Biological and Inflammatory Effects of Antigen 5 from Polybia paulista (Hymenoptera, Vespidae) Venom in Mouse Intraperitoneal Macrophages
Abstract
:1. Introduction
2. Results
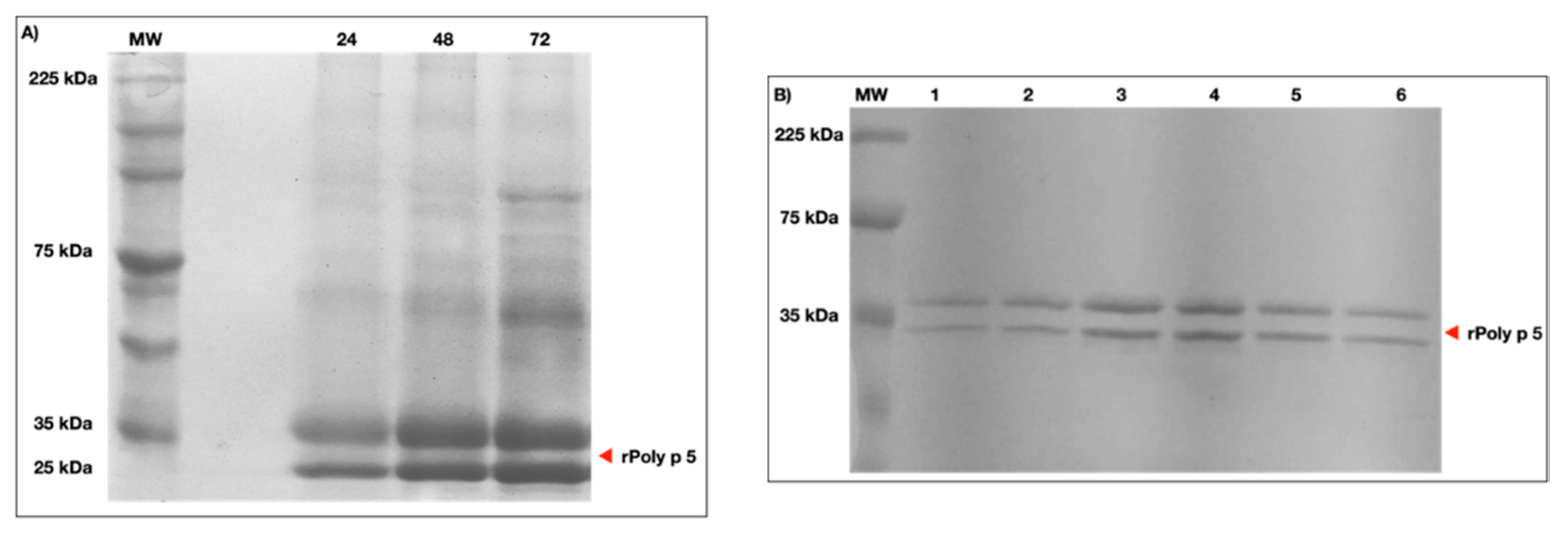
2.1. Expression and Purification of rPoly p 5
2.2. Nitric Oxide Production
2.3. Pro and Antiinflammatory Cytokine Production
2.4. Co-Stimulatory Molecules and MHCII Expression
2.5. Expression of p65 Phosphorylated Form of NF-kB
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Recombinant Antigen 5 (rPoly p 5) Obtention
5.2. SDS-PAGE and Protein Quantification
5.3. Macrophage Cell Culture
5.4. Determination of Nitrite Concentration in Culture Supernatants
5.5. Evaluation of CD80, CD86 and MHCII Cell Markers
5.6. Quantification of Cytokines in Culture Supernatants
5.7. Evaluation of p65 Phosphorylated Subunit Form of NF-κB
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bazon, M.L.; Perez-Riverol, A.; Dos Santos-Pinto, J.R.A.; Fernandes, L.G.R.; Lasa, A.M.; Justo-Jacomini, D.L.; Palma, M.S.; De Lima Zollner, R.; Brochetto-Braga, M.R. Heterologous expression, purification and immunoreactivity of the antigen 5 from polybia paulista wasp venom. Toxins 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, L.D.; da Silva Menegasso, A.R.; dos Santos Pinto, J.R.A.; Santos, K.S.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Proteomic characterization of the multiple forms of the PLAs from the venom of the social wasp Polybia paulista. Proteomics 2011, 11, 1403–1412. [Google Scholar] [CrossRef]
- Justo Jacomini, D.L.; Campos Pereira, F.D.; Aparecido dos Santos Pinto, J.R.; dos Santos, L.D.; da Silva Neto, A.J.; Giratto, D.T.; Palma, M.S.; de Lima Zollner, R.; Brochetto Braga, M.R. Hyaluronidase from the venom of the social wasp Polybia paulista (Hymenoptera, Vespidae): Cloning, structural modeling, purification, and immunological analysis. Toxicon 2013, 64, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos-Pinto, J.R.A.; Dos Santos, L.D.; Andrade Arcuri, H.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Using proteomic strategies for sequencing and post-translational modifications assignment of antigen-5, a major allergen from the venom of the social wasp polybia paulista. J. Proteome Res. 2014, 13, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, G.M.; Roelants, K.; O’Bryan, M.K. The CAP superfamily: Cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins - Roles in reproduction, cancer, and immune defense. Endocr. Rev. 2008, 29, 865–897. [Google Scholar] [CrossRef]
- Schiener, M.; Eberlein, B.; Moreno-Aguilar, C.; Pietsch, G.; Serrano, P.; McIntyre, M.; Schwarze, L.; Russkamp, D.; Biedermann, T.; Spillner, E.; et al. Application of recombinant antigen 5 allergens from seven allergy-relevant Hymenoptera species in diagnostics. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 98–108. [Google Scholar] [CrossRef]
- Blank, S.; Bazon, M.L.; Grosch, J.; Schmidt-Weber, C.B.; Brochetto-Braga, M.R.; Bilò, M.B.; Jakob, T. Antigen 5 Allergens of Hymenoptera Venoms and Their Role in Diagnosis and Therapy of Venom Allergy. Curr. Allergy Asthma Rep. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.C.F.; Wiezel, G.A.; Amorim, F.G.; Arantes, E.C. Arthropod venom Hyaluronidases: Biochemical properties and potential applications in medicine and biotechnology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Blank, S.; Grosch, J.; Ollert, M.; Bilò, M.B. Precision Medicine in Hymenoptera Venom Allergy: Diagnostics, Biomarkers, and Therapy of Different Endotypes and Phenotypes. Front. Immunol. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Müller, U.; Schmid-Grendelmeier, P.; Hausmann, O.; Helbling, A. IgE to recombinant allergens Api m 1, Ves v 1, and Ves v 5 distinguish double sensitization from crossreaction in venom allergy. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Vinzón, S.E.; Pirpignani, M.L.; Nowicki, C.; De Jimènez Bonino, M.B. Molecular cloning and expression in Pichia pastoris of a hypoallergenic antigen 5. Protein Expr. Purif. 2010, 73, 23–30. [Google Scholar] [CrossRef] [PubMed]
- King, T.P.; Jim, S.Y.; Monsalve, R.I.; Kagey-Sobotka, A.; Lichtenstein, L.M.; Spangfort, M.D. Recombinant Allergens with Reduced Allergenicity but Retaining Immunogenicity of the Natural Allergens: Hybrids of Yellow Jacket and Paper Wasp Venom Allergen Antigen 5s. J. Immunol. 2001, 166, 6057–6065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Riverol, A.; Miehe, M.; Jabs, F.; Seismman, H.; Romani Fernandes, L.G.; de Lima Zollner, R.; Jakob, T.; Brochetto Braga, M.R.; Spillner, E. Venoms of Neotropical wasps lack cross-reactive carbohydrate determinants enabling reliable protein-based specific IgE determination. J. Allergy Clin. Immunol. 2018, 141, 1917–1919. [Google Scholar] [CrossRef] [Green Version]
- Lina, W.W.; Chen, B.C.; Hsu, Y.W.; Lee, C.M.; Shyue, S.K. Modulation of inducible nitric oxide synthase induction by prostaglandin E2 in macrophages: Distinct susceptibility in murine J774 and RAW 264.7 macrophages. Prostaglandins Other Lipid Mediat. 1999, 58, 87–101. [Google Scholar] [CrossRef]
- Wu, T.M.; Chou, T.C.; Ding, Y.A.; Li, M.L. Stimulation of TNF-α, IL-1β and nitrite release from mouse cultured spleen cells and lavaged peritoneal cells by mastoparan M. Immunol. Cell Biol. 1999, 77, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Kim, S.K.; Han, J.B.; Ahn, H.J.; Bae, H.; Min, B. Il Effects of bee venom on the pro-inflammatory responses in RAW264.7 macrophage cell line. J. Ethnopharmacol. 2005, 99, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Kweon, H.Y.; Woo, S.O.; Lee, M.L.; Baek, H.J.; Kim, S.Y.; Park, K.K. Effect of honey bee venom on microglial cells nitric oxide and tumor necrosis factor-α production stimulated by LPS. J. Ethnopharmacol. 2007, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.; Shafeeq, T.; Irfan, M.; Lee, Y.Y.; Kwon, H.W.; Seo, M.G.; Park, S.J.; Lee, K.Y.; Rhee, M.H. Anti-Inflammatory Activity of Crude Venom Isolated from Parasitoid Wasp, Bracon hebetor Say. Mediators Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, D.K.; Thounaojam, M.C.; Mitra, A.; Basu, A. Vespa tropica venom suppresses lipopolysaccharide-mediated secretion of pro-inflammatory cyto-chemokines by abrogating nuclear factor-κ B activation in microglia. Inflamm. Res. 2014, 63, 657–665. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. ™ Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Eidlen, D.; Arend, W.P. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J. Clin. Invest. 1997, 99, 2930–2940. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.W.; Malefyt, R.D.W.; Robert, L.; Garra, A.O. I Nterleukin -10 and the I Nterleukin -10. Mol. Cell. Biol. 2001, 1, 683–765. [Google Scholar]
- Tavano, R.; Segat, D.; Gobbo, M.; Papini, E. The honeybee antimicrobial peptide apidaecin differentially immunomodulates human macrophages, monocytes and dendritic cells. J. Innate Immun. 2011, 3, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Feng, Y.; Yang, Y.; Jingliu; Zhou, W.; He, P.; Zhou, R.; Li, X.; Zou, J. Kinetics of the phenotype and function of murine peritoneal macrophages following acute inflammation. Cell. Mol. Immunol. 2004, 1, 57–62. [Google Scholar] [PubMed]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S. NF-≃B antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c- IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.I.; Cidlowski, J.A. Molecular control of immune/inflammatory responses: Interactions between nuclear factor-κB and steroid receptor-signaling pathways. Endocr. Rev. 1999, 20, 435–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Acquisto, F.; May, M.J.; Ghosh, S. Inhibition of nuclear factor kappa B (NF-B): An emerging theme in anti-inflammatory therapies. Mol. Interv. 2002, 2, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Gerstberger, S.; Carlson, L.; Franzoso, G.; Siebenlist, U. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 1995, 267, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.; Moskaug, J.Ø.; Fromm, S.H.; Blomhoff, R. In Vivo Imaging of NF-κB Activity. J. Immunol. 2002, 168, 1441–1446. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.D. Anatomy of a discovery: M1 and M2 macrophages. Front. Immunol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Dang, L.; Xi, G.S.; Wang, F. Molecular cloning, characterization and expression analysis of calreticulin gene in the ant polyrhachis vicina roger (hymenoptera: SsFormicidae). Sociobiology 2013, 60, 355–361. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef]
- Justo, O.R.; Simioni, P.U.; Gabriel, D.L.; Tamashiro, W.M.d.S.C.; Rosa, P.d.T.V.; Moraes, A.M. Evaluation of in vitro anti-inflammatory effects of crude ginger and rosemary extracts obtained through supercritical CO2 extraction on macrophage and tumor cell line: The influence of vehicle type. BMC Complement. Altern. Med. 2015, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazon, M.L.; Fernandes, L.G.R.; Assugeni, I.O.S.; Pinto, L.M.; Simioni, P.U.; Zollner, R.d.L.; Braga, M.R.B. Biological and Inflammatory Effects of Antigen 5 from Polybia paulista (Hymenoptera, Vespidae) Venom in Mouse Intraperitoneal Macrophages. Toxins 2021, 13, 850. https://doi.org/10.3390/toxins13120850
Bazon ML, Fernandes LGR, Assugeni IOS, Pinto LM, Simioni PU, Zollner RdL, Braga MRB. Biological and Inflammatory Effects of Antigen 5 from Polybia paulista (Hymenoptera, Vespidae) Venom in Mouse Intraperitoneal Macrophages. Toxins. 2021; 13(12):850. https://doi.org/10.3390/toxins13120850
Chicago/Turabian StyleBazon, Murilo Luiz, Luis Gustavo Romani Fernandes, Isabela Oliveira Sandrini Assugeni, Lucas Machado Pinto, Patrícia Ucelli Simioni, Ricardo de Lima Zollner, and Márcia Regina Brochetto Braga. 2021. "Biological and Inflammatory Effects of Antigen 5 from Polybia paulista (Hymenoptera, Vespidae) Venom in Mouse Intraperitoneal Macrophages" Toxins 13, no. 12: 850. https://doi.org/10.3390/toxins13120850
APA StyleBazon, M. L., Fernandes, L. G. R., Assugeni, I. O. S., Pinto, L. M., Simioni, P. U., Zollner, R. d. L., & Braga, M. R. B. (2021). Biological and Inflammatory Effects of Antigen 5 from Polybia paulista (Hymenoptera, Vespidae) Venom in Mouse Intraperitoneal Macrophages. Toxins, 13(12), 850. https://doi.org/10.3390/toxins13120850





