Bee Venom Prevents Mucin 5AC Production through Inhibition of AKT and SPDEF Activation in Airway Epithelia Cells
Abstract
:1. Introduction
2. Results
2.1. Effect of BV on the Viability of A549 Cells
2.2. Effect of BV on the Phosphorylation of STAT6 in IL-13-Treated A549 Cells
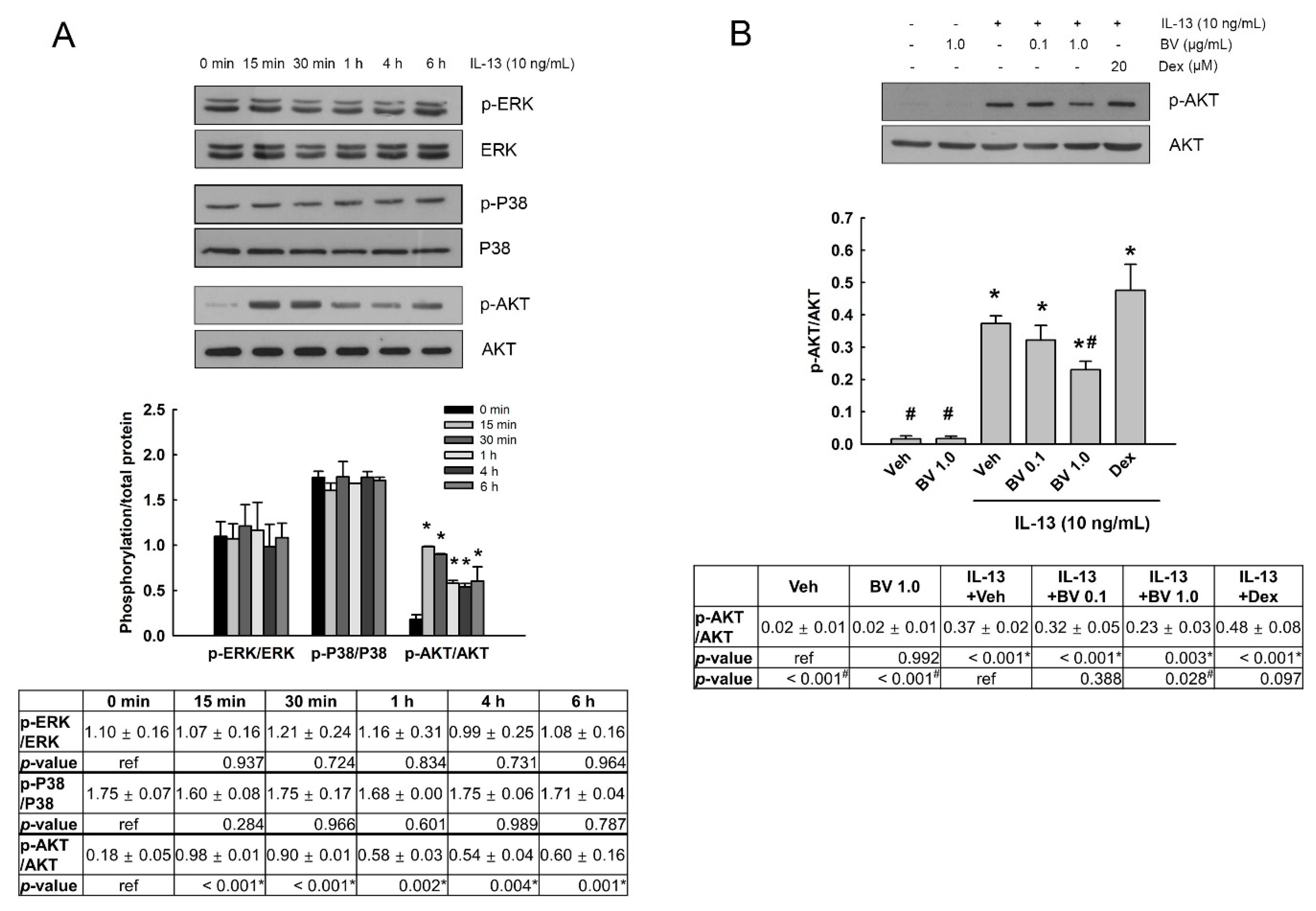
2.3. Effect of BV on the Phosphorylation of Mitogen-Activated Protein Kinases (MAPKs) and AKT in IL-13-Treated A549 Cells
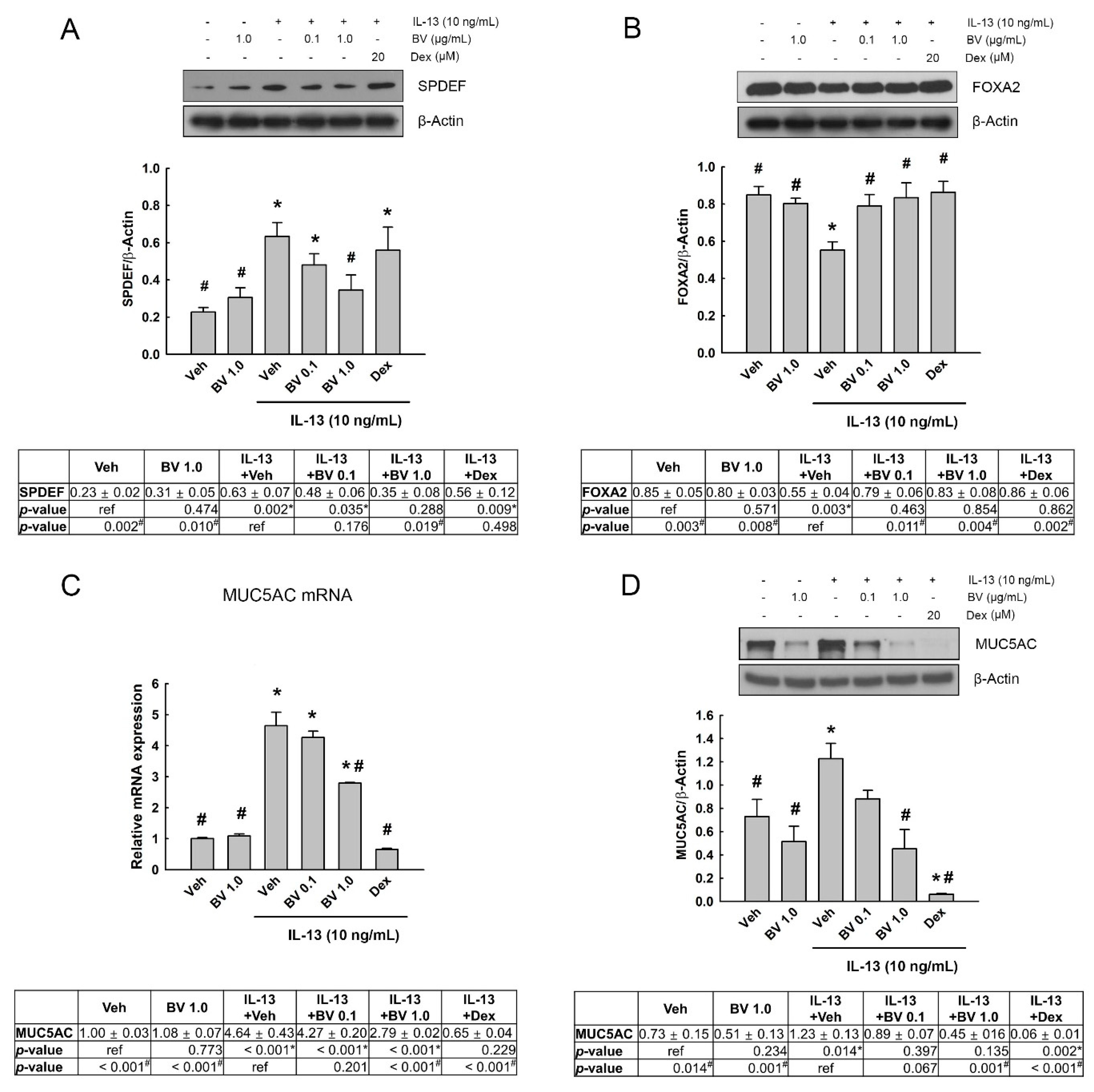
2.4. Effect of BV on the MUC5AC Expression in IL-13-Treated A549 Cells
2.5. Effect of Phosphoinositide 3-Kinase (PI3K)/AKT Inhibition on SPDEF, FOXA2 and MUC5AC Expressions in IL-13- and BV-Treated A549 Cells
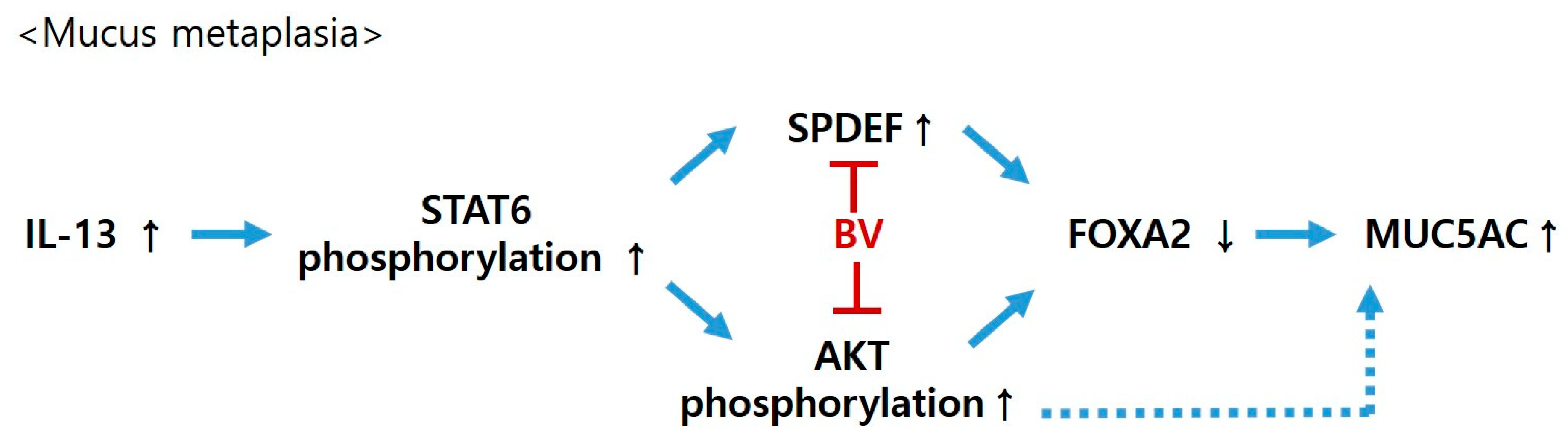
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture and Treatment
5.2. Cell Viability Assay
5.3. Western Blot Analysis
5.4. Quantitative Real-Time PCR (qRT-PCR)
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bousquet, J.; Jeffery, P.K.; Busse, W.W.; Johnson, M.; Vignola, A.M. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med. 2000, 161, 1720–1745. [Google Scholar] [CrossRef] [Green Version]
- Foster, P.S.; Maltby, S.; Rosenberg, H.F.; Tay, H.L.; Hogan, S.P.; Collison, A.M.; Yang, M.; Kaiko, G.E.; Hansbro, P.M.; Kumar, R.K.; et al. Modeling T(H) 2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol. Rev. 2017, 278, 20–40. [Google Scholar] [CrossRef]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef]
- Kuperman, D.A.; Schleimer, R.P. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Curr. Mol. Med. 2008, 8, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Erle, D.J.; Sheppard, D. The cell Biol.ogy of asthma. J. Cell Biol. 2014, 205, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Williams, O.W.; Sharafkhaneh, A.; Kim, V.; Dickey, B.F.; Evans, C.M. Airway mucus: From production to secretion. Am. J. Respir. Cell Mol. Biol. 2006, 34, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.M.; Williams, O.W.; Tuvim, M.J.; Nigam, R.; Mixides, G.P.; Blackburn, M.R.; DeMayo, F.J.; Burns, A.R.; Smith, C.; Reynolds, S.D.; et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol. 2004, 31, 382–394. [Google Scholar] [CrossRef] [Green Version]
- Pardo-Saganta, A.; Law, B.M.; Gonzalez-Celeiro, M.; Vinarsky, V.; Rajagopal, J. Ciliated cells of pseudostratified airway epithelium do not become mucous cells after ovalbumin challenge. Am. J. Respir. Cell Mol. Biol. 2013, 48, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Tyner, J.W.; Kim, E.Y.; Ide, K.; Pelletier, M.R.; Roswit, W.T.; Morton, J.D.; Battaile, J.T.; Patel, A.C.; Patterson, G.A.; Castro, M.; et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J. Clin. Investig. 2006, 116, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Park, S.W.; Nguyenvu, L.T.; Rodriguez, M.W.; Barbeau, R.; Paquet, A.C.; Erle, D.J. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am. J. Respir. Cell Mol. Biol. 2007, 36, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Koth, L.L.; Arron, J.R.; Fahy, J.V. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit Care Med. 2009, 180, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Atherton, H.C.; Jones, G.; Danahay, H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 285, L730–L739. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S.; Korfhagen, T.R.; Bruno, M.D.; Kitzmiller, J.A.; Wan, H.; Wert, S.E.; Khurana Hershey, G.K.; Chen, G.; Whitsett, J.A. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Investig. 2007, 117, 978–988. [Google Scholar] [CrossRef]
- Chen, G.; Korfhagen, T.R.; Xu, Y.; Kitzmiller, J.; Wert, S.E.; Maeda, Y.; Gregorieff, A.; Clevers, H.; Whitsett, J.A. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Investig. 2009, 119, 2914–2924. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Li, Q.; Kolosov, V.P.; Perelman, J.M.; Zhou, X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun. Adhes. 2010, 17, 83–92. [Google Scholar] [CrossRef]
- Wan, H.; Kaestner, K.H.; Ang, S.L.; Ikegami, M.; Finkelman, F.D.; Stahlman, M.T.; Fulkerson, P.C.; Rothenberg, M.E.; Whitsett, J.A. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 2004, 131, 953–964. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wan, H.; Luo, F.; Zhang, L.; Xu, Y.; Lewkowich, I.; Wills-Karp, M.; Whitsett, J.A. Foxa2 programs Th2 cell-mediated innate immunity in the developing lung. J. Immunol. 2010, 184, 6133–6141. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.B.; Lee, J.D.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Beitz, A.J.; Lee, J.H. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain 2001, 90, 271–280. [Google Scholar] [CrossRef]
- Lee, J.A.; Son, M.J.; Choi, J.; Jun, J.H.; Kim, J.I.; Lee, M.S. Bee venom acupuncture for rheumatoid arthritis: A systematic review of randomised clinical trials. BMJ Open 2014, 4, e006140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.R.; Lin, L.T.; Xiao, L.Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef]
- Lee, Y.M.; Cho, S.N.; Son, E.; Song, C.H.; Kim, D.S. Apamin from bee venom suppresses inflammation in a murine model of gouty arthritis. J. Ethnopharmacol. 2020, 257, 112860. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Anti-Inflammatory Applications of Melittin, a Major Component of Bee Venom: Detailed Mechanism of Action and Adverse Effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.Y.; Shin, J.; Hwang, J.T.; Jeon, H.N.; Bae, H. Dose-Dependent Neuroprotective Effect of Standardized Bee Venom Phospholipase A(2) Against MPTP-Induced Parkinson’s Disease in Mice. Front. Aging Neurosci. 2019, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.S.; Park, S.; Choi, T.; Lee, G.; Haam, K.K.; Hong, M.C.; Min, B.I.; Bae, H. Bee venom ameliorates ovalbumin induced allergic asthma via modulating CD4+CD25+ regulatory T cells in mice. Cytokine 2013, 61, 256–265. [Google Scholar] [CrossRef]
- Park, S.; Baek, H.; Jung, K.H.; Lee, G.; Lee, H.; Kang, G.H.; Lee, G.; Bae, H. Bee venom phospholipase A2 suppresses allergic airway inflammation in an ovalbumin-induced asthma model through the induction of regulatory T cells. Immun. Inflamm. Dis. 2015, 3, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Choi, W.; Bae, H. Bee Venom Phospholipase A2 Alleviate House Dust Mite-Induced Atopic Dermatitis-Like Skin Lesions by the CD206 Mannose Receptor. Toxins 2018, 10, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.H.; Baek, H.; Kang, M.; Kim, N.; Lee, S.Y.; Bae, H. Bee Venom Phospholipase A2 Ameliorates House Dust Mite Extract Induced Atopic Dermatitis Like Skin Lesions in Mice. Toxins 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Lee, S.J.; Park, J.Y.; Park, K.D.; Han, S.M.; Kim, M.K.; et al. Apamin inhibits TNF-α- and IFN-γ-induced inflammatory cytokines and chemokines via suppressions of NF-κB signaling pathway and STAT in human keratinocytes. Pharmacol. Rep. 2017, 69, 1030–1035. [Google Scholar] [CrossRef]
- Yoon, J.; Jeon, J.H.; Lee, Y.W.; Cho, C.K.; Kwon, K.R.; Shin, J.E.; Sagar, S.; Wong, R.; Yoo, H.S. Sweet bee venom pharmacopuncture for chemotherapy-induced peripheral neuropathy. J. Acupunct. Meridian. Stud. 2012, 5, 156–165. [Google Scholar] [CrossRef]
- Hartmann, A.; Müllner, J.; Meier, N.; Hesekamp, H.; van Meerbeeck, P.; Habert, M.O.; Kas, A.; Tanguy, M.L.; Mazmanian, M.; Oya, H.; et al. Bee Venom for the Treatment of Parkinson Disease—A Randomized Controlled Clinical Trial. PLoS ONE 2016, 11, e0158235. [Google Scholar] [CrossRef] [Green Version]
- Boucherat, O.; Boczkowski, J.; Jeannotte, L.; Delacourt, C. Cellular and molecular mechanisms of goblet cell metaplasia in the Respir.atory airways. Exp. Lung Res. 2013, 39, 207–216. [Google Scholar] [CrossRef]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, H.W.; Williams, O.W.; Chandra, D.; Bellinghausen, L.K.; Pérez, G.; Suárez, A.; Tuvim, M.J.; Roy, M.G.; Alexander, S.N.; Moghaddam, S.J.; et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5’ elements. Am. J. Respir. Cell Mol. Biol. 2007, 37, 273–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Shah, T.A.; Ustiyan, V.; Zhang, Y.; Shinn, J.; Chen, G.; Whitsett, J.A.; Kalin, T.V.; Kalinichenko, V.V. FOXM1 promotes allergen-induced goblet cell metaplasia and pulmonary inflammation. Mol. Cell Biol. 2013, 33, 371–386. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Li, W.; Zhou, H.; Wu, Y.; Ying, S.; Chen, Z.; Shen, H. Interleukin-13-induced MUC5AC expression is regulated by a PI3K-NFAT3 pathway in mouse tracheal epithelial cells. Biochem. Biophys. Res. Commun. 2014, 446, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Revuelta, P.; Madrigal-Burgaleta, R. Death due to Live Bee Acupuncture Apitherapy. J. Investig. Allergol. Clin. Immunol. 2018, 28, 45–46. [Google Scholar] [CrossRef] [Green Version]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, Y.W.; Kim, H.; Chung, D.K. Bee Venom Alleviates Atopic Dermatitis Symptoms through the Upregulation of Decay-Accelerating Factor (DAF/CD55). Toxins 2019, 11, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocyigit, A.; Guler, E.M.; Kaleli, S. Anti-inflammatory and antioxidative properties of honey bee venom on Freund’s Complete Adjuvant-induced arthritis model in rats. Toxicon 2019, 161, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Shim, S.R.; Rhee, H.Y.; Park, H.J.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Park, S.U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism. Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, Y.E.; Doo, K.H.; Lee, J.H.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Kim, H.; Rhee, H.Y.; et al. Efficacy of Combined Treatment with Acupuncture and Bee Venom Acupuncture as an Adjunctive Treatment for Parkinson’s Disease. J. Altern. Complement. Med. 2018, 24, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.-C.; Kong, J.C.; Park, T.-Y.; Yang, C.-Y.; Kang, K.-W.; Choi, S.-m. Bee venom acupuncture for chronic low back pain: A randomised, sham-controlled, triple-blind clinical trial. Eur. J. Integr. Med. 2012, 4, e271–e280. [Google Scholar] [CrossRef]
- Conrad, V.J.; Hazan, L.L.; Latorre, A.J.; Jakubowska, A.; Kim, C.M. Efficacy and safety of honey bee venom (Apis mellifera) dermal injections to treat osteoarthritis knee pain and physical disability: A randomized controlled trial. J. Altern. Complementary Med. 2019, 25, 845–855. [Google Scholar] [CrossRef]
- Choi, H.S.; Kang, S.Y.; Roh, D.H.; Choi, S.R.; Ryu, Y.; Lee, J.H. Bee venom stimulation of a lung meridian acupoint reduces inflammation in carrageenan-induced pleurisy: An alternative therapeutic approach for Respir.atory inflammation. J. Vet. Sci 2018, 19, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Jun, H.S.; Jeon, J.W.; Park, J.K.; Lee, B.J.; Suh, G.H.; Park, J.S.; Cho, C.W. Preparation and characterization of bee venom-loaded PLGA particles for sustained release. Pharm. Dev. Technol. 2018, 23, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.M.; Kim, J.H.; Cho, C.W.; Jeon, J.W.; Park, J.K.; Lee, S.H.; Jung, B.G.; Lee, B.J. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and Respir.atory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunopathol. 2018, 200, 40–51. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, H.-W.; Chang, S.-H.; Leem, K.-H.; Park, H.-J. Bee Venom Prevents Mucin 5AC Production through Inhibition of AKT and SPDEF Activation in Airway Epithelia Cells. Toxins 2021, 13, 773. https://doi.org/10.3390/toxins13110773
Kim S, Kim H-W, Chang S-H, Leem K-H, Park H-J. Bee Venom Prevents Mucin 5AC Production through Inhibition of AKT and SPDEF Activation in Airway Epithelia Cells. Toxins. 2021; 13(11):773. https://doi.org/10.3390/toxins13110773
Chicago/Turabian StyleKim, Sanga, Hee-Won Kim, Seok-Hwan Chang, Kang-Hyun Leem, and Hae-Jeong Park. 2021. "Bee Venom Prevents Mucin 5AC Production through Inhibition of AKT and SPDEF Activation in Airway Epithelia Cells" Toxins 13, no. 11: 773. https://doi.org/10.3390/toxins13110773
APA StyleKim, S., Kim, H.-W., Chang, S.-H., Leem, K.-H., & Park, H.-J. (2021). Bee Venom Prevents Mucin 5AC Production through Inhibition of AKT and SPDEF Activation in Airway Epithelia Cells. Toxins, 13(11), 773. https://doi.org/10.3390/toxins13110773




