Modulation of Adhesion Molecules Expression by Different Metalloproteases Isolated from Bothrops Snakes
Abstract
:1. Introduction
2. Results
2.1. Expression of the ICAM-1 and PECAM-1 Proteins in the Cremaster Muscle
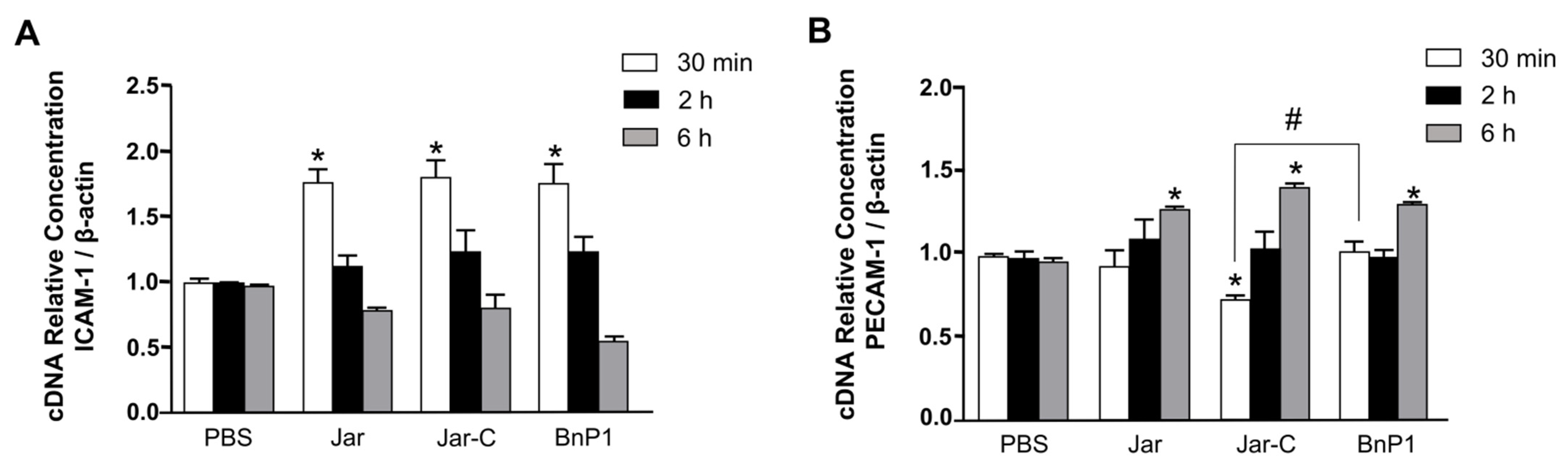
2.2. Expression of mRNAs Encoding the Adhesion Molecules ICAM-1 and PECAM-1 in the Cremaster Muscle of Mice
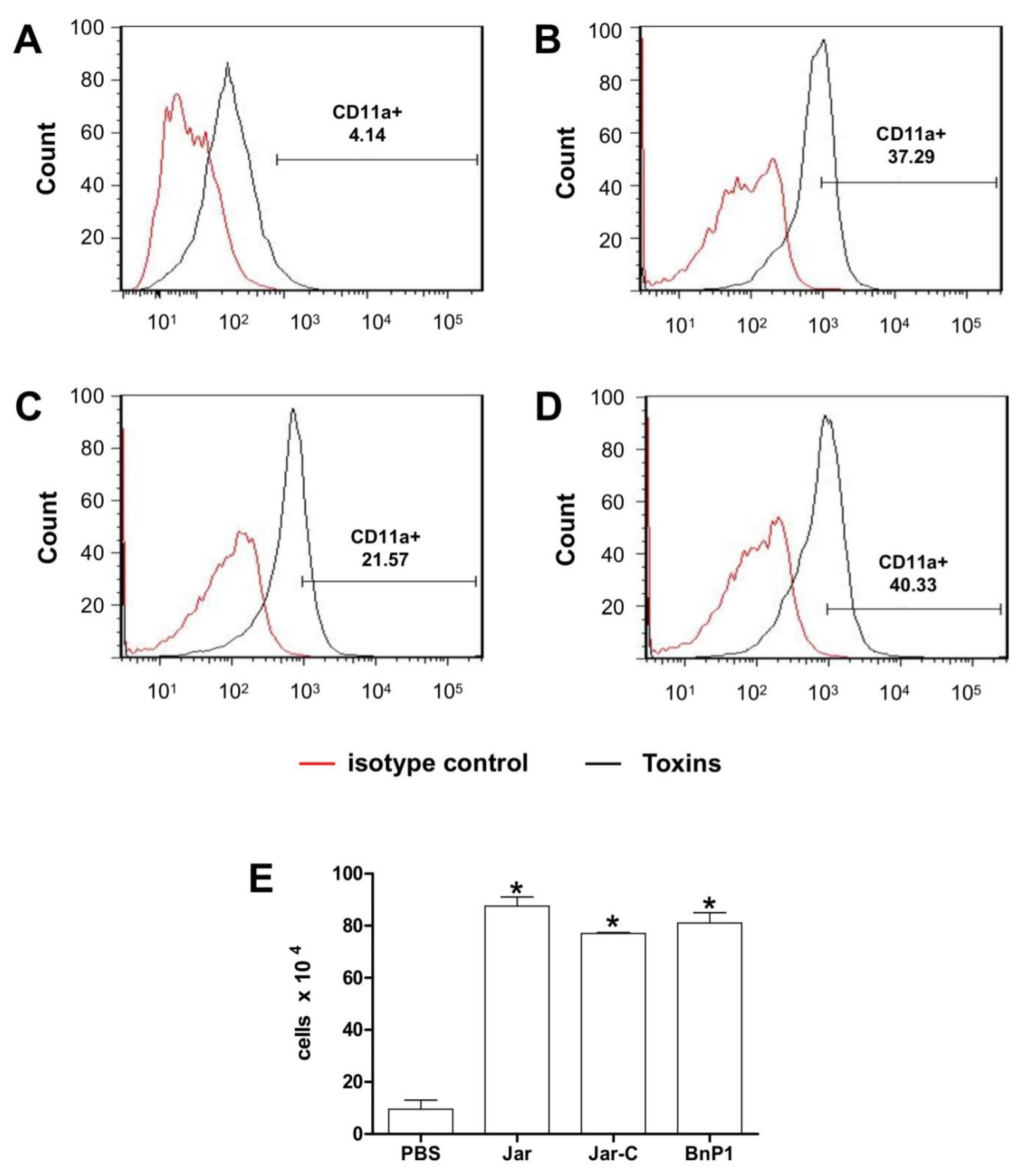
2.3. Expression of CD11a and CD11b on the Surface of Leukocytes Present in the Peritoneal Exudates from Mice Injected with Different Toxins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Toxins
5.3. Expression of Adhesion Molecules
5.3.1. Evaluation of Protein Expression in Endothelial Cells of the Cremaster Muscle Using Immunohistochemistry
5.3.2. Evaluation of Protein Expression in Endothelial Cells of the Cremaster Muscle Using Indirect ELISA Assays
5.4. Real-Time PCR Analysis of Gene Expression in Endothelial Cells of the Cremaster Muscle
5.5. Integrin Expression in Peritoneal Leucocytes
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Seventy-First World Health Assembly Resolution WHA71.5 on Addressing the Burden of Snakebite Envenoming; WHO: Geneva, Switzerland, 2018; Volume 1, pp. 24–26. Available online: http://apps.who.int/gb/ebwha/pdf_files/EB142/B142_R4-en.pdf (accessed on 17 October 2021).
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.; De Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministério da Saúde do Brasil. Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos; FUNASA: Brasilia, Brazil, 2001; p. 112. [Google Scholar]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0140673609617542 (accessed on 17 October 2021). [CrossRef]
- Moura-da Silva, A.M.; Laing, G.D.; Paine, M.J.; Dennison, J.M.; Politi, V.; Cramptom, J.M.; Theakston, R.D. Processing of pro-tumor necrosis factor-alpha by venom metalloproteinase: A hypothesis explaining local tissue damage follwing snakebite. Eur. J. Immunol. 1996, 26, 2000–2005. [Google Scholar] [CrossRef]
- Zychar, B.C.; Dale, C.S.; Demarchi, D.S.; Gonçalves, L.R.C. Contribution of metalloproteases, serine proteases and phospholipases A2 to the inflammatory reaction induced by Bothrops jararaca crude venom in mice. Toxicon 2010, 55, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zychar, B.C.; Clissa, P.B.; Carvalho, E.; Baldo, C.; Gonçalves, L.R.C. Leukocyte recruitment induced by snake venom metalloproteinases: Role of the catalytic domain. Biochem. Biophys. Res. Commun. 2020, 521, 402–407. [Google Scholar] [CrossRef]
- Cidade, D.A.; Simão, T.A.; Dávila, A.; Wagner, G.; Junqueira-De-Azevedo, I.D.L.; Ho, P.L.; Bon, C.; Zingali, R.B.; Albano, R.M. Bothrops jararaca venom gland transcriptome: Analysis of the gene expression pattern. Toxicon 2006, 48, 437–461. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef]
- Mandelbaum, F.R.; Reichel, A.P.; Assakura, M.T. Isolation and characterization of a proteolytic enzyme from the venom of the snake Bothrops jararaca (Jararaca). Toxicon 1982, 20, 955–972. [Google Scholar] [CrossRef]
- Paine, M.J.; Desmond, H.P.; Theakston, R.G.D.; Crampton, J.M. Purification, cloning and molecular characterization of high molecular weight hemorrhagic metalloproteinase, jararhagin, from Bothrops jararaca venom. J. Biol. Chem. 1992, 267, 22869–22876. [Google Scholar] [CrossRef]
- Maruyama, M.; Tanigawa, M.; Sugiki, M.; Yoshida, E.; Mihara, H. Purification and characterization of low molecular weight fibrinolytic/hemorrhagic enzymes from snake (Bothrops jararaca) venom. Enzyme Protein. 1993, 47, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.; Kim, J.; Wang, D.; Dragulev, B.; Shannon, J.D.; Mann, H.H.; Veit, G.; Wagener, R.; Koch, M.; Fox, J.W. The cysteine-rich domain of snake venom metalloproteinases is a ligand for von Willebrand factor a domains: Role in substrate targeting. J. Biol. Chem. 2006, 281, 39746–39756. [Google Scholar] [CrossRef] [Green Version]
- Moura-Da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of snake venom metalloproteinase in cell biology: Effects on platelets, inflammatory and endothelial cell. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Tanjoni, I.; Evangelista, K.; Della Casa, M.S.; Butera, D.; Magalhães, G.S.; Baldo, C.; Clissa, P.B.; Fernandes, I.; Eble, J.; Moura-Da-Silva, A.M. Different regions of the class PIII snake venom metalloproteinase jararhagin are involved in binding to α2β1 integrin and collagen. Toxicon 2010, 55, 1093–1099. [Google Scholar] [CrossRef]
- Moura-da Silva, A.M.; Della-Casa, M.S.; David, A.S.; Assakura, M.T.; Butera, D.; Lebrun, I.; Shannon, J.D.; Serrano, S.M.T.; Fox, J.W. Evidence of heterogeneous forms of the snake venom metalloproteinase jararhagin: A factor contributing to snake venom variability. Arch. Bioch. Biophys. 2003, 409, 395. [Google Scholar] [CrossRef]
- Clissa, P.B.; Lopes-Ferreira, M.; Della-Casa, M.S.; Farsky, S.H.P.; Moura-da Silva, A.M. Importance of jararhagin disintegrin-like and cysteine-rich domains in the early events of local inflammatory response. Toxicon 2006, 47, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.D.M.; Soares, A.; Guerra-Sá, R.; Rodrigues, V.; Fontes, M.; Giglio, J.R. Structural and functional characterization of neuwidase, anon-hemorrhagic fibrin (ogen)olyitc metalloprotease from Bothrops neuwiedi snake venom. Arch. Bioch. Biophys. 2000, 381, 213–224. [Google Scholar] [CrossRef]
- Baldo, C.; Tanjoni, I.; León, I.; Batista, I.; Della-Casa, M.; Clissa, P.; Weinlich, R.; Lopes-Ferreira, M.; Lebrun, I.; Amarante-Mendes, G.P.; et al. BnP1, a novel P-I metalloproteinase from Bothrops neuwiedi venom: Biological effects benchmarking relatively to jararhagin, a P-III SVMP. Toxicon 2008, 51, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Hermant, B.; Bibert, S.; Concord, E.; Dublet, B.; Weidenhaupt, M.; Vernet, T.; Gulino-Debrac, D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J. Biol. Chem. 2003, 18, 14002–14012. [Google Scholar] [CrossRef]
- Nolte, D.; Kuebler, W.M.; Muller, W.A.; Wolff, K.D.; Messmer, K. Attenuation of leukocyte sequestration by selective blockade of PECAM-1 or VCAM-1 in murine endotoxemia. Eur. Surg. Res. 2004, 26, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Biol. 2008, 121, 2115–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V.; Springer, T.A. A Transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 2004, 167, 377–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, E.C. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell 1991, 67, 1033–1036. [Google Scholar] [CrossRef]
- Rose, D.M.; Alon, R.; Ginsberg, M.H. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev. 2007, 218, 126–134. [Google Scholar] [CrossRef]
- Baldo, C.; Jamora, C.; Yamanouye, N.; Zorn, T.M.; Moura-da-Silva, A.M. Mechanisms of vascular damage by hemorrhagic snake venom metalloproteinases: Tissue distribution and in situ hydrolysis. PLoS Negl. Trop. Dis. 2010, 4, e727. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.; Voisin, M.B.; Escalante, T.; Rucavado, A.; Nourshargh, S.; Gutierrez, J.M. Effects of PI and PIII Snake Venom Haemorrhagic Metalloproteinases on the Microvasculature: A Confocal Microscopy Study on the Mouse Cremaster Muscle. PLoS ONE 2016, 11, e0168643. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Zamuner, S.R.; Zuliani, J.P.; Rucavado, A.; Gutiérrez, J.M.; Teixeira, C.F.P. Inflammatory effects of BaP1 a metalloproteinase isolated from Bothrops asper snake venom: Leukocyte recruitment and release of cytokines. Toxicon 2006, 47, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.R.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef]
- Moreira, V.; Teixeira, C.; Borges da Silva, H.; D’Império Lima, M.R.; Dos-Santos, M.C. The role of TLR2 in the acute inflammatory response induced by Bothrops atrox snake venom. Toxicon 2016, 118, 121–128. [Google Scholar] [CrossRef]
- Zamunér, R.S.; Zuliani, J.P.; Fernandes, C.M.; Gutiérrez, J.M.; Teixeira, F.P.C. Inflammation induced by Bothrops asper venom: Release of proinflammatory cytokines and eicosanoids, and role of adhesion molecules in leukocyte infiltration. Toxicon 2005, 46, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Rucavado, A.; Escalante, T.; Herrera, C.; Fernandez, J.; Lomonte, B.; Fox, J.W. Unresolved issues in the understanding of the pathogenesis of local tissue damage induced by snake venoms. Toxicon 2018, 148, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Farsky, S.H.; Goncalves, L.R.C.; Cury, Y. Characterization of local tissue damage evoked by Bothrops jararaca venom in the rat connective tissue microcirculation: An intravital microscopic study. Toxicon 1999, 37, 1079–1083. [Google Scholar] [CrossRef]
- Zychar, B.C.; Castro, N.C., Jr.; Marcelino, J.R.; Gonçalves, L.R.C. Phenol used as a preservative in Bothrops antivenom induces impairment leukocyte-endothelium alteraction. Toxicon 2008, 51, 1151–1157. [Google Scholar] [CrossRef]
- Peichoto, M.C.; Zychar, B.C.; Tavares, F.L.; Gonçalves, L.R.C.; Acosta, O.; Santoro, M.L. Inflammatory effects of patagonfibrase, a metalloproteinase from Philodryas patagoniensis (Patagonia Green Racer; Dipsadidae) venom. Exp. Biol. Med. 2011, 236, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.C.; Paes Leme, A.F.; Melo, R.L.; Silva, C.A.; Della Casa, M.; Bruni, F.M.; Lima, C.; Lopes-Ferreira, M.; Camargo, A.C.M.; Fox, J.W.; et al. Activation of leukocyte rolling by the cysteine-rich domain and the hyper-variable region of HF3, a snake venom hemorrhagic metalloproteinase. FEBS Lett. 2008, 582, 3915–3921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, eaat4579. [Google Scholar] [CrossRef] [Green Version]
- Clissa, P.B.; Laing, G.D.; Theakston, R.D.; Mota, I.; Taylor, M.J.; Moura-da-Silva, A.M. The effect of jararhagin, a metalloproteinase from Bothrops jararaca venom, on pro-inflammatory cytokines released by murine peritoneal adherent cells. Toxicon 2001, 39, 1567–1573. [Google Scholar] [CrossRef]
- Muller, W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 323–344. [Google Scholar] [CrossRef] [Green Version]
- Phillipson, M.; Heit, B.; Colarusso, P.; Liu, L.; Ballantyne, C.M.; Kubes, P. Intraluminal crawling of neutrophils to emigration sites: A molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 2006, 203, 2569–2575. [Google Scholar] [CrossRef]
- Sumagin, R.; Prizant, H.; Lomakina, E.; Waugh, R.E.; Sarelius, I.H. LFA-1 and Mac-1 Define characteristically different intraluminal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immun. 2010, 185, 7057–7066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisco, M.; Chao, J.D.; Kim, I.; Mogford, J.E.; Mayadas, T.N.; Mustoe, T.A. Delayed wound healing in Mac-1-deficient mice is associated with normal monocyte recruitment. Wound Repair. Regen. 2007, 15, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Anto-Michel, N.; Blankenbach, H.; Wiedemann, A.; Buscher, K.; Hohmann, J.D.; Lim, B.; Bäuml, M.; Marki, A.; Mauler, M.; et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat. Commun. 2018, 9, 525. [Google Scholar] [CrossRef]
- Nourshargh, S.; Hordijk, P.L.; Sixt, M. Breaching multiple barriers: Leukocyte motility through venular walls and the interstitium. Nat. Rev. 2010, 11, 366–378. [Google Scholar] [CrossRef]
- Zamuner, S.R.; Teixeira, C.F.P. Cell adhesion molecules involved in the leukocyte recruitment induced by venom of the snake Bothrops jararaca. Mediators Inflamm. 2002, 11, 351–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, C.S.; Goncalves, L.R.C.; Juliano, L.; Juliano, M.A.; Moura-da-Silva, A.M.; Giorgi, R. The C-terminus of murine S100A9 inhibits hyperalgesia and edema induced by jararhagin. Peptides 2004, 25, 81–89. [Google Scholar] [CrossRef]
- Stroka, A.; Donato, J.L.; Bon, C.; Hyslop, S.; De Araujo, A.L. Purification and characterization of a hemorrhagic metalloproteinase from Bothrops lanceolatus (Fer-de-lance) snake venom. Toxicon 2005, 45, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Aida, Y.; Pabst, M.J. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 1990, 132, 191–195. [Google Scholar] [CrossRef]
- De Castro, K.L.P.; Lopes-de-Souza, L.; de Oliveira, D.; Machado-de-Ávila, R.A.; Paiva, A.L.B.; de Freitas, C.F.; Ho, P.L.; Chávez-Olórtegui, C.; Duarte, C.G. A Combined Strategy to Improve the Development of a Coral Antivenom against Micrurus spp. Front. Immunol. 2019, 10, 2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guri, A.J.; Hotencillas, R.; Bassaganya-Riera, J. Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clin. Nutr. 2010, 286, 2504–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zychar, B.C.; Clissa, P.B.; Carvalho, E.; Alves, A.S.; Baldo, C.; Faquim-Mauro, E.L.; Gonçalves, L.R.C. Modulation of Adhesion Molecules Expression by Different Metalloproteases Isolated from Bothrops Snakes. Toxins 2021, 13, 803. https://doi.org/10.3390/toxins13110803
Zychar BC, Clissa PB, Carvalho E, Alves AS, Baldo C, Faquim-Mauro EL, Gonçalves LRC. Modulation of Adhesion Molecules Expression by Different Metalloproteases Isolated from Bothrops Snakes. Toxins. 2021; 13(11):803. https://doi.org/10.3390/toxins13110803
Chicago/Turabian StyleZychar, Bianca C., Patrícia B. Clissa, Eneas Carvalho, Adilson S. Alves, Cristiani Baldo, Eliana L. Faquim-Mauro, and Luís Roberto C. Gonçalves. 2021. "Modulation of Adhesion Molecules Expression by Different Metalloproteases Isolated from Bothrops Snakes" Toxins 13, no. 11: 803. https://doi.org/10.3390/toxins13110803
APA StyleZychar, B. C., Clissa, P. B., Carvalho, E., Alves, A. S., Baldo, C., Faquim-Mauro, E. L., & Gonçalves, L. R. C. (2021). Modulation of Adhesion Molecules Expression by Different Metalloproteases Isolated from Bothrops Snakes. Toxins, 13(11), 803. https://doi.org/10.3390/toxins13110803




