Lonomia obliqua Envenoming and Innovative Research
Abstract
1. Introduction
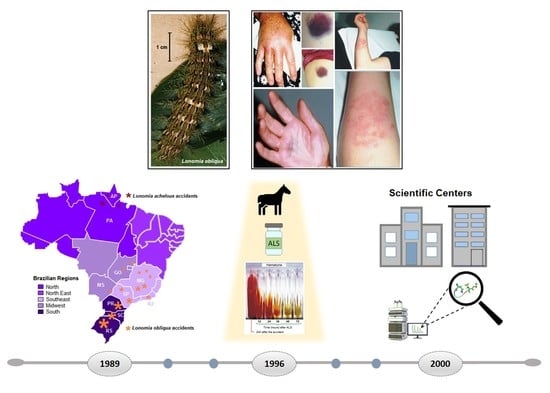
2. Lonomia spp. Epidemiology and Impact on Public Health
3. Clinical Manifestations and Complications

- (a)
- Mild: patient with local envenomation and without coagulation changes or bleeding within 12 h after the accident, confirmed with the identification of the agent.
- (b)
- Moderate: patient with local manifestations, alterations in global coagulation tests, or hemorrhagic manifestations in the skin and/or mucous membranes (gingivorrhagia, ecchymosis, hematoma), and hematuria, without hemodynamic alterations (hypotension, tachycardia, or shock) (see Figure 2b).
- (c)
- Severe: patient with impaired coagulation, hemorrhagic manifestations in the viscera (hematemesis, hypermenorrhagia, pulmonary bleeding, and intracranial hemorrhage), hemodynamic changes, and/or failure of multiple organs or systems.
4. The Importance of Butantan Institute in Antilonomic Serum Treatment
5. Blood Coagulation Alterations
6. Toxins Identified in LOCBE
6.1. Expressed Genes in LOCBE
6.2. Proteomic Analysis of LOCBE
6.3. Procoagulant Toxins from LOCBE
7. Effect of LOCBE and Toxins in the Inflammatory Response
8. Innovation in Toxinology: Research Centers and New Molecule Development
8.1. Research Centers Specialized in Toxinology
8.2. New Molecular Entities for the Study of Anti-Inflammatory Diseases
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villas-Boas, I.M.; Alvarez-Flores, M.P.; Chudzinski-Tavassi, A.M.; Tambourgi, D.V. Envenomation by caterpillars. In Clinical Toxinology; Gopalakrishnakone, P., Faiz, S., Gnanathasan, C., Habib, A., Fernando, R., Yang, C.C., Eds.; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Seldeslachts, A.; Peigneur, S.; Tytgat, J. Caterpillar Venom: A Health Hazard of the 21st Century. Biomedicines 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.D.; Campos, C.M.; Gonçalves, L.R.; Sousa-E-Silva, M.C.; Higashi, H.G.; Yamagushi, I.K.; Kelen, E.M. Development of an antivenom against toxins of Lonomia obliqua caterpillars. Toxicon 1996, 34, 1045–1049. [Google Scholar] [CrossRef]
- Rocha-Campos, A.C.; Gonçalves, L.R.; Higashi, H.G.; Yamagushi, I.K.; Fernandes, I.; Oliveira, J.E.; Ribela, M.T.; Sousa-E-Silva, M.C.; da Silva, W.D. Specific heterologous F(ab’)2 antibodies revert blood incoagulability resulting from envenoming by Lonomia obliqua caterpillars. Am. J. Trop. Med. Hyg. 2001, 64, 283–289. [Google Scholar] [CrossRef]
- Caovilla, J.J.; José Guardão Barros, E. Efficacy of two different doses of antilonomic serum in the resolution of hemorrhagic syndrome resulting from envenoming by Lonomia obliqua caterpillars: A randomized controlled trial. Toxicon 2004, 43, 811–818. [Google Scholar] [CrossRef]
- Gonçalves, L.R.; Souza-E-Silva, M.C.; Tomy, S.C.; Sano-Martins, I.S. Efficacy of serum therapy on the treatment of rats experimentally envenomed by bristle extract of the caterpillar Lonomia obliqua: Comparison with epsilon-aminocaproic acid therapy. Toxicon 2007, 50, 349–356. [Google Scholar] [CrossRef]
- Sano-Martins, I.S.; González, C.; Anjos, I.V.; Díaz, J.; Gonçalves, L.R.C. Effectiveness of Lonomia antivenom in recovery from the coagulopathy induced by Lonomia orientoandensis and Lonomia casanarensis caterpillars in rats. PLoS Negl. Trop. Dis. 2018, 12, e0006721. [Google Scholar] [CrossRef]
- Zannin, M.; Lourenço, D.M.; Motta, G.; Dalla Costa, L.R.; Grando, M.; Gamborgi, G.P.; Noguti, M.A.; Chudzinski-Tavassi, A.M. Blood coagulation and fibrinolytic factors in 105 patients with hemorrhagic syndrome caused by accidental contact with Lonomia obliqua caterpillar in Santa Catarina, southern Brazil. Thromb. Haemost. 2003, 89, 355–364. [Google Scholar] [CrossRef]
- Favalesso, M.M.; Cuervo, P.F.; Casafús, M.G.; Guimarães, A.T.B.; Peichoto, M.E. Lonomia envenomation in Brazil: An epidemiological overview for the period 2007–2018. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 9–19. [Google Scholar] [CrossRef]
- Brazilian Ministry of Health. SINAN, Sistema de Informação de Agravos de Notificação (Information System for Notification of Diseases). Available online: http://tabnet.datasus.gov.br/cgi/menu_tabnet_php.htm (accessed on 31 August 2021).
- Alvarenga, Z. A taturana. In VII Congresso Brasileiro de Medicina Cirúrgica; Anais da Sociedade de Medicina e Cirurgia: Belo Horizonte, Brazil, 1912; pp. 132–135. [Google Scholar]
- Fraiha-Neto, H.; Ballarini, A.J.; Leão, R.N.Q.; Costa, J.R.D.; Dias, L.B. Síndrome hemorrágica por contato com larvas de mariposa (Lepidoptera, Saturniidae). In Instituto Evandro Chagas: 50 Anos de Contribuição às Ciências Biológicas e à Medicina Tropical; Fundação Serviços de Saúde Pública: Belém, Brazil, 1986; Volume 2, pp. 811–820. [Google Scholar]
- Fan, H.W.; Cardoso, J.L.C.; Olmos, R.D.; Almeida, F.J.; Viana, R.P.; Martinez, A.P.P. Hemorrhagic syndrome and Acute renal failure in a pregnant woman after contact with Lonomia caterpillars: A case report. Rev. Inst. Med. Trop. São Paulo 1998, 40, 119–120. [Google Scholar] [CrossRef][Green Version]
- Arocha-Piñango, C.L.; de Bosch, N.B.; Torres, A.; Goldstein, C.; Nouel, A.; Argüello, A.; Carvajal, Z.; Guerrero, B.; Ojeda, A.; Rodriguez, A. Six New Cases of a Caterpillar-Induced Bleeding Syndrome. Thromb. Haemost. 1992, 67, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Kelen, E.M.A.; Picarelli, Z.P.; Duarte, A.C. Hemorrhagic syndrome induced by contact with caterpillars of the genus Lonomia (saturniidae, hemileucinae). J. Toxicol.-Toxin Rev. 1995, 14, 283–308. [Google Scholar] [CrossRef]
- Duarte, A.C.; Crusius, P.S.; Pires, C.A.; Schilling, M.A.; Fan, H.W. Intracerebral haemorrhage after contact with Lonomia caterpillars. Lancet 1996, 348, 9033. [Google Scholar] [CrossRef]
- Arocha-Piñango, C.L.; Guerrero, B. Lonomia obliqua and haemorrhagic syndrome. Lancet 1999, 354, 9186. [Google Scholar] [CrossRef]
- Pineda, D.; Amarillo, A.; Becerra, J.; Montenegro, G. Síndrome hemorrágico por contacto con orugas del género Lonomia (Saturniidae) en Casanare, Colombia: Informe de dos casos. Biomédica 2001, 21, 328. [Google Scholar] [CrossRef][Green Version]
- Sano-Martins, I.S.; Duarte, A.C.; Guerrero, B.; Moraes, R.H.P.; Barros, E.J.G.; Arocha-Piñango, C.L. Hemostatic disorders induced by skin contact with Lonomia obliqua (Lepidoptera, Saturniidae) caterpillars. Rev. Inst. Med. Trop. Sao Paulo 2017, 59. [Google Scholar] [CrossRef] [PubMed]
- Spadacci-Morena, D.D.; Soares, M.A.; Moraes, R.H.; Sano-Martins, I.S.; Sciani, J.M. The urticating apparatus in the caterpillar of Lonomia obliqua (Lepidoptera: Saturniidae). Toxicon 2016, 119, 218–224. [Google Scholar] [CrossRef]
- Arocha-Piñango, C.L. Fibrinólisis producida por contacto con orugas. Acta Cient. Venez. 1967, 18, 136–139. [Google Scholar]
- Cardoso, A.E.C.; Junior, V.H. Accidents caused by lepidopterans (moth larvae and adult): Study on the epidemiological, clinical and therapeutic aspects. An. Bras. Dermatol. 2005, 80, 571–578. [Google Scholar] [CrossRef]
- Arocha-Piñango, C.L.; Layrisse, M. Fibrinolysis Produced by contact with a caterpillar. Lancet 1969, 293, 810–812. [Google Scholar] [CrossRef]
- Duarte, A.C.; Caovilla, J.; Lorini, I.; Lorini, D.; Mantovani, G.; Sumida, J.; Manfre, P.C.; Silveira, R.C.; Moura, S.P. Insuficiência renal aguda por acidentes com lagartas. J. Bras. Nefrol. 1990, 12, 1033–1187. [Google Scholar]
- Secretária da Saúde do Paraná. SESA/PR. Available online: https://www.saude.pr.gov.br/ (accessed on 31 August 2001).
- CIT/RS. Centro de Informação Toxológica do Rio Grande do Sul. 2005. Available online: http://www.cit.rs.gov.br/ (accessed on 31 August 2001).
- Ministério da Saúde. Acidentes por lepidópteros. In Manual de Diagnóstico e Tratamento de Acidentes por Animais peçoNhentos, 2nd ed.; Assessoria de Comunicação e Educação em Saúde/Ascom/Pre/FUNASA, Ed.; Fundação Nacional de Saúde (FUNASA): Brasilia, Brazil, 2001; pp. 67–76. [Google Scholar]
- Chudzinski-Tavassi, A.M.; Zannin, M. Aspectos clínicos y epidemiológicos del envenenamiento por Lonomia obliqua. In Emergencias por Animales Ponzoñosos en las Américas; D’Suze, G., Corzo Burguete, G.A., Paniagua Solis, J.F., Eds.; Instituto Bioclon: Mexico City, Mexico, 2011; pp. 303–321. [Google Scholar]
- Burdmann, E.A.; Antunes, I.; Saldanha, L.B.; Abdulkader, R.C. Severe acute renal failure induced by the venom of Lonomia caterpillars. Clin. Nephrol. 1996, 46, 337–339. [Google Scholar] [PubMed]
- Schmitberger, P.A.; Fernandes, T.C.; Santos, R.C.; de Assis, R.C.; Gomes, A.P.; Siqueira, P.K.; Vitorino, R.R.; de Mendonça, E.G.; de Almeida Oliveira, M.G.; Siqueira-Batista, R. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Chudzinski-Tavassi, A.M.; Alvarez Flores, M.P. South American Lonomia Obliqua Caterpillars: Morphologic Aspects and Venom Biochemistry. In Lepidoptera; Guerritore, E., DeSare, J., Eds.; Nova Science Publishers: New York, NY, USA, 2013; pp. 169–185. [Google Scholar]
- Berger, M.; de Moraes, J.A.; Beys-da-Silva, W.O.; Santi, L.; Terraciano, P.B.; Driemeier, D.; Cirne-Lima, E.O.; Passos, E.P.; Vieira, M.; Barja-Fidalgo, T.C.; et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl. Trop. Dis. 2019, 13, e0007197. [Google Scholar] [CrossRef] [PubMed]
- Kowacs, P.A.; Cardoso, J.; Entres, M.; Novak, E.M.; Werneck, L.C. Fatal intracerebral hemorrhage secondary to Lonomia obliqua caterpillar envenoming: Case report. Arq. Neuro-Psiquiatr. 2006, 64, 1030–1032. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos, J.H.A.; Oliveira, S.S.; Alves, E.C.; Mendonça-da-Silva, I.; Sachett, J.A.G.; Tavares, A.; Ferreira, L.C.; Fan, H.W.; Lacerda, M.; Monteiro, W.M. Severe Hemorrhagic Syndrome After Lonomia Caterpillar Envenomation in the Western Brazilian Amazon: How Many More Cases Are There? Wilderness Environ. Med. 2017, 28, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, C.B.; Reck Junior, J.; Fernandes, D.; Sordi, R.; Guimarães, J.A.; Assreuy, J.; Termignoni, C. Kallikrein–kinin system activation by Lonomia obliqua caterpillar bristles: Involvement in edema and hypotension responses to envenomation. Toxicon 2007, 49, 663–669. [Google Scholar] [CrossRef]
- Alvarez Flores, M.P.; Zannin, M.; Chudzinski-Tavassi, A.M. New Insight into the Mechanism of Lonomia obliqua envenoming: Toxin involvement and molecular approach. Pathophysiol. Haemost. Thromb. 2010, 37, 1–16. [Google Scholar] [CrossRef]
- Gando, S.; Levi, M.; Toh, C.-H. Disseminated intravascular coagulation. Nat. Rev. Dis. Primers 2016, 2, 16037. [Google Scholar] [CrossRef]
- Wada, H.; Sakuragawa, N.; Mori, Y.; Takagi, M.; Nakasaki, T.; Shimura, M.; Hiyoyama, K.; Nisikawa, M.; Gabazza, E.C.; Deguchi, K.; et al. Hemostatic molecular markers before the onset of disseminated intravascular coagulation. Am. J. Hematol. 1999, 60, 273–278. [Google Scholar] [CrossRef]
- Spero, J.A.; Lewis, J.H.; Hasiba, U. Disseminated intravascular coagulation. Findings in 346 patients. Thromb. Haemost. 1980, 43, 28–33. [Google Scholar]
- Guerrero, B.A.; Arocha-Piñango, C.L.; San Juan, A.G. Lonomia achelous caterpillar venom (LACV) selectively inactivates blood clotting factor XIII. Thromb. Res. 1997, 87, 83–93. [Google Scholar] [CrossRef]
- Bauer, K.A. Activation markers of coagulation. Best Pract. Res. Clin. Haematol. 1999, 12, 387–406. [Google Scholar] [CrossRef]
- Delabranche, X.; Helms, J.; Meziani, F. Immunohaemostasis: A new view on haemostasis during sepsis. Ann. Intensive Care 2017, 7, 117. [Google Scholar] [CrossRef]
- Alvarez-Flores, M.P.; Furlin, D.; Ramos, O.H.P.; Balan, A.; Konno, K.; Chudzinski-Tavassi, A.M. Losac, the First Hemolin that Exhibits Procogulant Activity through Selective Factor X Proteolytic Activation. J. Biol. Chem. 2011, 286, 6918–6928. [Google Scholar] [CrossRef] [PubMed]
- Donato, J.L.; Moreno, R.A.; Hyslop, S.; Duarte, A.; Antunes, E.; Le Bonniec, B.F.; Rendu, F.; de Nucci, G. Lonomia obliqua caterpillar spicules trigger human blood coagulation via activation of factor X and prothrombin. Thromb. Haemost. 1998, 79, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.V.; Kelen, E.; Farsky, S.; Portaro, F.; Sampaio, C.; Fernandes, B.; Camargo, A.C.; Chudzinski-Tavassi, A.M. A Ca++ activated serine protease (LOPAP) could be responsible for the haemorrhagic syndrome caused by the caterpillar Lonomia obliqua. Lancet 1999, 353, 1942. [Google Scholar] [CrossRef]
- Reis, C.V.; Farsky, S.H.P.; Fernandes, B.L.; Santoro, M.L.; Oliva, M.L.V.; Mariano, M.; Chudzinski-Tavassi, A.M. In Vivo Characterization of Lopap, a Prothrombin Activator Serine Protease from the Lonomia obliqua Caterpillar Venom. Thromb. Res. 2001, 102, 437–443. [Google Scholar] [CrossRef]
- Reis, C.V.; Andrade, S.A.; Ramos, O.H.P.; Ramos, C.R.R.; Ho, P.L.; de Batista, I.F.C.; Chudzinski-Tavassi, A.M. Lopap, a prothrombin activator from Lonomia obliqua belonging to the lipocalin family: Recombinant production, biochemical characterization and structure–function insights. Biochem. J. 2006, 398, 295–302. [Google Scholar] [CrossRef]
- Chudzinski-Tavassi, A.M.; Alvarez-Flores, M.P.; Carrijo-Carvalho, L.C.; Ricci-Silva, M.E. Toxins from Lonomia obliqua: Recombinant production and molecular approach. In An Integrated View of the Molecular Recognition and Toxinology—From Analytical Procedures to Biomedical Applications; Baptista, G.R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 1–32. [Google Scholar]
- Veiga, A.B.G.; Ribeiro, J.M.C.; Guimarães, J.A.; Francischetti, I.M.B. A catalog for the transcripts from the venomous structures of the caterpillar Lonomia obliqua: Identification of the proteins potentially involved in the coagulation disorder and hemorrhagic syndrome. Gene 2005, 355, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Silva, M.E.; Valente, R.H.; León, I.R.; Tambourgi, D.V.; Ramos, O.H.P.; Perales, J.; Chudzinski-Tavassi, A.M. Immunochemical and proteomic technologies as tools for unravelling toxins involved in envenoming by accidental contact with Lonomia obliqua caterpillars. Toxicon 2008, 51, 1017–1028. [Google Scholar] [CrossRef]
- Chudzinski-Tavassi, A.M.; Alvarez Flores, M.P. Exploring new molecules and activities from Lonomia obliqua caterpillars. Pathophysiol. Haemost. Thromb. 2005, 34, 228–233. [Google Scholar] [CrossRef]
- Seibert, C.S.; Shinohara, E.M.G.; Sano-Martins, I.S. In vitro hemolytic activity of Lonomia obliqua caterpillar bristle extract on human and Wistar rat erythrocytes. Toxicon 2003, 41, 831–839. [Google Scholar] [CrossRef]
- Seibert, C.S.; Oliveira, M.R.L.; Gonçalves, L.R.C.; Santoro, M.L.; Sano-Martins, I.S. Intravascular hemolysis induced by Lonomia obliqua caterpillar bristle extract: An experimental model of envenomation in rats. Toxicon 2004, 44, 793–799. [Google Scholar] [CrossRef]
- Seibert, C.S.; Santoro, M.L.; Tambourgi, D.V.; Sampaio, S.C.; Takahashi, H.K.; Peres, C.M.; Curi, R.; Sano-Martins, I.S. Lonomia obliqua (Lepidoptera, Saturniidae) caterpillar bristle extract induces direct lysis by cleaving erythrocyte membrane glycoproteins. Toxicon 2010, 55, 1323–1330. [Google Scholar] [CrossRef]
- Seibert, C.S.; Tanaka-Azevedo, A.M.; Santoro, M.L.; Mackessy, S.P.; Soares Torquato, R.J.; Lebrun, I.; Tanaka, A.S.; Sano-Martins, I.S. Purification of a phospholipase A2 from Lonomia obliqua caterpillar bristle extract. Biochem. Biophys. Res. Commun. 2006, 342, 1027–1033. [Google Scholar] [CrossRef]
- Fritzen, M.; Flores, M.P.A.; Reis, C.V.; Chudzinski-Tavassi, A.M. A prothrombin activator (Lopap) modulating inflammation, coagulation and cell survival mechanisms. Biochem. Biophys. Res. Commun. 2005, 333, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.V.; Portaro, F.C.V.; Andrade, S.A.; Fritzen, M.; Fernandes, B.L.; Sampaio, C.A.; Camargo, A.C.; Chudzinski-Tavassi, A.M. A Prothrombin Activator Serine Protease from the Lonomia obliqua Caterpillar Venom (Lopap). Thromb. Res. 2001, 102, 427–436. [Google Scholar] [CrossRef]
- Veiga, A.B.G.; Ribeiro, J.M.C.; Francischetti, I.M.B.; Xu, X.; Guimarães, J.A.; Andersen, J.F. Examination of the Ligand-Binding and Enzymatic Properties of a Bilin-Binding Protein from the Poisonous Caterpillar Lonomia obliqua. PLoS ONE 2014, 9, e95424. [Google Scholar] [CrossRef]
- Sato, A.C.; Bosch, R.V.; Will, S.E.; Alvarez-Flores, M.P.; Goldfeder, M.B.; Pasqualoto, K.F.; da Silva, B.A.; de Andrade, S.A.; Chudzinski-Tavassi, A.M. Exploring the in vivo wound healing effects of a recombinant hemolin from the caterpillar Lonomia obliqua. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.V.; Alvarez-Flores, M.P.; Maria, D.A.; Chudzinski-Tavassi, A.M. Hemolin triggers cell survival on fibroblasts in response to serum deprivation by inhibition of apoptosis. Biomed. Pharmacother. 2016, 82, 537–546. [Google Scholar] [CrossRef]
- Chudzinski Tavassi, A.M.; Alvarez-Flores, M.P. Lonomia obliqua brasileña: Bioquímica y fisiopatologia del veneno. In Emergencias por Animales Ponzoñosos en las Américas; D’Suze, G., Corzo Burguete, G.A., Paniagua Solis, J.F., Eds.; Instituto Bioclon: Mexico City, Mexico, 2011; pp. 323–369. [Google Scholar]
- Alvarez-Flores, M.P.; Hébert, A.; Gouelle, C.; Geller, S.; Chudzinski-Tavassi, A.M.; Pellerin, L. Neuroprotective effect of rLosac on supplement-deprived mouse cultured cortical neurons involves maintenance of monocarboxylate transporter MCT2 protein levels. J. Neurochem. 2019, 148, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Flores, M.P.; Fritzen, M.; Reis, C.V.; Chudzinski-Tavassi, A.M. Losac, a factor X activator from Lonomia obliqua bristle extract: Its role in the pathophysiological mechanisms and cell survival. Biochem. Biophys. Res. Commun. 2006, 343, 1216–1223. [Google Scholar] [CrossRef]
- Lilla, S.; Pereira, R.; Hyslop, S.; Donato, J.L.; Le Bonniec, B.F.; de Nucci, G. Purification and initial characterization of a novel protein with factor Xa activity from Lonomia obliqua caterpillar spicules. J. Mass Spectrom. 2005, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.F.; Dobrovolski, R.; Veiga, A.B.; Guimarães, J.A. Lonofibrase, a novel alpha-fibrinogenase from Lonomia obliqua caterpillars. Thromb. Res. 2004, 113, 147–154. [Google Scholar] [CrossRef]
- da CB Gouveia, A.I.; da Silveira, R.B.; Nader, H.B.; Dietrich, C.P.; Gremski, W.; Veiga, S.S. Identification and partial characterisation of hyaluronidases in Lonomia obliqua venom. Toxicon 2005, 45, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Heinen, T.E.; de Farias, C.B.; Abujamra, A.L.; Mendonça, R.Z.; Roesler, R.; da Veiga, A.B.G. Effects of Lonomia obliqua caterpillar venom upon the proliferation and viability of cell lines. Cytotechnology 2014, 66, 63–74. [Google Scholar] [CrossRef][Green Version]
- Souza, A.P.; Peixoto, C.C.; Maranga, L.; Carvalhal, A.V.; Moraes, R.H.; Mendonça, R.M.; Pereira, C.A.; Carrondo, M.J.; Mendonça, R.Z. Purification and characterization of an anti-apoptotic protein isolated from Lonomia obliqua hemolymph. Biotechnol. Prog. 2005, 21, 99–105. [Google Scholar] [CrossRef]
- Carmo, A.C.V.; Yamasaki, L.H.T.; Figueiredo, C.A.; da Silva Giovanni, D.N.; de Oliveira, M.I.; Dos Santos, F.C.P.; Curti, S.P.; Rahal, P.; Mendonça, R.Z. Discovery of a new antiviral protein isolated Lonomia obliqua analysed by bioinformatics and real-time approaches. Cytotechnology 2015, 67, 1011–1022. [Google Scholar] [CrossRef]
- Sousa, Á.P.B.; Moraes, R.H.P.; Mendonça, R.Z. Improved replication of the baculovirus Anticarsia gemmatalis nucleopolyhedrovirus (AgMNPV) in vitro using proteins from Lonomia obliqua hemolymph. Cytotechnology 2015, 67, 331–342. [Google Scholar] [CrossRef]
- de Castro Bastos, L.; Veiga, A.B.; Guimarães, J.A.; Tonussi, C.R. Nociceptive and edematogenic responses elicited by a crude bristle extract of Lonomia obliqua caterpillars. Toxicon 2004, 43, 273–278. [Google Scholar] [CrossRef]
- Nascimento-Silva, V.; Rodrigues da Silva, G.; Moraes, J.A.; Cyrino, F.Z.; Seabra, S.H.; Bouskela, E.; Almeida Guimarães, J.; Barja-Fidalgo, C. A pro-inflammatory profile of endothelial cell in Lonomia obliqua envenomation. Toxicon 2012, 60, 50–60. [Google Scholar] [CrossRef]
- Oliveira, D.S.; de Souza, J.G.; Alvarez-Flores, M.P.; Cunegundes, P.S.; DeOcesano-Pereira, C.; Lobba, A.M.; Gomes, R.N.; Chudzinski-Tavassi, A.M. Lonomia obliqua Venom Induces NF-κB Activation and a Pro-Inflammatory Profile in THP-1-Derived Macrophage. Toxins 2021, 13, 462. [Google Scholar] [CrossRef]
- Pinto, A.F.M.; Dragulev, B.; Guimarães, J.A.; Fox, J.W. Novel perspectives on the pathogenesis of Lonomia obliqua caterpillar envenomation based on assessment of host response by gene expression analysis. Toxicon 2008, 51, 1119–1128. [Google Scholar] [CrossRef]
- Bernardi, L.; Pinto, A.F.M.; Mendes, E.; Yates, J.R.; Lamers, M.L. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon 2019, 162, 32–39. [Google Scholar] [CrossRef]
- Chudzinski-Tavassi, A.M.; Schattner, M.; Fritzen, M.; Pozner, R.G.; Reis, C.V.; Lourenço, D.; Lazzari, M.A. Effects of Lopap on Human Endothelial Cells and Platelets. Pathophysiol. Haemost. Thromb. 2001, 31, 257–265. [Google Scholar] [CrossRef]
- Berger, M.; Reck, J.; Terra, R.M.S.; Pinto, A.F.M.; Termignoni, C.; Guimarães, J.A. Lonomia obliqua caterpillar envenomation causes platelet hypoaggregation and blood incoagulability in rats. Toxicon 2010, 55, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Reck, J.; Terra, R.M.S.; Beys da Silva, W.O.; Santi, L.; Pinto, A.F.; Vainstein, M.H.; Termignoni, C.; Guimarães, J.A. Lonomia obliqua venomous secretion induces human platelet adhesion and aggregation. J. Thromb. Thrombolysis 2010, 30, 300–310. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Schmidt, O.; Söderhäll, K.; Theopold, U.; Faye, I. Role of adhesion in arthropod immune recognition. Ann. Rev. Entomol. 2010, 55, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Waismam, K.; Chudzinski-Tavassi, A.M.; Carrijo-Carvalho, L.C.; Fernandes Pacheco, M.T.; Farsky, S.H.P. Lopap: A non-inflammatory and cytoprotective molecule in neutrophils and endothelial cells. Toxicon 2009, 53, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Chudzinski-Tavassi, A.M.; Carrijo-Carvalho, L.C.; Waismam, K.; Farsky, S.H.P.; Ramos, O.H.P.; Reis, C.V. A lipocalin sequence signature modulates cell survival. FEBS Lett. 2010, 584, 2896–2900. [Google Scholar] [CrossRef] [PubMed]
- Carrijo-Carvalho, L.C.; Maria, D.A.; Ventura, J.S.; Morais, K.L.P.; Melo, R.L.; Rodrigues, C.J.; Chudzinski-Tavassi, A.M. A Lipocalin-Derived Peptide Modulating Fibroblasts and Extracellular Matrix Proteins. J. Toxicol. 2012, 2012, 325250. [Google Scholar] [CrossRef]
- Wlian, L.; Carrijo-Carvalho, L.C.; Andrade, S.A.; Lourenço, S.V.; Rodrigues, C.J.; Maria, D.A.; Chudzinski-Tavassi, A.M. Wound Healing Effects of a Lipocalin-Derived Peptide. J. Clin. Toxicon 2014, 4, 2–6. [Google Scholar]
- Mesquita Pasqualoto, K.F.; Carrijo-Carvalho, L.C.; Chudzinski-Tavassi, A.M. Rational development of novel leads from animal secretion based on coagulation and cell targets: 1. In silico analysis to explore a peptide derivative as lipocalins’ signature. Toxicon 2013, 69, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Björkqvist, J.; Jämsä, A.; Renné, T. Plasma kallikrein: The bradykinin-producing enzyme. Thromb. Haemost. 2013, 110, 399–407. [Google Scholar] [CrossRef]
- Zanon, P.; Pizzato, S.B.; da Rosa, R.L.; Terraciano, P.B.; Moraes, J.A.; Beys-da-Silva, W.O.; Santi, L.; Yates, J.R., 3rd; Passos, E.P.; Barja-Fidalgo, C.; et al. Urine proteomic analysis reveals alterations in heme/hemoglobin and aminopeptidase metabolism during Lonomia obliqua venom-induced acute kidney injury. Toxicol. Lett. 2021, 341, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Santi, L.; Beys-da-Silva, W.O.; Oliveira, F.M.S.; Caliari, M.V.; Yates, J.R., 3rd; Vieira, M.A.; Guimarães, J.A. Mechanisms of acute kidney injury induced by experimental Lonomia obliqua envenomation. Arch. Toxicol. 2015, 89, 459–483. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte Migration into Inflamed Tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Claesson-Welsh, L. Vascular permeability—The essentials. Upsala J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef]
- Moraes, J.; Rodrigues, G.; Nascimento-Silva, V.; Renovato-Martins, M.; Berger, M.; Guimarães, J.; Barja-Fidalgo, C. Effects of Lonomia obliqua Venom on Vascular Smooth Muscle Cells: Contribution of NADPH Oxidase-Derived Reactive Oxygen Species. Toxins 2017, 9, 360. [Google Scholar] [CrossRef]
- Berger, M.; Beys-da-Silva, W.O.; Santi, L.; de Oliveira, I.M.; Jorge, P.M.; Henriques, J.A.; Driemeier, D.; Vieira, M.A.; Guimarães, J.A. Acute Lonomia obliqua caterpillar envenomation-induced physiopathological alterations in rats: Evidence of new toxic venom activities and the efficacy of serum therapy to counteract systemic tissue damage. Toxicon 2013, 74, 179–192. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, A.C.M. Perspective for pharmaceutical innovation in Brazil—center for applied toxinology (CEPID- center for research, innovation and dissemination—FAPESP). J. Venom. Anim. Toxins Incl. Trop. Dis. 2005, 11, 384–390. [Google Scholar] [CrossRef]
- Kim, H.J.; Je, H.J.; Cheon, H.M.; Kong, S.Y.; Han, J.; Yun, C.Y.; Han, Y.S.; Lee, I.H.; Kang, Y.J.; Seo, S.J. Accumulation of 23kDa lipocalin during brain development and injury in Hyphantria cunea. Insect Biochem. Mol. Biol. 2005, 35, 1133–1141. [Google Scholar] [CrossRef]
- Spreyer, P.; Schaal, H.; Kuhn, G.; Rothe, T.; Unterbeck, A.; Olek, K.; Müller, H.W. Regeneration-associated high level expression of apolipoprotein D mRNA in endoneurial fibroblasts of peripheral nerve. EMBO J. 1990, 9, 2479–2484. [Google Scholar] [CrossRef]
- Playford, R.J.; Belo, A.; Poulsom, R.; Fitzgerald, A.J.; Harris, K.; Pawluczyk, I.; Ryon, J.; Darby, T.; Nilsen-Hamilton, M.; Ghosh, S.; et al. Effects of Mouse and Human Lipocalin Homologues 24p3/lcn2 and Neutrophil Gelatinase–Associated Lipocalin on Gastrointestinal Mucosal Integrity and Repair. Gastroenterology 2006, 131, 809–817. [Google Scholar] [CrossRef]
- Flower, D.R. The lipocalin protein family: Structure and function. Biochem. J. 1996, 318, 1–14. [Google Scholar] [CrossRef]
- Rassart, E.; Desmarais, F.; Najyb, O.; Bergeron, K.-F.; Mounier, C. Apolipoprotein D. Gene 2020, 756, 144874. [Google Scholar] [CrossRef] [PubMed]
- Flower, D.R.; North, A.C.T.; Attwood, T.K. Structure and sequence relationships in the lipocalins and related proteins. Protein Sci. 1993, 2, 753–761. [Google Scholar] [CrossRef]
- Li, W.; Terenius, O.; Hirai, M.; Nilsson, A.S.; Faye, I. Cloning, expression and phylogenetic analysis of Hemolin, from the Chinese oak silkmoth, Antheraea pernyi. Dev. Comp. Immunol. 2005, 29, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, R.; Lanz-Mendoza, H.; Lindquist, K.R.; Faye, I. Cell Adhesion Properties of Hemolin, an Insect Immune Protein in the Ig Superfamily. Eur. J. Biochem. 1997, 250, 630–637. [Google Scholar] [CrossRef]
- Bettencourt, R.; Gunne, H.; Gastinel, L.; Steiner, H.; Faye, I. Implications of hemolin glycosylation and Ca2+-binding on homophilic and cellular interactions. Eur. J. Biochem. 1999, 266, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Maurer, P.; Hohenester, E.; Engel, J. Extracellular calcium-binding proteins. Curr. Opin. Cell Biol. 1996, 8, 609–617. [Google Scholar] [CrossRef]
- Tedder, T.F.; Steeber, D.A.; Chen, A.; Engel, P. The selectins: Vascular adhesion molecules. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 866–873. [Google Scholar]
- WHO. Ten Threats to Global Health in 2019. 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 31 August 2021).
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef]

| Manifestations of Severity | Clinical Local Picture | Coagulation Time | Bleeding | Treatment |
|---|---|---|---|---|
| Mild | Present | Normal | Absent | No ALS required |
| Moderate | Present or absent | Altered | Absent or present in skin/mucous membranes | Serotherapy: 5 ampoules of ALS |
| Severe | Present or absent | Altered | Present in viscera. Life-threatening | Serotherapy: 10 ampoules of ALS |
| Toxin/Biological Activity | Source | Method of Detection/Characteristic | References |
|---|---|---|---|
| Lipocalin | LOCBE Hemolymph | Edman sequencing cDNA library More abundant | [47,49] |
| LOCBE | Proteome | [50] | |
| Tegument Cryosecretion | Edman sequencing | [49] | |
| Hemolin | LOCBE | Edman sequencing cDNA library | [47,49] |
| Serpin | LOCBE | Edman sequencing cDNA library | [49,51] |
| Proteome | [50] | ||
| Tegument | Edman sequencing cDNA library | [49] | |
| Hemolymph Cryosecretion | Edman sequencing | [49] | |
| Kininogen | LOCBE | cDNA library | [49,51] |
| Trypsin | LOCBE | Edman sequencing | [49,51] |
| Lectin | LOCBE | Edman sequencing cDNA library | [49,51] |
| Tegument | cDNA library | [49] | |
| Transferrin | LOCBE Tegument Hemolymph Cryosecretion | Edman sequencing | [49] |
| Laminin | LOCBE | Edman sequencing | [49] |
| Protease inhibitor | Hemolymph | Edman sequencing | [49] |
| Tegument | cDNA Library | [49] | |
| Serine proteases | LOCBE | cDNA Library | [49] |
| Phospholipase A2 (PLA-2)/hemolytic activity | LOCBE | cDNA Library | [49,51] |
| Purified protein with Indirect hemolytic activity | [52,53,54,55] | ||
| Tegument | cDNA Library | [49] | |
| Lopap | LOCBE | Proteoma cDNA library Native and recombinant protein Lipocalin-like Prothrombin activator Cytoprotector | [45,46,47,56,57] |
| Bilin-binding proteins (BBP) | LOCBE | Recombinant Lipocalin Similar to Lopap No prothrombinase activity | [58] |
| Losac | LOCBE | Native and recombinant protein Hemolin-like Factor X activator Neuroprotection Antiapoptotic | [59,60,61,62,63] |
| Factor Xa-like | LOCBE | Sequence similar to Lopap Enzymatic activity on S-2222 chromogenic substrate | [64] |
| Lonofibrase | Hemolymph | Fibrinogenolytic activity | [48,65] |
| Lonoglyases | LOCBE | Hyaluronidase Degradation of ECM | [66] |
| Antiapoptotic/proliferative | LOCBE | Activity on several cell cultures | [56,60,62,63,67] |
| Hemolymph | Activity on Spodoptera frugiperda (Sf-9) | [68] | |
| Antiviral | Hemolymph | Recombinant protein Effect of several virus | [69,70] |
| Nociceptive and edematogenic | LOCBE | Prostaglandins favors nociception Kallikrein inhibitor or bradykinin B2 receptor antagonists reduces the edema and hypotension | [35,71] |
| Kallikrein–kinin system activation | LOCBE | In vitro and in vivo studies showed activation of kinin system | [32,35,71] |
| Proinflammatory response | LOCBE | Proinflammatory phenotype in macrophages and endothelial cells NF-κB pathway activation | [72,73,74] |
| Modulation of cell adhesion/cytoskeleton dynamics | LOCBE | Changes in cell–ECM interaction | [74,75] |
| Platelet adhesion and aggregation | LOCBE | Platelet aggregation in vitro inhibited by PLA-2 inhibitors | [76,77,78] |
| Molecule | Patent Number | Claim |
|---|---|---|
| Lopap | INPI (PI0200269-8, Brazil, 26 July 2016). | Lopap obtention process |
| WO03/070746 (WIPO) | ||
| AU2003208190 (Australia) | ||
| CAN2,471,410 (Canada) | ||
| EP1482969 (German) | ||
| JP2003-569653 (Japan) | ||
| MX04007344 (Mexico) | ||
| US10/501,238 (USA) | ||
| P4 | INPI (PCT/IB2009/05023, Brazil, 21 July 2010) | Tissue remodeling and tissue repair |
| 101965398B, 2015 (China) | ||
| JP5777886B2, 2015 (Japan) | ||
| EP2245149B1, 2015 (German) | ||
| US8,883,740 B2, 2014 (USA) | ||
| Losac-derived peptides | INPI (BR10201901866, Brazil, 9 September 2019) | Anti-inflammatory agents |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Flores, M.P.; Gomes, R.N.; Trevisan-Silva, D.; Oliveira, D.S.; Batista, I.d.F.C.; Buri, M.V.; Alvarez, A.M.; DeOcesano-Pereira, C.; de Souza, M.M.; Chudzinski-Tavassi, A.M. Lonomia obliqua Envenoming and Innovative Research. Toxins 2021, 13, 832. https://doi.org/10.3390/toxins13120832
Alvarez-Flores MP, Gomes RN, Trevisan-Silva D, Oliveira DS, Batista IdFC, Buri MV, Alvarez AM, DeOcesano-Pereira C, de Souza MM, Chudzinski-Tavassi AM. Lonomia obliqua Envenoming and Innovative Research. Toxins. 2021; 13(12):832. https://doi.org/10.3390/toxins13120832
Chicago/Turabian StyleAlvarez-Flores, Miryam Paola, Renata Nascimento Gomes, Dilza Trevisan-Silva, Douglas Souza Oliveira, Isabel de Fátima Correia Batista, Marcus Vinicius Buri, Angela Maria Alvarez, Carlos DeOcesano-Pereira, Marcelo Medina de Souza, and Ana Marisa Chudzinski-Tavassi. 2021. "Lonomia obliqua Envenoming and Innovative Research" Toxins 13, no. 12: 832. https://doi.org/10.3390/toxins13120832
APA StyleAlvarez-Flores, M. P., Gomes, R. N., Trevisan-Silva, D., Oliveira, D. S., Batista, I. d. F. C., Buri, M. V., Alvarez, A. M., DeOcesano-Pereira, C., de Souza, M. M., & Chudzinski-Tavassi, A. M. (2021). Lonomia obliqua Envenoming and Innovative Research. Toxins, 13(12), 832. https://doi.org/10.3390/toxins13120832