar] [CrossRef]
- Chen, H.; Heinemann, S.H. Interaction of scorpion α-toxins with cardiac sodium channels: Binding properties and enhancement of slow inactivation. J. Gen. Physiol. 2001, 117, 505–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, S.; Zoukimian, C.; Meudal, H.; De Waard, S.; Ait Ouares, K.; Canepari, M.; Beroud, R.; Landon, C.; De Waard, M.; Boturyn, D. In vitro and in vivo characterization of asynthetic scorpion toxin AmmTx3, a potentinhibitor of cardiac voltage-gated potassiumchannel Kv4.2. Arch. Cardiovasc. Dis. Suppl. 2019, 11, 259–260. [Google Scholar]
- Dehesa-Dávila, M.; Possani, L.D. Scorpionism and serotherapy in Mexico. Toxicon 1994, 32, 1015–1018. [Google Scholar] [CrossRef]
- Bolaños, C.; Beltran, J.T.; Guerrero-Vargas, J.A. Cardiotoxic evaluation of the Tityus sp. Scorpion venom on Rattus norvegicus. (Wistar rats). In Proceedings of the XI Congress of the Pan-American Section of the International Society on Toxinology, Guaruya Sao Pablo, Brasil, 3–8 November 2013. p. Poster. [Google Scholar]
- Otero, R.; Uribe, F.; Sierra, A. Envenenamiento escorpiónico en niños. Actual. Pediátr 1998, 8, 88–92. [Google Scholar]
- Beltran, J.T.; Ayerbe, A.; Arenas, C.; Segura, B.; Torres, M.; Hurtado, F.; Morales, H.; Tobar, Y.; Coral, R. Evaluación Cardiotóxica del Veneno del escorpion Tityus pachyurus (Pocock, 1897), en ratas Rattus norvegicus. In Proceedings of the VIII simposio de Investigaciones en Ciencias Biológicas, Popayán, Colombia, 13–15 September 2011. [Google Scholar]
- De Oliveira, N.A.; Cardoso, S.C.; Barbosa, D.A.; da Fonseca, C.D. Acute kidney injury caused by venomous animals: In-flammatory mechanisms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, 1–12. [Google Scholar] [CrossRef]
- Saidani, C.; Béchohra, L.; Laraba-Djebari, F.; Hammoudi-Triki, D. Kidney inflammation and tissue injury induced by scorpion venom: Comparison with a nephrotoxic model. Toxin Rev. 2019, 38, 240–247. [Google Scholar] [CrossRef]
- El Hidan, M.A.; Touloun, O.; El Hiba, O.; Boumezzough, A. Pathophysiological and neurobehavioral injuries in mice experimentally envenomed with Androctonus liouvillei (Pallary, 1928) scorpion venom. Exp. Toxicol. Pathol. 2016, 68, 133–141. [Google Scholar] [CrossRef]
- Portillo, A.; Sojo, I.; Zerpa, J. Alteraciones Histopatológicas Causadas por el Veneno del Escorpión Tityus Caripitensis (Familia: Buthidae) sobre Hígado y riñón de Ratón; Escuela de Medicina, Universidad de Oriente: Barcelona, Brazil, 1996. [Google Scholar]
- Galíndez-Cerón, J.D.; Jorge, R.J.B.; Chavez-Acosta, M.H.; Jorge, A.R.C.; Alves, N.T.Q.; Prata, M.M.G.; Rodrigues, F.A.; Havt, A.; Sampaio, T.L.; Martins, A.M.C.; et al. Renal Alterations Induced by the Venom of Colombian Scorpion Centruroides Margaritatus. Curr. Top. Med. Chem. 2019, 19, 2049–2057. [Google Scholar] [CrossRef]
- El Hidan, M.A.; Touloun, O.; El Hiba, O.; Chait, A.; Eddine Hafid, J.; Boumezzough, A. Behavioral, histopathological and biochemical impairments observed in mice envenomed by the scorpion: Hottentota gentili (Pallary, 1924). Toxicon 2015, 103, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Salomón, L. Cambios Histopatológicos Agudos Causados por el Veneno de Tityus Nororientalis (Scorpiones, Buthidae) en Riñones de Ratones NMRI, BALBc y C57BL/6.; Universidad de Oriente: Barcelona, Brazil, 2009. [Google Scholar]
- Alves, R.D.S.; Falcão Do Nascimento, N.R.; Ferreira Barbosa, P.S.; Kerntopf, M.R.; Abreu Lessa, L.M.; De Sousa, C.M.; Martins, R.D.; Sousa, D.F.; Rodrigues De Queiroz, M.G.; Toyama, M.H.; et al. Renal effects and vascular reactivity induced by Tityus serrulatus venom. Toxicon 2005, 46, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Abd-Elsalam, M.A. Are the toxicological effects of scorpion envenomation related to tissue venom concentration? Toxicon 1988, 26, 233–256. [Google Scholar] [CrossRef]
- Murugesan, S.; Radha Krishna Murthy, K.; Noronha, O.P.D.; Samuel, A.M. Tc 99m—Scorpion Venom: Labelling, Biodistribution and Scintiimaging. J. Venom. Anim. Toxins 1999, 5, 35–46. [Google Scholar] [CrossRef]
- Ait Laaradia, M.; El Hidan, M.A.; Marhoume, F.; Bouimeja, B.; Oufquir, S.; Sokar, Z.; Boumezzough, A.; Chait, A. Buthus lienhardi venom and pathophysiological effects at the histological, hematological, biochemical and motor skills levels. Toxicon 2018, 146, 106–113. [Google Scholar] [CrossRef]
- El-Asmar, M.F.; Soliman, S.F.; Ismail, M.; Osman, O.H. Glycemic effect of venom from the scorpion Buthus minax (L. Koch). Toxicon 1974, 12, 249–251. [Google Scholar] [CrossRef]
- Muhammad, S. The acute effects of scorpion (Leiurus quinquestriatus) venom on some clinicalpathological parameters in Guinea pigs Muhammad. J. Am. Sci. 2011, 7, 794–801. [Google Scholar]
- Ghersy de Nieto, M.T.; D’Suze, G.; Sevcik, C.; Salazar, V.; Silva, V.; Urbina, H.; Pardo, R. Diabetes inducida por emponzoñamiento escorpiónico grave en un lactante de un año. Medicrit 2004, 16, 14–19. [Google Scholar]
- Gordillo, M. Escorpionismo en Pediatría. Arch. Argent. Pediatr 2000, 98, 296–303. [Google Scholar]
- Bacon, F. Apararto Digestivo. In Histología; Greneser, F., Ed.; Médica Panamericana: Buenos Aires, Argentina, 2001; pp. 465–534. [Google Scholar]
- Babloul, M.; Kallel, H.; Rekik, N.; Hamida, C.; Chelly, H.; Bouaziz, M. Atteinte cardiovasculaire lors d’envenimation scorpionique grave: Mécanismes et physiopathologie. Press. Med. 2005, 34, 115–120. [Google Scholar] [CrossRef]
- De Roodt, A.R. Veneno de escorpiones (alacranes) y envenenamiento. Acta Bioquímica Clínica Lat. 2015, 49, 55–71. [Google Scholar]
- Murthy, K.R.K.; Rao, R.P.; Natu, V.S.; Kumar, Z.N.; Lavanya, M. Suppressed Insulin Secretion, Elevated Mediators of Inflammation, Hyper-Insulinemia-Insulin Resistance: Insulin Administration Reverses Cardiovascular, Metabolic Changes, Pulmonary Edema and All Other Clinical Manifestations in Scorpion Envenoming Syndrom. Indian J. Mednodent Allied Sci. 2015, 3, 90. [Google Scholar] [CrossRef]
- Murthy, K.R.K.; Hase, N.K. Scorpion envenoming and the role of insulin. Toxicon 1994, 32, 1041–1044. [Google Scholar] [CrossRef]
- D’Suze, G.; Moncada, S.; González, C.; Sevcik, C.; Aguilar, V.; Alagón, A. Relationship between plasmatic levels of various cytokines, tumour necrosis factor, enzymes, glucose and venom concentration following Tityus scorpion sting. Toxicon 2003, 41, 367–375. [Google Scholar] [CrossRef]
- Mazzei, C.A.; Dàvila, D.F.; Donis, J.H.; Arata, G.; Villarreal, V.; Barboza, J.S.; Mazzei de Dàvila, C.A.; Dàvila, D.F.; Donis, J.H.; De Bellabarba, G.A.; et al. Sympathetic nervous system activation, antivenin administration and cardiovascular manifestations of scorpion envenomation. Toxicon 2002, 40, 1339–1346. [Google Scholar] [CrossRef]
- Sofer, S.; Gueron, M.; White, R.M.; Lifshitz, M.; Apte, R.N. Interleukin-6 release following scorpion sting in children. Toxicon 1996, 34, 389–392. [Google Scholar] [CrossRef]
- Ismail, M. The scorpion envenoming syndrome. Toxicon 1995, 33, 825–858. [Google Scholar] [CrossRef]
- De la Vega, R.C.; Possani, L.D. Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure–function relationships and evolution. Toxicon 2005, 46, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.; Possani, L.D. Scorpion venom components that affect ion-channels function. Toxicon 2013, 15, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Carmo, A.O.; Oliveira-Mendes, B.B.R.R.; Horta, C.C.R.R.; Magalhães, B.F.; Dantas, A.E.; Chaves, L.M.; Chavez-Olortegui, C.; Kalapothakis, E.; Chávez-Olórtegui, C.; Kalapothakis, E.; et al. Molecular and functional characterization of metalloserrulases, new metalloproteases from the Tityus serrulatus venom gland. Toxicon 2014, 90, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.; Rendón-Anaya, M.; Rego, S.C.; Schwartz, E.F.; Possani, L.D. Antarease-like Zn-metalloproteases are ubiquitous in the venom of different scorpion genera. Biochim. Biophys. Acta (BBA)-General Subj. 2014, 1840, 1738–1746. [Google Scholar] [CrossRef]
- Fletcher, P.L.; Fletcher, M.D.; Weninger, K.; Anderson, T.E.; Martin, B.M. Vesicle-associated membrane protein (VAMP) cleavage by a new metalloprotease from the Brazilian scorpion Tityus serrulatus. J. Biol. Chem. 2010, 285, 7405–7416. [Google Scholar] [PubMed] [Green Version]
- Zamudio, F.Z.; Conde, R.; Arévalo, C.; Becerril, B.; Martin, B.M.; Valdivia, H.H.; Possani, L.D. The mechanism of inhibition of ryanodine receptor channels by imperatoxin I, a heterodimeric protein from the scorpion Pandinus imperator. J. Biol. Chem. 1997, 272, 11886–11894. [Google Scholar] [CrossRef] [Green Version]
- Conde, R.; Zamudio, F.Z.; Becerril, B.; Possani, L.D. Phospholipin, a novel heterodimeric phospholipase A2 from Pandinus imperator scorpion venom. FEBS Lett. 1999, 460, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Valdez-Cruz, N.A.; Batista, C.V.F.; Possani, L.D. Phaiodactylipin, a glycosylated heterodimeric phospholipase A2 from the venom of the scorpion Anuroctonus phaiodactylus. Eur. J. Biochem. 2004, 271, 1453–1464. [Google Scholar] [CrossRef]
- Romero-Imbachi, M.R.; Cupitra, N.; Ángel, K.; González, B.; Estrada, O.; Calderón, J.C.; Guerrero-Vargas, J.; Beltrán, J.; Narvaez-Sanchez, R. Centruroides margaritatus scorpion complete venom exerts cardiovascular effects through alpha-1 adrenergic receptors. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108939. [Google Scholar] [CrossRef]
- Beltrán-Vidal, J.; Carcamo-Noriega, E.; Pastor, N.; Zamudio-Zuñiga, F.; Guerrero-Vargas, J.A.; Castaño, S.; Possani, L.D.; Restano-Cassulini, R. Colombian Scorpion Centruroides margaritatus: Purification and Characterization of a Gamma Potassium Toxin with Full-Block Activity on the hERG1 Channel. Toxins 2021, 13, 407. [Google Scholar] [CrossRef]
- Barona, J.; Batista, C.V.F.; Zamudio, F.Z.; Gomez-Lagunas, F.; Wanke, E.; Otero, R.; Possani, L.D. Proteomic analysis of the venom and characterization of toxins specific for Na+-and K+-channels from the Colombian scorpion Tityus pachyurus. Biochim. Biophys. Acta (BBA)-Proteins Proteomics 2006, 1764, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Vargas, J.A.; Mourão, C.B.; Quintero-Hernández, V.; Possani, L.D.; Schwartz, E.F. Identification and phylogenetic analysis of Tityus pachyurus and Tityus obscurus novel putative Na-channel scorpion toxins. PLoS ONE 2012, 7, e30478. [Google Scholar]
- Bohórquez-Gómez, R.M. Revisión Taxonómica y Distribución Geográfica de las especies de Tityus, Subgénero Atreus, (Scorpiones, Buthidae) Presentes en Colombia; Universidad Nacional de Colombia: Bogotá, Colombia, 2016. [Google Scholar]
- Hamilton, M.A.; Russo, R.C.; Thurston, R.V. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977, 11, 714–719. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; Lacroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–452. [Google Scholar] [CrossRef]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice, 3th ed.; Dries, D.J., Ed.; Butterworth Heinemann: Oxford, UK, 1999; ISBN 9781907904325. [Google Scholar]
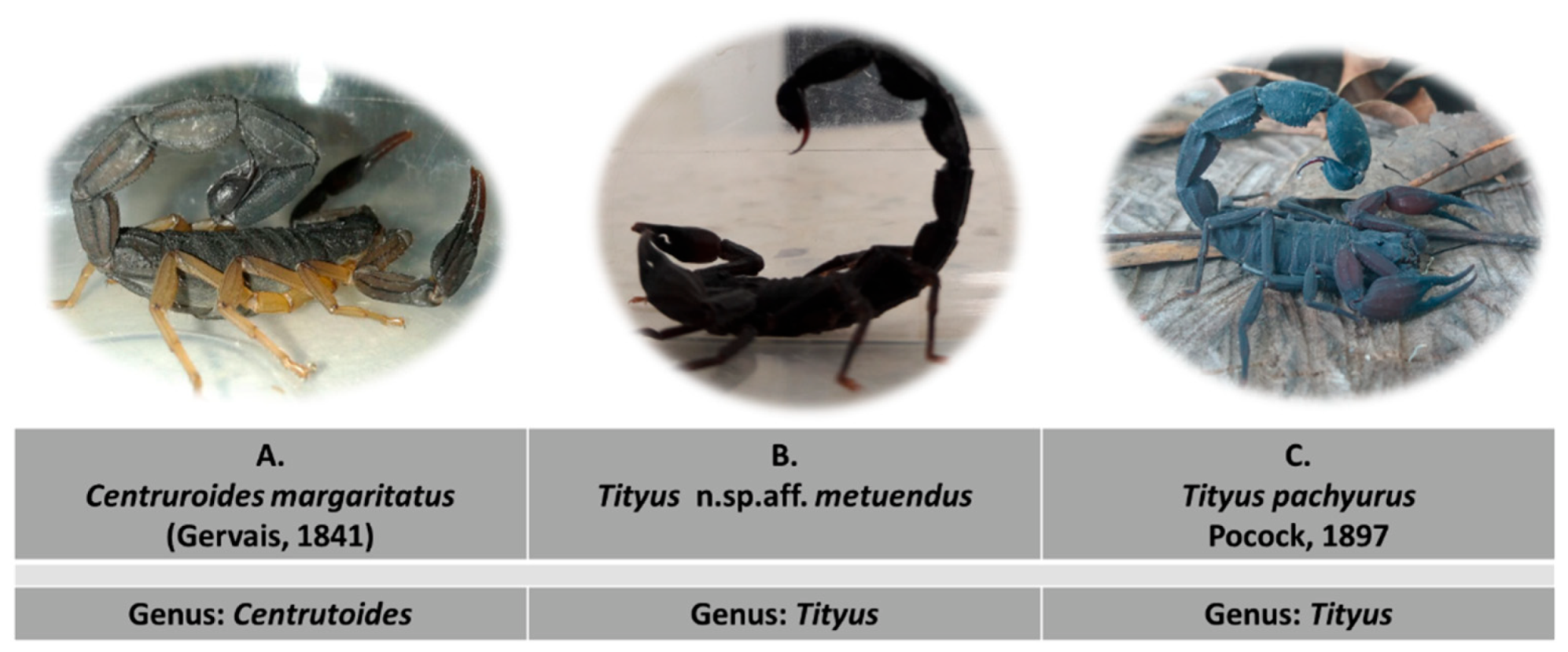







| Scorpion Specie | LD50 Total Venom (mg/kg) | LD50 Soluble Venom (mg/kg) | Administration |
|---|---|---|---|
| C. margaritatus | 19.56 | 42.8 [26] | Intraperitoneal |
| T. pachyurus | 2.92 | 4.8 [24] | Intraperitoneal |
| T. n. sp. aff metuendus | 1.02 | 3.5 [28] | Intraperitoneal |
| Centruroides margaritatus | Tityus pachyurus | T. n. sp. aff. metuendus |
|---|---|---|
| Piloerection | Piloerection | Piloerection |
| Priapism | Priapism | |
| Hypoactivity | Hypoactivity | Hypoactivity (70% LD50) |
| Tachypnea | Tachypnea | Tachypnea |
| Sialorrhea | Sialorrhea | |
| Epiphora (70% LD50) | Epiphora (70% LD50) | |
| Grimace–pain score: 2 Lordosis | Grimace -pain score: 2 Lordosis | Grimace pain score: 1 |
| Milky ocular secretion | Milky ocular secretion | |
| No Grooming | No Grooming | No Grooming (70% LD50) |
| Death (2–70% LD50) | ||
| Seizures | ||
| Fast limb shiverings (70% LD50) | Fast limb shiverings (70% LD50) | |
| Diarrhea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Tobar, L.L.; Meza-Cabrera, I.A.; Sepúlveda-Arias, J.C.; Guerrero-Vargas, J.A. Comparison of the Scorpionism Caused by Centruroides margaritatus, Tityus pachyurus and Tityus n. sp. aff. metuendus Scorpion Venoms in Colombia. Toxins 2021, 13, 757. https://doi.org/10.3390/toxins13110757
Mendoza-Tobar LL, Meza-Cabrera IA, Sepúlveda-Arias JC, Guerrero-Vargas JA. Comparison of the Scorpionism Caused by Centruroides margaritatus, Tityus pachyurus and Tityus n. sp. aff. metuendus Scorpion Venoms in Colombia. Toxins. 2021; 13(11):757. https://doi.org/10.3390/toxins13110757
Chicago/Turabian StyleMendoza-Tobar, Leydy Lorena, Ivonne Alejandra Meza-Cabrera, Juan C. Sepúlveda-Arias, and Jimmy Alexander Guerrero-Vargas. 2021. "Comparison of the Scorpionism Caused by Centruroides margaritatus, Tityus pachyurus and Tityus n. sp. aff. metuendus Scorpion Venoms in Colombia" Toxins 13, no. 11: 757. https://doi.org/10.3390/toxins13110757
APA StyleMendoza-Tobar, L. L., Meza-Cabrera, I. A., Sepúlveda-Arias, J. C., & Guerrero-Vargas, J. A. (2021). Comparison of the Scorpionism Caused by Centruroides margaritatus, Tityus pachyurus and Tityus n. sp. aff. metuendus Scorpion Venoms in Colombia. Toxins, 13(11), 757. https://doi.org/10.3390/toxins13110757






