Simultaneous Detection of Seven Alternaria Toxins in Mixed Fruit Puree by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Modified QuEChERS
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Water Addition
2.2. Optimization of Extraction Solvent
2.3. Optimization of Dehydrating Agent and Salting Out Agent
2.4. Optimization of the QuEChERS Purification
2.5. Optimization of the Extraction Method
2.6. Optimization of Chromatography and Mass Spectrometry Conditions
2.7. Method Validation
2.7.1. Matrix Effects (MEs)
2.7.2. Linearity and Detectability of the Method
2.7.3. Trueness and Precision of Standard Addition
2.7.4. Analysis of Fruit Puree Samples
3. Conclusions
4. Materials and Methods
4.1. Sample Collection
4.2. Chemicals, Reagents, and Standards
4.3. Detection and Quantification Method
4.4. Sample Pretreatment Method
4.5. Method Validation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.M. Determination of Alternaria Toxins in Fruits by Liquid Chromatography. Master’s Thesis, Shanxi Normal University, Xi’an, China, 2012. [Google Scholar]
- King, A.D.; Schade, J.E. Alternaria Toxins and Their Importance in Food. J. Food Prot. 1984, 47, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Puntscher, H.; Kutt, M.L.; Skrinjar, P.; Mikula, H.; Podlech, J.; Frohlich, J.; Marko, D.; Warth, B. Tracking emerging mycotoxins in food: Development of an LC-MS/MS method for free and modified Alternaria toxins. Anal. Bioanal. Chem. 2018, 410, 4481–4494. [Google Scholar] [CrossRef] [PubMed]
- Puntscher, H.; Cobankovic, I.; Marko, D.; Warth, B. Quantitation of free and modified Alternaria mycotoxins in European food products by LC-MS/MS. Food Control 2019, 102, 157–165. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Testempasis, S.; Vryzas, Z.; Karaoglanidis, G.S.; Papadopoulou-Mourkidou, E. Determination of mycotoxins in pomegranate fruits and juices using a QuEChERS-based method. Food Chem. 2015, 182, 81–88. [Google Scholar] [CrossRef]
- De Berardis, S.; De Paola, E.L.; Montevecchi, G.; Garbini, D.; Masino, F.; Antonelli, A.; Melucci, D. Determination of four Alternaria alternata mycotoxins by QuEChERS approach coupled with liquid chromatography-tandem mass spectrometry in tomato-based and fruit-based products. Food Res. Int. 2018, 106, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, J.J.; Cai, Z.X.; Huang, B.F.; Jin, M.C.; Ren, Y.P. Simultaneous determination of five Alternaria toxins in cereals using QuEChERS-based methodology. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1068, 15–23. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. A new method for detection of five alternaria toxins in food matrices based on LC–APCI-MS. Food Chem. 2013, 140, 161–167. [Google Scholar] [CrossRef]
- Tölgyesi, Á.; Kozma, L.; Sharma, V.K.; Kuo, P.-C. Determination of Alternaria Toxins in Sunflower Oil by Liquid Chromatography Isotope Dilution Tandem Mass Spectrometry. Molecules 2020, 25, 1685. [Google Scholar] [CrossRef]
- Wang, Z.Z. Processing of four kinds of jam food. Agric. Consult. 2016, 7, 54. [Google Scholar]
- Rose, M.D. Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar]
- Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, 4654. [Google Scholar]
- Tanaka, F.; Shoji, Y.; Okazaki, K.; Miyazawa, T. Sensory Attributes of Apple Products Enhanced with Reconstituted Ethyl Esters that are Aroma Components of Watercored Apples. J. Jpn. Soc. Food Sci. Technol. 2017, 64, 34–37. [Google Scholar] [CrossRef][Green Version]
- Scott, P.M.; Weber, D.; Kanhere, S.R. Gas chromatography-mass spectrometry of Alternaria mycotoxins. J. Chromatogr. A 1997, 765, 255–263. [Google Scholar] [CrossRef]
- Delgado, T.; Gómez-Cordovés, C.; Scott, P.M. Determination of alternariol and alternariol methyl ether in apple juice using solid-phase extraction and high-performance liquid chromatography. J. Chromatogr. A 1996, 731, 109–114. [Google Scholar] [CrossRef]
- Michele, S.; Annalisa, D.G.; Carolina, V.; Angelo, V.; van den Bulk, R. Liquid chromatographic determination of Alternaria toxins in carrots. J. AOAC Int. 2004, 87, 101–106. [Google Scholar]
- Gross, M.; Curtui, V.; Ackermann, Y.; Latif, H.; Usleber, E. Enzyme Immunoassay for Tenuazonic Acid in Apple and Tomato Products. J. Agric. Food Chem. 2011, 59, 12317–12322. [Google Scholar] [CrossRef]
- Rubert, J.; Dzuman, Z.; Vaclavikova, M.; Zachariasova, M.; Soler, C.; Hajslova, J. Analysis of mycotoxins in barley using ultra high liquid chromatography high resolution mass spectrometry: Comparison of efficiency and efficacy of different extraction procedures. Talanta 2012, 99, 712–719. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Li, H.; Zhang, J.; Shao, B. Determination of Alternaria toxins in drinking water by ultra-performance liquid chromatography tandem mass spectrometry. Environ. Sci. Pollut. Res. Int. 2019, 26, 22485–22493. [Google Scholar] [CrossRef]
- Xing, L.J.; Zou, L.J.; Luo, R.F.; Wang, Y. Determination of five Alternaria toxins in wolfberry using modified QuEChERS and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2020, 311, 1–27. [Google Scholar] [CrossRef]
- Jeroen, W.; Hannes, M.; Michael, R.; Stefan, A.; Njumbe, E.E.; Diana, D.M.J.; Anita, V.L.; Lynn, V.; Sarah, D.S. Development and validation of an ultra-high-performance liquid chromatography tandem mass spectrometric method for the simultaneous determination of free and conjugated Alternaria toxins in cereal-based foodstuffs. J. Chromatogr. A 2014, 1372, 91–101. [Google Scholar]
- Noser, J.; Schneider, P.; Rother, M.; Schmutz, H. Determination of six Alternaria toxins with UPLC-MS/MS and their occurrence in tomatoes and tomato products from the Swiss market. Mycotoxin Res. 2011, 27, 265–271. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Li, L.; Wu, Y.T.; Lin, Y.; Zhang, Q.; Bi, S.; Liu, W.Z.; Liu, L.Y. QuEChERS purification—ULTRA high performance liquid chromatography—tandem mass spectrometry for the determination of 5 kinds of Alternaria toxin in tomato. Phys. Chem. Exam. 2019, 55, 1036–1041. [Google Scholar]
- Dong, L.N.; Chen, J.B.; Liu, J.; Zhao, M.M.; Ding, H.; Wang, X.H.; Dingjin, H.U.; Zhou, Y.X. Advanced in Application of QuEChERS for Multi-Mycotoxins Analysis in Food. Acta Polym. Sin. 2014, 116, 121–127. [Google Scholar]
- Sun, J.; Li, W.X.; Zhang, Y.; Hu, X.X.; Wu, L.; Wang, B.J. QuEChERS Purification Combined with Ultrahigh-Performance Liquid Chromatography Tandem Mass Spectrometry for Simultaneous Quantification of 25 Mycotoxins in Cereals. Toxins 2016, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, A.Z.; Xing, J.L.; Cheng, H.; Zhang, S.F.; Zheng, R.H.; Dai, X.J. Determination residues of six alternaria toxins in mango by HPLC—MS. Sci. Technol. Food Ind. 2020, 41, 199–205, 211. [Google Scholar]
- Stachniuk, A.; Fornal, E. Liquid Chromatography-Mass Spectrometry in the Analysis of Pesticide Residues in Food. Food Anal. Methods 2016, 9, 1654–1665. [Google Scholar] [CrossRef]
- Mandal, S.; Poi, R.; Ansary, I.; Hazra, D.K.; Bhattacharyya, S.; Karmaker, R. Validation of a modified QuEChERS method to determine multiclass multipesticide residues in apple, banana and guava using GC–MS and LC–MS/MS and its application in real sample analysis. SN Appl. Sci. 2020, 2, 188. [Google Scholar] [CrossRef]
- Shen, H.H.; Li, X.Y.; Su, M.; Hei, W.; He, T.T.; Wang, C. Rapid determination of 29 pesticide residues in Aksu jujube by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry. J. Food Saf. Qual. 2019, 10, 8410–8417. [Google Scholar]
- Scott, P.M. Analysis of agricultural commodities and foods for Alternaria mycotoxins. J. AOAC Int. 2001, 84, 1809–1817. [Google Scholar] [CrossRef]
- Dong, H.; Xian, Y.P.; Xiao, K.J.; Wu, Y.L.; Zhu, L.; He, J.P. Development and comparison of single-step solid phase extraction and QuEChERS clean-up for the analysis of 7 mycotoxins in fruits and vegetables during storage by UHPLC-MS/MS. Food Chem. 2019, 274, 471–479. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Zhao, Q.Y.; Gong, L.; Liu, Y.Y.; Zhang, Y.H.; Ma, L.; Jiao, B.N. Rapid determination of five Alternaria toxins species in citrus by SUPER-high performance liquid chromatography (HPLC) tandem mass spectrometry. Chin. J. Anal. Chem. 2015, 43, 1851–1858. [Google Scholar]
- Cheng, J.X.; Li, Y.M.; Yang, Y.X.; Yang, Q.; Yuan, D.; Yang, J.; Wang, Y.C.; Zhang, R.L.; Du, Z.X. Determination and Risk Assessment of Four Alternaria Mycotoxins in Jujube. J. Instrum. Anal. 2018, 37, 1334–1338. [Google Scholar]
- Kamikata, K.; Vicente, E.; Arisseto-Bragotto, A.P.; de Oliveira Miguel, A.M.R.; Milani, R.F.; Tfouni, S.A.V. Occurrence of 3-MCPD, 2-MCPD and glycidyl esters in extra virgin olive oils, olive oils and oil blends and correlation with identity and quality parameters. Food Control 2019, 95, 135–141. [Google Scholar] [CrossRef]
- Hemmati, M.; Tejada-Casado, C.; Lara, F.J.; García-Campaña, A.M.; Rajabi, M.; del Olmo-Iruela, M. Monitoring of cyanotoxins in water from hypersaline microalgae colonies by ultra high performance liquid chromatography with diode array and tandem mass spectrometry detection following salting-out liquid-liquid extraction. J. Chromatogr. A 2019, 1608, 1–31. [Google Scholar] [CrossRef] [PubMed]
- González-Jartín, J.M.; Alfonso, A.; Rodríguez, I.; Sainz, M.J.; Vieytes, M.R.; Botana, L.M. A QuEChERS based extraction procedure coupled to UPLC-MS/MS detection for mycotoxins analysis in beer. Food Chem. 2019, 275, 703–710. [Google Scholar] [CrossRef]
- Guo, W.; Fan, K.; Nie, D.; Meng, J.; Huang, Q.; Yang, J.; Shen, Y.; Tangni, E.K.; Zhao, Z.; Wu, Y.; et al. Development of a QuEChERS-Based UHPLC-MS/MS Method for Simultaneous Determination of Six Alternaria Toxins in Grapes. Toxins 2019, 11, 87. [Google Scholar] [CrossRef]
- Wang, C.C. Determination of Pesticide Residues in Fruits and Vegetables by Liquid Chromatography-Tandem Mass Spectrometry. Master’s Thesis, Shandong University, Jinan, China, 2017. [Google Scholar]
- Wang, C.; Fan, Y.; He, W.; Hu, D.; Wu, A.; Wu, W. Development and Application of a QuEChERS-Based Liquid Chromatography Tandem Mass Spectrometry Method to Quantitate Multi-Component Alternaria Toxins in Jujube. Toxins 2018, 10, 382. [Google Scholar] [CrossRef]
- Wu, C.J.; Wu, L.; Li, H.; Yu, S.J. Determination of 4(5)-methylimidazole in foods and beverages by modified QuEChERS extraction and liquid chromatography-tandem mass spectrometry analysis. Food Chem. 2019, 280, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Xian, H.; Wei, D.; Shi, L.; Feng, X. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1429, 22–29. [Google Scholar] [CrossRef]
- De Smedt, J.M. AOAC validation of qualitative and quantitative methods for microbiology in foods. Association of Official Agricultural Chemists. Int. J. Food Microbiol. 1998, 45, 25–28. [Google Scholar] [CrossRef]
- Marina, G.; Stefan, A.; Klara, G.; Fooladi, M.A.; Elisabeth, B.; Roland, K.; Michael, R. Quantitation of Six Alternaria Toxins in Infant Foods Applying Stable Isotope Labeled Standards. Front. Microbiol. 2019, 10, 1–14. [Google Scholar]

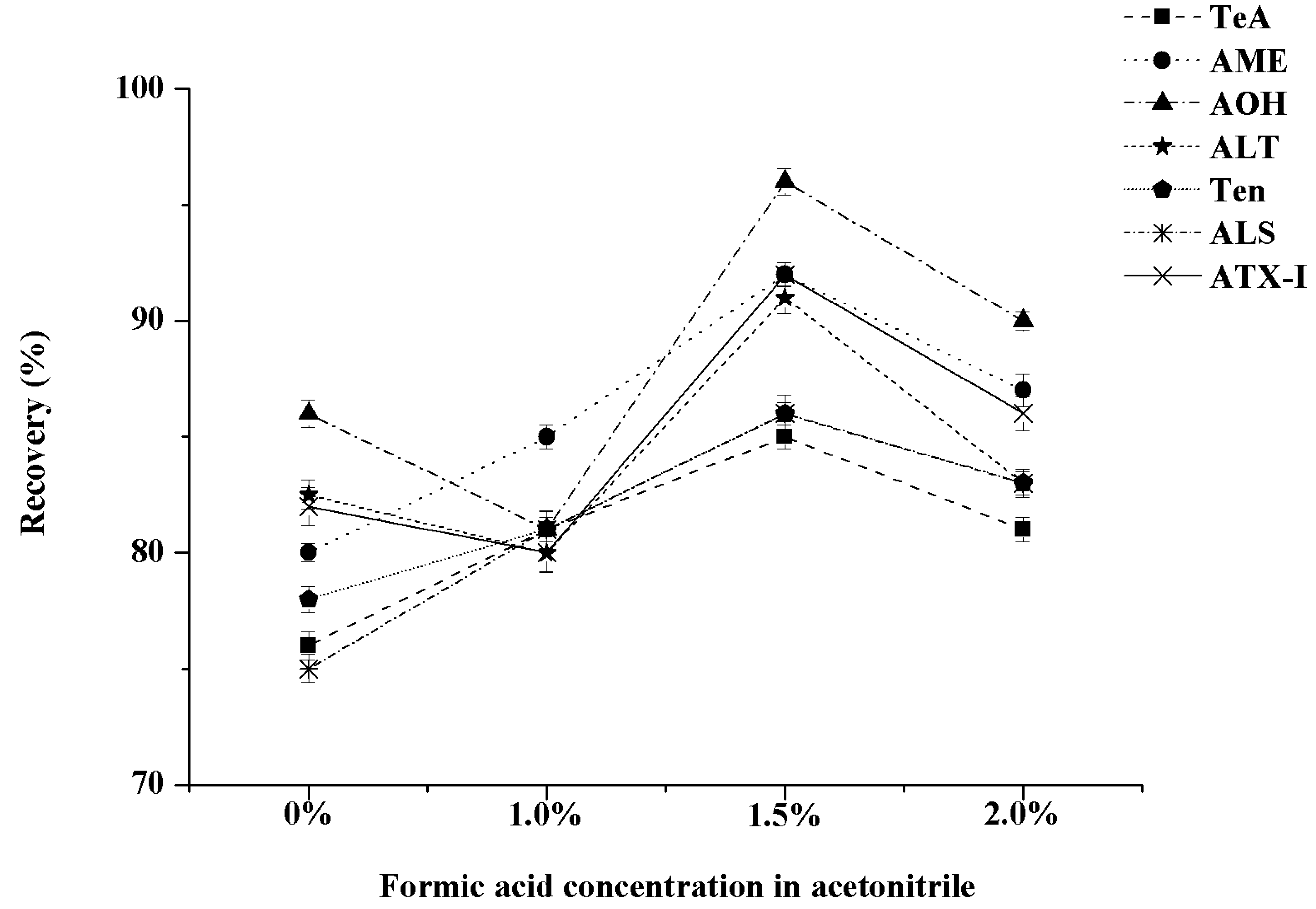



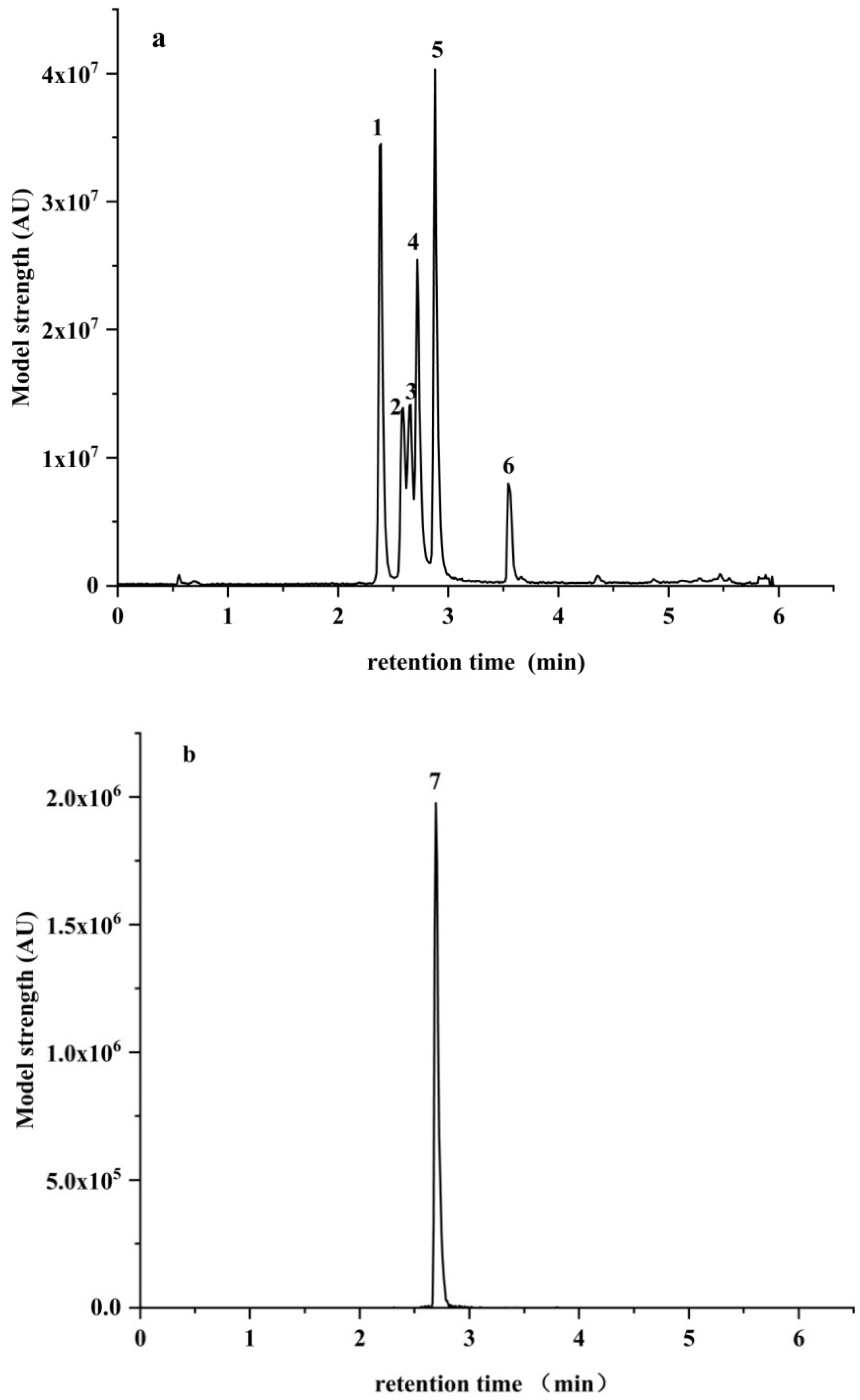
| Adsorbent | Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|
| TeA | AME | AOH | ALT | Ten | ALS | ATX-I | |
| 0 mg | 84.6 ± 1.2 a | 92.1 ± 1.6 a | 95.6 ± 2.1 a | 90.3 ± 0.9 c | 88.2 ± 1.7 a | 86.5 ± 2.8 b | 91.4 ± 1.7 a |
| 50 mg C18 | 80.0 ± 3.8 d | 86.9 ± 2.9 g | 90.1 ± 3.6 e | 87.7 ± 4.4 f | 80.4 ± 4.6 g | 82.6 ± 5.1 e | 85.3 ± 3.8 g |
| 100 mg C18 | 81.4 ± 3.3 c | 88.9 ± 4.6 e | 92.5 ± 2.9 d | 90.3 ± 4.6 c | 83.6 ± 3.1 d | 84.3 ± 2.9 d | 87.7 ± 3.9 e |
| 150 mg C18 | 75.8 ± 4.0 f | 87.2 ± 3.8 f | 86.4 ± 3.1 g | 88.6 ± 2.9 e | 81.9 ± 2.2 f | 78.9 ± 3.4 g | 86.7 ± 2.2 f |
| 50 mg PSA | 80.1 ± 3.1 d | 89.7 ± 3.6 d | 93.2 ± 2.2 c | 90.5 ± 2.8 b | 84.4 ± 2.9 c | 85.6 ± 3.1 c | 89.1 ± 3.2 c |
| 100 mg PSA | 83.7 ± 3.9 b | 91.6 ± 4.5 b | 94.1 ± 5.2 b | 91.2 ± 4.3 a | 85.9 ± 4.6 b | 87.7 ± 2.9 a | 90.3 ± 5.6 b |
| 150 mg PSA | 77.5 ± 2.8 e | 90.2 ± 5.4 c | 87.6 ± 4.2 f | 89.3 ± 3.9 d | 83.4 ± 4.1 e | 82.3 ± 3.3 f | 88.6 ± 4.1 d |
| Target Analyte | Matrix Effects before Dilution (Injection Volume: 10 μL) | Matrix Effects after Dilution (Injection Volume: 10 μL) | Matrix Effects after Dilution (Injection Volume: 3 μL) |
|---|---|---|---|
| TeA | 69.7 ± 2.4 a | 79.2 ± 1.4 b | 85.9 ± 1.9 c |
| AME | 78.6 ± 4.6 a | 82.3 ± 3.2 a | 88.3 ± 2.1 b |
| AOH | 75.3 ± 3.1 a | 87.1 ± 2.8 b | 88.6 ± 1.7 b |
| ALT | 123.3 ± 2.5 c | 112.4 ± 3.2 b | 98.7 ± 0.9 a |
| Ten | 88.4 ± 1.8 a | 90.3 ± 2.7 ab | 92.2 ± 1.1 b |
| ALS | 136.5 ± 3.4 c | 120.5 ± 1.5 b | 96.3 ± 1.8 a |
| ATX-I | 90.1 ± 2.0 a | 91.7 ± 1.3 a | 93.1 ± 2.3 b |
| Component | Linear Range (ng/mL) | Linear Equation | R2 | LODs (μg/kg) | LOQs (μg/kg) |
|---|---|---|---|---|---|
| TeA | 0.5–200 | y = 41232.3x − 3133.37 | 0.9963 | 0.46 | 1.47 |
| AME | 0.5–200 | y = 2828.31x − 893.32 | 0.9998 | 0.37 | 1.22 |
| AOH | 0.5–200 | y = 2503.73x − 1257.22 | 0.9997 | 0.53 | 2.17 |
| ALT | 0.5–200 | y = 7573.01x + 248.023 | 0.9996 | 0.22 | 0.77 |
| Ten | 0.5–200 | y = 16149.8x − 3371.39 | 0.9998 | 0.18 | 0.56 |
| ALS | 0.5–200 | y = 1398.39x + 618.519 | 0.9925 | 0.39 | 1.25 |
| ATX-I | 0.5–200 | y = 3136.11x − 1402.31 | 0.9996 | 0.27 | 0.89 |
| Component | Spiked (μg/kg) | Average Recovery (%) | RSD (%) |
|---|---|---|---|
| TeA | 5 | 85.3 | 9.78 |
| 10 | 88.2 | 8.65 | |
| 20 | 79.5 | 9.65 | |
| AME | 5 | 93.0 | 8.85 |
| 10 | 93.5 | 6.54 | |
| 20 | 106.7 | 5.63 | |
| AOH | 5 | 87.2 | 5.36 |
| 10 | 96.1 | 2.35 | |
| 20 | 102.8 | 7.21 | |
| ALT | 5 | 85.6 | 6.08 |
| 10 | 90.2 | 4.68 | |
| 20 | 98.6 | 6.31 | |
| Ten | 5 | 90.3 | 3.67 |
| 10 | 88.9 | 3.69 | |
| 20 | 101.5 | 5.48 | |
| ALS | 5 | 86.0 | 4.56 |
| 10 | 86.3 | 5.13 | |
| 20 | 83.2 | 5.48 | |
| ATX-I | 5 | 91.1 | 3.43 |
| 10 | 98.7 | 2.68 | |
| 20 | 96.5 | 5.45 |
| Samples | TeA (μg/kg) | AME (μg/kg) | AOH (μg/kg) | ALT (μg/kg) | Ten (μg/kg) | ALS (μg/kg) | ATX-I (μg/kg) |
|---|---|---|---|---|---|---|---|
| 6 | 38.92 | ND | ND | ND | ND | ND | ND |
| 9 | ND | ND | ND | ND | 3.26 | ND | ND |
| 11 | 43.31 | 9.83 | 7.15 | ND | ND | ND | ND |
| 17 | ND | ND | ND | ND | 5.21 | 6.56 | ND |
| 20 | ND | ND | ND | ND | 4.73 | ND | ND |
| 24 | ND | ND | ND | ND | 2.11 | ND | ND |
| 27 | 47.96 | ND | 8.11 | ND | 4.39 | ND | ND |
| 28 | ND | ND | ND | ND | 5.51 | ND | ND |
| 31 | ND | ND | ND | ND | 2.66 | ND | ND |
| 32 | 52.68 | ND | ND | ND | 3.67 | ND | 7.54 |
| 35 | ND | 6.32 | 7.49 | ND | 1.66 | ND | ND |
| 38 | ND | ND | ND | ND | 6.32 | ND | ND |
| 39 | ND | ND | ND | ND | ND | 4.11 | ND |
| 41 | 38.99 | ND | ND | ND | 4.68 | ND | ND |
| 43 | 44.77 | ND | ND | ND | 8.37 | ND | ND |
| 44 | ND | ND | 4.17 | ND | ND | ND | ND |
| 45 | ND | ND | ND | 2.66 | 5.18 | ND | ND |
| 48 | ND | 2.28 | ND | ND | 4.89 | ND | ND |
| 50 | 54.89 | ND | ND | ND | 2.56 | ND | ND |
| 51 | 43.32 | ND | ND | ND | 1.69 | ND | ND |
| 52 | ND | ND | ND | ND | 4.67 | ND | ND |
| 55 | ND | 2.61 | 3.75 | ND | 4.33 | ND | ND |
| 59 | ND | ND | ND | ND | 5.68 | ND | ND |
| 62 | 34.44 | ND | ND | ND | 3.65 | ND | ND |
| 66 | ND | ND | ND | ND | 4.66 | ND | ND |
| 68 | 23.32 | ND | ND | ND | 2.37 | ND | ND |
| 70 | 36.98 | ND | 5.99 | ND | ND | ND | ND |
| 71 | ND | ND | ND | ND | 1.32 | 15.48 | ND |
| 74 | 45.67 | ND | ND | ND | ND | ND | 6.43 |
| 76 | ND | 3.92 | 4.21 | ND | ND | ND | ND |
| 79 | 33.29 | ND | ND | ND | 6.98 | ND | ND |
| Component | Ionization Mode | Parent (m/z) | Daughter (m/z) | Dwell Time (s) | Cone Voltage (V) | Collision Voltage (V) |
|---|---|---|---|---|---|---|
| Ten | ESI+ | 415.4 | 199.2 * 171.2 | 0.012 | 25 | 13 18 |
| AME | ESI+ | 273.2 | 258.2 128.1 * | 0.012 | 25 | 25 40 |
| AOH | ESI+ | 259.2 | 213.2 185.1 * | 0.012 | 25 | 25 30 |
| TeA | ESI+ | 198.2 | 125.1 * 153.1 | 0.012 | 25 | 15 12 |
| ALT | ESI+ | 293.2 | 257.2 * 275.4 | 0.012 | 25 | 12 8 |
| ALS | ESI+ | 291.2 | 255.2 199.2 * | 0.012 | 25 | 18 30 |
| ATX-I | ESI− | 351.3 | 315.25 * 333.3 | 0.0.12 | 25 | 8 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, J.; Zhang, Z.; Zheng, R.; Xu, X.; Mao, L.; Lu, J.; Shen, J.; Dai, X.; Yang, Z. Simultaneous Detection of Seven Alternaria Toxins in Mixed Fruit Puree by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Modified QuEChERS. Toxins 2021, 13, 808. https://doi.org/10.3390/toxins13110808
Xing J, Zhang Z, Zheng R, Xu X, Mao L, Lu J, Shen J, Dai X, Yang Z. Simultaneous Detection of Seven Alternaria Toxins in Mixed Fruit Puree by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Modified QuEChERS. Toxins. 2021; 13(11):808. https://doi.org/10.3390/toxins13110808
Chicago/Turabian StyleXing, Jiali, Zigeng Zhang, Ruihang Zheng, Xiaorong Xu, Lingyan Mao, Jingping Lu, Jian Shen, Xianjun Dai, and Zhenfeng Yang. 2021. "Simultaneous Detection of Seven Alternaria Toxins in Mixed Fruit Puree by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Modified QuEChERS" Toxins 13, no. 11: 808. https://doi.org/10.3390/toxins13110808
APA StyleXing, J., Zhang, Z., Zheng, R., Xu, X., Mao, L., Lu, J., Shen, J., Dai, X., & Yang, Z. (2021). Simultaneous Detection of Seven Alternaria Toxins in Mixed Fruit Puree by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Modified QuEChERS. Toxins, 13(11), 808. https://doi.org/10.3390/toxins13110808




