Botulinum Neurotoxin Type A in the Treatment of Facial Seborrhea and Acne: Evidence and a Proposed Mechanism
Abstract
:1. Introduction
2. Search Strategy
3. Botulinum Neurotoxin for Sebum Reduction
3.1. Anecdotal Clinical Reports
3.2. Extraneuronal Cholinergic System of Sebaceous Glands
3.3. Early Observations
3.4. OnabotulinumtoxinA
3.5. IncobotulinumtoxinA and AbobotulinumtoxinA
3.6. Enlarged Facial Pores
3.7. Limitations of the Procedure
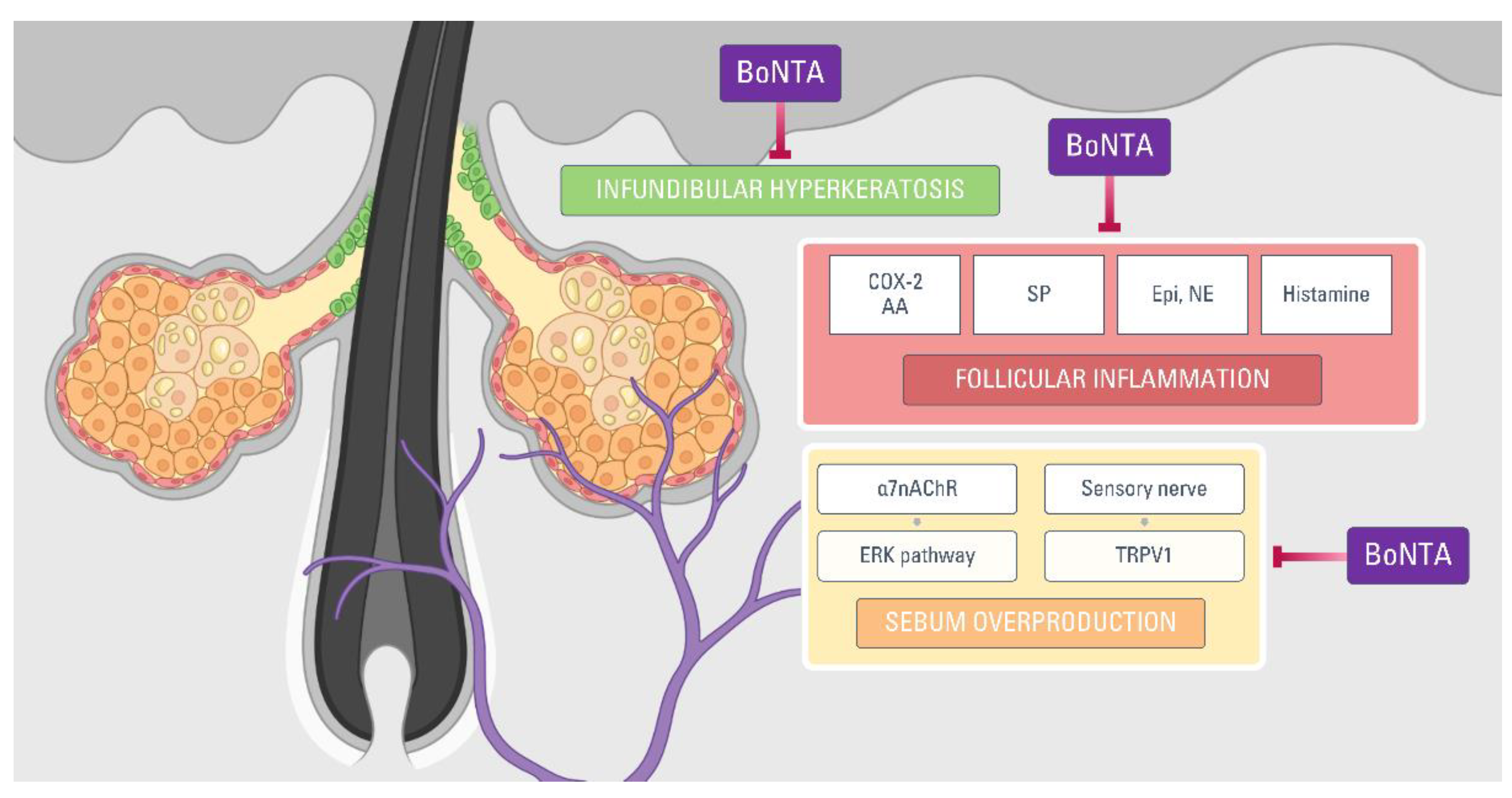
4. Botulinum Neurotoxin for Acne Treatment: Possible Mechanisms
4.1. Pathogenesis of Acne
4.2. Acne and Cholinergic Signaling
4.3. Catecholamines
4.4. Mediators of Inflammation in Acne
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbuckle, R.; Atkinson, M.J.; Clark, M.; Abetz, L.; Lohs, J.; Kuhagen, I.; Harness, J.; Draelos, Z.; Thiboutot, D.; Blume-Peytavi, U.; et al. Patient experiences with oily skin: The qualitative development of content for two new patient reported outcome questionnaires. Health Qual. Life Outcomes 2008, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.E.; Goldberg, D.J. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol. Surg. 2013, 39, 443–448. [Google Scholar] [CrossRef]
- Endly, D.C.; Miller, R.A. Oily skin: A review of treatment options. J. Clin. Aesthet. Dermatol. 2017, 10, 49–55. [Google Scholar]
- Burton, J.L.; Cunliffe, W.J.; Saunders, I.G.G.; Shuster, S. The effect of facial nerve paresis on sebum excretion. Br. J. Dermatol. 1971, 84, 135–138. [Google Scholar] [CrossRef]
- Sudy, E.; Urbina, F. Unilateral acne after facial palsy. An. Bras. Dermatol. 2018, 93, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.E.; Conway, J.; Ebling, F.J.G.; Harrington, C.I. Measurement of sebum excretion rate and skin temperature above and below the neurological lesion in paraplegic patients. Br. J. Dermatol. 1985, 112, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Cartlidge, M.; Burton, J.L.; Shuster, S. The effect of prolonged topical application of an anticholinergic agent on the sebaceous glands. Br. J. Dermatol. 1972, 86, 61–63. [Google Scholar] [CrossRef]
- Shuo, L.; Ting, Y.; KeLun, W.; Rui, Z.; Rui, Z.; Hang, W. Efficacy and possible mechanisms of botulinum toxin treatment of oily skin. J. Cosmet. Dermatol. 2019, 18, 451–457. [Google Scholar] [CrossRef]
- Martignoni, E.; Godi, L.; Pacchetti, C.; Berardesca, E.; Vignoli, G.P.; Albani, G.; Mancini, F.; Nappi, G. Is seborrhea a sign of autonomic impairment in Parkinson’s disease? J. Neural Transm. 1997, 104, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Kurzen, H.; Berger, H.; Jäger, C.; Hartschuh, W.; Näher, H.; Gratchev, A.; Goerdt, S.; Deichmann, M. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J. Investig. Dermatol. 2004, 123, 937–949. [Google Scholar] [CrossRef]
- Kurzen, H.; Wessler, I.; Kirkpatrick, C.J.; Kawashima, K.; Grando, S.A. The non-neuronal cholinergic system of human skin. Horm. Metab. Res. 2007, 39, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Grando, S.A.; Zelickson, B.D.; Kist, D.A.; Weinshenker, D.; Bigliardi, P.L.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Dahl, M.V. Keratinocyte muscarinic acetylcholine receptors: Immunolocalization and partial characterization. J. Investig. Dermatol. 1995, 104, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Zouboulis, C.C. The brain of the skin: Sebaceous gland. In Lipids and Skin Health; Pappas, A., Ed.; Springer: New York, NY, USA, 2015; pp. 109–125. ISBN 9783319099439. [Google Scholar]
- Makrantonaki, E.; Adjaye, J.; Herwig, R.; Brink, T.C.; Groth, D.; Hultschig, C.; Lehrach, H.; Zouboulis, C.C. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell 2006, 5, 331–344. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Brink, T.C.; Zampeli, V.; Elewa, R.M.; Mlody, B.; Hossini, A.M.; Hermes, B.; Krause, U.; Knolle, J.; Abdallah, M.; et al. Identification of biomarkers of human skin ageing in both genders. Wnt Signalling—A label of skin ageing? PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Ramot, Y.; Böhm, M.; Paus, R. Translational neuroendocrinology of human skin: Concepts and perspectives. Trends Mol. Med. 2021, 27, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Baron, J.M.; Böhm, M.; Kippenberger, S.; Kurzen, H.; Reichrath, J.; Thielitz, A. Frontiers in sebaceous gland biology and pathology. Exp. Dermatol. 2008, 17, 542–551. [Google Scholar] [CrossRef]
- Stegemann, A.; Böhm, M. Targeting the α7 nicotinic acetylcholine receptor—A novel road towards the future treatment of skin diseases. Exp. Dermatol. 2020, 29, 924–931. [Google Scholar] [CrossRef]
- Li, Z.J.; Park, S.B.; Sohn, K.C.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Kim, Y.S.; Lee, J.H.; Im, M. Regulation of lipid production by acetylcholine signalling in human sebaceous glands. J. Dermatol. Sci. 2013, 72, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kurzen, H.; Schallreuter, K.U. Novel aspects in cutaneous biology of acetylcholine synthesis and acetylcholine receptors. Exp. Dermatol. 2004, 13, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Valente Duarte De Sousa, I.C. Novel pharmacological approaches for the treatment of acne vulgaris. Expert Opin. Investig. Drugs 2014, 23, 1389–1410. [Google Scholar] [CrossRef]
- Shi, V.Y.; Leo, M.; Hassoun, L.; Chahal, D.S.; Maibach, H.I.; Sivamani, R.K. Role of sebaceous glands in inflammatory dermatoses. J. Am. Acad. Dermatol. 2015, 73, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Kucukkaya, D.; Irkoren, S.; Ozkan, S.; Sivrioglu, N. The effects of botulinum toxin A on the wound and skin graft contraction. J. Craniofac. Surg. 2014, 25, 1908–1911. [Google Scholar] [CrossRef]
- Turner, I.M.; Agrillo, T. Migraine, botulinum toxin type-A, and the disappearing sebaceous cyst. Headache 2005, 45, 166–167. [Google Scholar] [CrossRef]
- Jankovic, J. Disease-oriented approach to botulinum toxin use. Toxicon 2009, 54, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Bulstrode, N.W.; Grobbelaar, A.O. Long-term prospective follow-up of botulinum toxin treatment for facial rhytides. Aesthet. Plast. Surg. 2002, 26, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.L. Microbotox of the lower face and neck: Evolution of a personal technique and its clinical effects. Plast. Reconstr. Surg. 2015, 136, 92S–100S. [Google Scholar] [CrossRef]
- Shah, A.R. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J. Drugs Dermatol. 2008, 7, 847–850. [Google Scholar]
- Pande, S.Y.; Misri, R. Sebumeter. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 444–446. [Google Scholar] [CrossRef]
- Hathout, H.M. Intradermal Injection of Botulinum Toxin a in Oily Skin; Ain Shams University: Cairo, Egypt, 2014. [Google Scholar]
- Shirshakova, M.; Morozova, E.; Sokolova, D.; Pervykh, S.; Smirnova, L. The effectiveness of botulinum toxin type A (BTX-A) in the treatment of facial skin oily seborrhea, enlarged pores, and symptom complex of post-acne. Int. J. Dermatol. 2021, 60, 1232–1241. [Google Scholar] [CrossRef]
- Min, P.; Xi, W.; Grassetti, L.; Trisliana Perdanasari, A.; Torresetti, M.; Feng, S.; Su, W.; Pu, Z.; Zhang, Y.; Han, S.; et al. Sebum production alteration after botulinum toxin type a injections for the treatment of forehead rhytides: A prospective randomized double-blind dose-comparative clinical investigation. Aesthet. Surg. J. 2015, 35, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Kondrateva, N.; Galkina, E.; Karakaeva, A.; Morrison, A.; Morrison, V. The effect of botulinum toxin type A injections on production of sebum in seborrhea. Saratov J. Med. Sci. Res. 2018, 14, 740–744. [Google Scholar]
- Kesty, K.; Goldberg, D.J. A randomized, double-blinded study evaluating the safety and efficacy of abobotulinumtoxinA injections for oily skin of the forehead: A dose-response analysis. Dermatol. Surg. 2021, 47, 56–60. [Google Scholar] [CrossRef]
- Park, J.-Y.; Cho, S.I.; Hur, K.; Lee, D.H. Intradermal microdroplet injection of diluted incobotulinumtoxin-A for sebum control, face lifting, and pore size improvement. J. Drugs Dermatol. 2021, 20, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Liew, S. Discussion: Microbotox of the lower face and neck: Evolution of a personal technique and its clinical effects. Plast. Reconstr. Surg. 2015, 136, 101S–103S. [Google Scholar] [CrossRef]
- Roh, M.; Han, M.; Kim, D.; Chung, K. Sebum output as a factor contributing to the size of facial pores. Br. J. Dermatol. 2006, 155, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P.; Demay, S.; Sapra, S.; Khanna, J.; Mraud, K.; Bonadonna, J. A single-blind, split-face, randomized, pilot study comparing the effects of intradermal and intramuscular injection of two commercially available botulinum toxin A formulas to reduce signs of facial aging. J. Clin. Aesthet. Dermatol. 2017, 10, 34–44. [Google Scholar] [PubMed]
- Sayed, K.S.; Hegazy, R.; Gawdat, H.I.; Abdel Hay, R.M.; Ahmed, M.M.; Mohammed, F.N.; Allam, R.; Fahim, A. The efficacy of intradermal injections of botulinum toxin in the management of enlarged facial pores and seborrhea: A split face-controlled study. J. Dermatol. Treat. 2020, 32, 771–777. [Google Scholar] [CrossRef]
- Arredondo, J.; Hall, L.L.; Ndoye, A.; Nguyen, V.T.; Chernyavsky, A.I.; Bercovich, D.; Orr-Urtreger, A.; Beaudet, A.L.; Grando, S.A. Central role of fibroblast α3 nicotinic acetylcholine receptor in mediating cutaneous effects of nicotine. Lab. Invest. 2003, 83, 207–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed El Attar, Y.; Nofal, A. Microbotox for the treatment of wide facial pores: A promising therapeutic approach. J. Cosmet. Dermatol. 2020, 20, 1361–1366. [Google Scholar] [CrossRef]
- Diaspro, A.; Calvisi, L.; Manzoni, V.; Sito, G. Microbotulinum: A quantitative evaluation of aesthetic skin improvement in 62 patients. Plast. Reconstr. Surg. 2020, 146, 987–994. [Google Scholar] [CrossRef]
- Kapoor, R.; Shome, D.; Jain, V.; Dikshit, R. Facial rejuvenation after intradermal botulinum toxin: Is it really the botulinum toxin or is it the pricks? Dermatol. Surg. 2010, 36, 2098–2105. [Google Scholar] [CrossRef]
- Briganti, S.; Flori, E.; Mastrofrancesco, A.; Ottaviani, M. Acne as an altered dermato-endocrine response problem. Exp. Dermatol. 2020, 29, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Plewig, G.; Melnik, B.; Chen, W. Plewig and Kligman´s Acne and Rosacea, 4th ed.; Springer: Berlin, Germany, 2019. [Google Scholar]
- Cunliffe, W.J. Acne; Martin Dunitz: London, UK, 1989. [Google Scholar]
- Ross, E.V. Optical treatments for acne. Dermatol. Ther. 2005, 18, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Hana, A.; Booken, D.; Henrich, C.; Gratchev, A.; Maas-Szabowski, N.; Goerdt, S.; Kurzen, H. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007, 80, 2214–2220. [Google Scholar] [CrossRef]
- Klaz, I.; Kochba, I.; Shohat, T.; Zarka, S.; Brenner, S. Severe acne vulgaris and tobacco smoking in young men. J. Investig. Dermatol. 2006, 126, 1749–1752. [Google Scholar] [CrossRef] [Green Version]
- Capitanio, B.; Sinagra, J.L.; Ottaviani, M.; Bordignon, V.; Amantea, A.; Picardo, M. Acne and smoking. Dermatoendocrinology 2009, 1, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K. Multiple intradermal small bolus injection of botulinum toxin: The limit and the potentiality. J. Cosmet. Laser Ther. 2012, 14, 304–306. [Google Scholar] [CrossRef]
- Diamond, A.; Jankovic, J. Botulinum toxin in dermatology—Beyond wrinkles and sweat. J. Cosmet. Dermatol. 2006, 5, 169. [Google Scholar] [CrossRef]
- Brin, M.F.; Boodhoo, T.I.; Pogoda, J.M.; James, L.M.; Demos, G.; Terashima, Y.; Gu, J.; Eadie, N.; Bowen, B.L. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: A meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J. Am. Acad. Dermatol. 2009, 61, 961–970.e11. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.; Chon, S.Y.; Kimball, A.B. The response of skin disease to stress: Changes in the severity of acne vulgaris as affected by examination stress. Arch. Dermatol. 2003, 139, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Zouboulis, C.C.; Böhm, M. Neuroendocrine regulation of sebocytes—A pathogenetic link between stress and acne. Exp. Dermatol., Suppl. 2004, 13, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, K.; Schnitger, A.; Glass, E.; Zouboulis, C.C. Corticotropin-releasing hormone skin signalling is receptor mediated and is predominant in the sebaceous glands. Exp. Dermatol. 2008, 13, 591–591. [Google Scholar] [CrossRef]
- Mizuno, K.; Okuyama, K.; Akimoto, N.; Sato, T. Involvement of catecholamine in the differentiation of sebaceous glands in vitro. J. Investig. Dermatol. 2017, 137, s77. [Google Scholar] [CrossRef]
- Borrel, V.; Thomas, P.; Catovic, C.; Racine, P.-J.; Konto-Ghiorghi, Y.; Lefeuvre, L.; Duclairoir-Poc, C.; Zouboulis, C.C.; Feuilloley, M.G.J. Acne and stress: Impact of catecholamines on Cutibacterium acnes. Front. Med. 2019, 6, 155. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Hao, Y.; Feng, Y.; Pan, L.; Liu, W.; Li, B.; Xiao, L.; Jin, L.; Nie, Z. The mechanism of botulinum A on Raynaud syndrome. Drug Des. Devel. Ther. 2018, 12, 1905–1915. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Seo, K.; Yum, K.W.; Oh, Y.S.; Yoon, T.G.; Yoon, S.M. Effects of botulinum toxin type A on the superior cervical ganglia in rabbits. Auton. Neurosci. Basic Clin. 2002, 102, 8–12. [Google Scholar] [CrossRef]
- Carroll, I.; Clark, J.D.; Mackey, S. Sympathetic block with botulinum toxin to treat complex regional pain syndrome. Ann. Neurol. 2009, 65, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Lomneth, R.; Martin, T.F.J.; DasGupta, B.R. Botulinum neurotoxin light chain inhibits norepinephrine secretion in PC12 cells at an intracellular membranous or cytoskeletal site. J. Neurochem. 1991, 57, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Martin, T.F.J.; DasGupta, B.R. Nerve growth factor induces sensitivity to botulinum neurotoxin type A in norepinephrine-secreting PC12 cells. Neurosci. Lett. 1993, 164, 93–96. [Google Scholar] [CrossRef]
- Morris, J.L.; Jobling, P.; Gibbins, I.L. Botulinum neurotoxin A attenuates release of norepinephrine but not NPY from vasoconstrictor neurons. Am. J. Physiol. Hear. Circ. Physiol. 2002, 283, 2627–2635. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, M.; Morohashi, M. New aspects in acne inflammation. Dermatology 2003, 206, 17–23. [Google Scholar] [CrossRef]
- Matak, I.; Tékus, V.; Bölcskei, K.; Lacković, Z.; Helyes, Z. Involvement of substance P in the antinociceptive effect of botulinum toxin type A: Evidence from knockout mice. Neuroscience 2017, 358, 137–145. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.S.; Alghanemy, N.; Joharji, H.; Al-Qahtani, D.; Alghamdi, H. Botulinum toxin: Non cosmetic and off-label dermatological uses. J. Dermatol. Dermatol. Surg. 2015, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.R.; Lew, B.L.; Lew, H.; Sim, W.Y. Treatment effects of intradermal botulinum toxin type A injection on alopecia areata. Dermatol. Surg. 2010, 36, 2175–2181. [Google Scholar] [CrossRef]
- Tamura, B.M.; Sortino-Rachou, A.M.; Cucé, L.C. Folliculitis responds to botulinum toxin: Is it possible? Dermatol. Surg. 2007, 33, 1398–1400. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Chen, Z.; Zhou, C.; Gan, Y.; Jia, Y. A review of the role of sebum in the mechanism of acne pathogenesis. J. Cosmet. Dermatol. 2017, 16, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Alestas, T.; Ganceviciene, R.; Fimmel, S.; Müller-Decker, K.; Zouboulis, C.C. Enzymes involved in the biosynthesis of leukotriene B 4 and prostaglandin E 2 are active in sebaceous glands. J. Mol. Med. 2006, 84, 75–87. [Google Scholar] [CrossRef]
- Ray, P.; Berman, J.D.; Middleton, W.; Brendle, J. Botulinum toxin inhibits arachidonic acid release associated with acetylcholine release from PC12 cells. J. Biol. Chem. 1993, 268, 11057–11064. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Yoshimura, N.; Huang, C.C.; Wu, M.; Chiang, P.H.; Chancellor, M.B. Intraprostatic botulinum toxin A injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J. Urol. 2008, 180, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H. The effects of botulinum toxin A on mast cell activity: Preliminary results. Burns 2013, 39, 816–817. [Google Scholar] [CrossRef]
- Ramachandran, R.; Marino, M.J.; Paul, S.; Wang, Z.; Mascarenhas, N.L.; Pellett, S.; Johnson, E.A.; Dinardo, A.; Yaksh, T.L. A study and review of effects of botulinum toxins on mast cell dependent and independent pruritus. Toxins 2018, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.F.; Si, M.; Huang, Y.; Chen, L.H.; Peng, X.Y.; Qin, Y.Q.; Liu, T.T.; Zhou, Y.; Liu, T.; Luo, W.F. Long-term anti-itch effect of botulinum neurotoxin A is associated with downregulation of TRPV1 and TRPA1 in the dorsal root ganglia in mice. Neuroreport 2017, 28, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.I.; Géczy, T.; Griger, Z.; Dózsa, A.; Seltmann, H.; Kovács, L.; Nagy, L.; Zouboulis, C.C.; Paus, R.; Bíró, T. Transient receptor potential vanilloid-1 signaling as a regulator of human sebocyte biology. J. Investig. Dermatol. 2009, 129, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermatoendocrinology 2011, 3, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| First Author | Product | Injection | n | Findings (As Described in the Paper) |
|---|---|---|---|---|
| Min [32] | ONA | IM | 42 | A significant decrease in sebum production A sebum gradient surrounding the injection point No difference in the efficacy between the 10 units group and the 20 units group Recovery of the sebum production at the 16-week follow-up |
| Kondrateva [33] | ONA | IM | 15 | Maximum decrease in sebum production at the 4-week follow-up A return-to-baseline at 16 weeks after injection |
| Hathout [30] | ONA | ID | 20 | A significant decrease in sebum production Patient satisfaction up to 3 months Associated improvement in the skin tone and facial pores No influence of age, gender, or skin types |
| Shirshakova [31] | ONA | ID | 12 | A decrease of skin surface sebum amount at 1 week and 2 weeks after injection |
| Li [19] | MTX | ID | 20 | A marked decrease in sebum production on the BoNTA-treated side in facial seborrhea group |
| Rose [2] | ABO | ID | 25 | A significantly lower sebum production at 1, 4, 8, and 12 weeks after injection |
| Kesty [34] | ABO | ID | 50 | A significant decrease in sebum production in the treatment groups 30 units and 45 units The effect lasted for 6 months after injection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rho, N.-K.; Gil, Y.-C. Botulinum Neurotoxin Type A in the Treatment of Facial Seborrhea and Acne: Evidence and a Proposed Mechanism. Toxins 2021, 13, 817. https://doi.org/10.3390/toxins13110817
Rho N-K, Gil Y-C. Botulinum Neurotoxin Type A in the Treatment of Facial Seborrhea and Acne: Evidence and a Proposed Mechanism. Toxins. 2021; 13(11):817. https://doi.org/10.3390/toxins13110817
Chicago/Turabian StyleRho, Nark-Kyoung, and Young-Chun Gil. 2021. "Botulinum Neurotoxin Type A in the Treatment of Facial Seborrhea and Acne: Evidence and a Proposed Mechanism" Toxins 13, no. 11: 817. https://doi.org/10.3390/toxins13110817
APA StyleRho, N. -K., & Gil, Y. -C. (2021). Botulinum Neurotoxin Type A in the Treatment of Facial Seborrhea and Acne: Evidence and a Proposed Mechanism. Toxins, 13(11), 817. https://doi.org/10.3390/toxins13110817






