Combined Developmental Toxicity of the Pesticides Difenoconazole and Dimethomorph on Embryonic Zebrafish
Abstract
:1. Introduction
2. Results
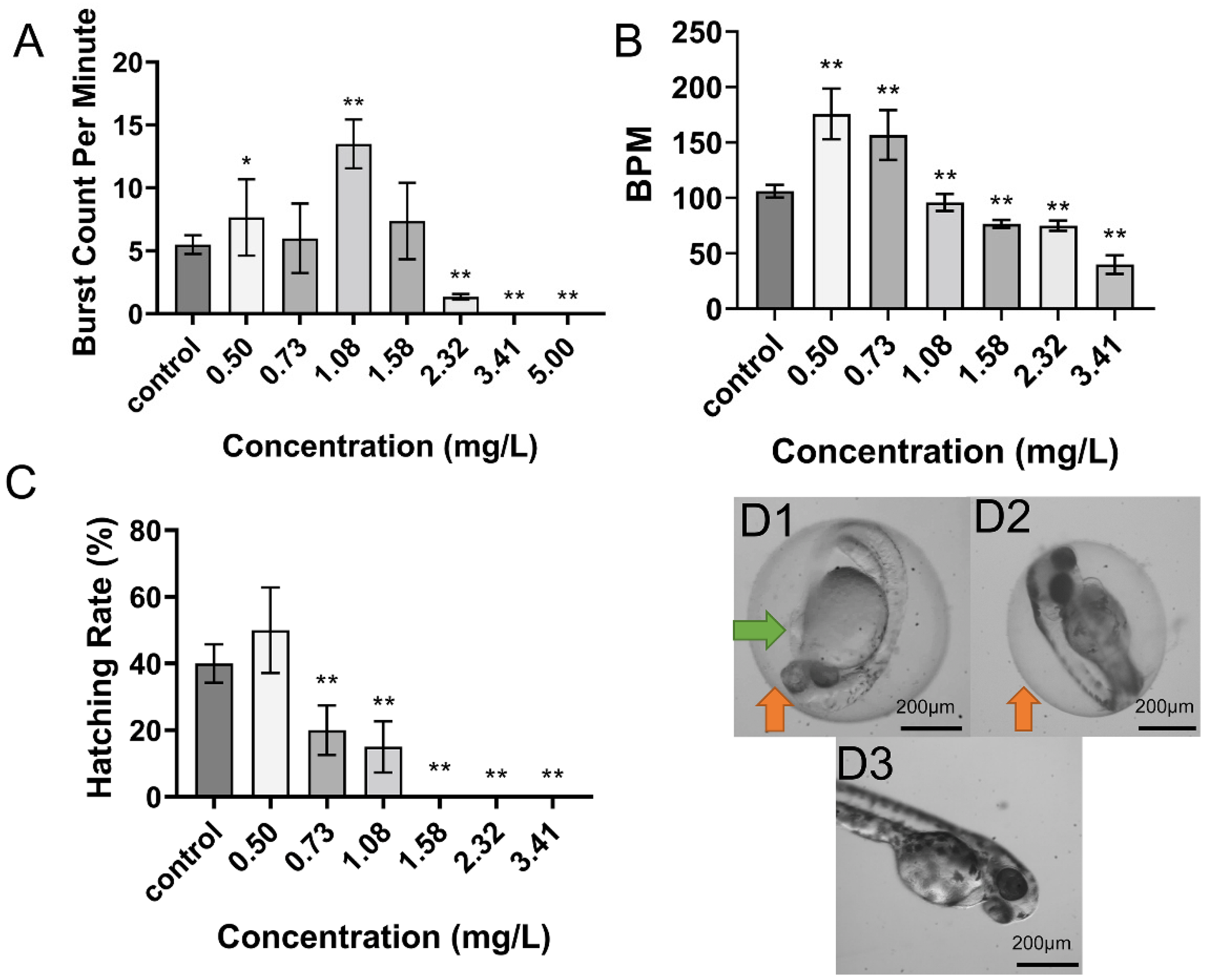
2.1. Toxicity of DIF on Zebrafish Embryos
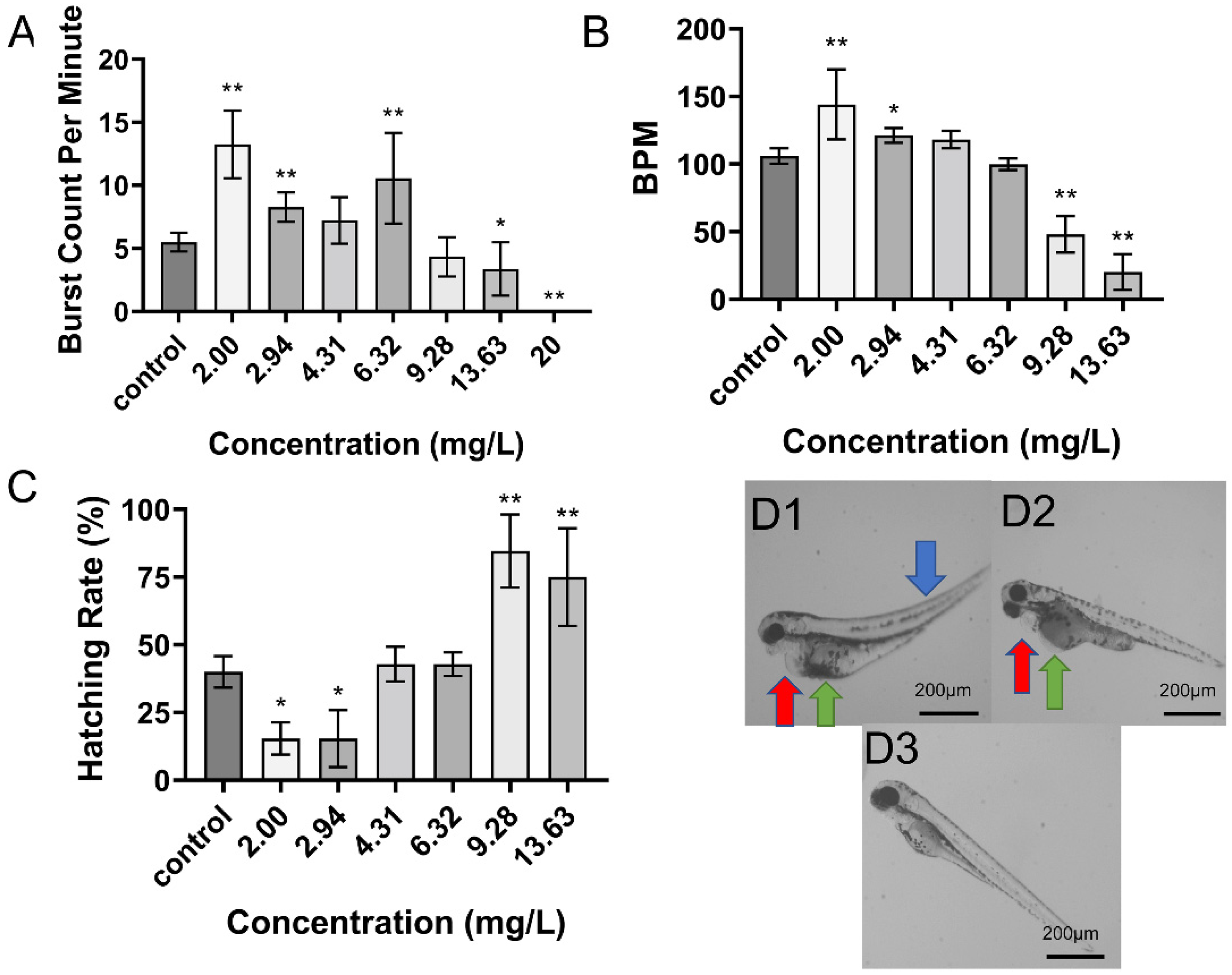
2.2. Toxicity of DIM on Zebrafish Embryo Development
2.3. Toxicity of Combined Exposure to DIF and DIM
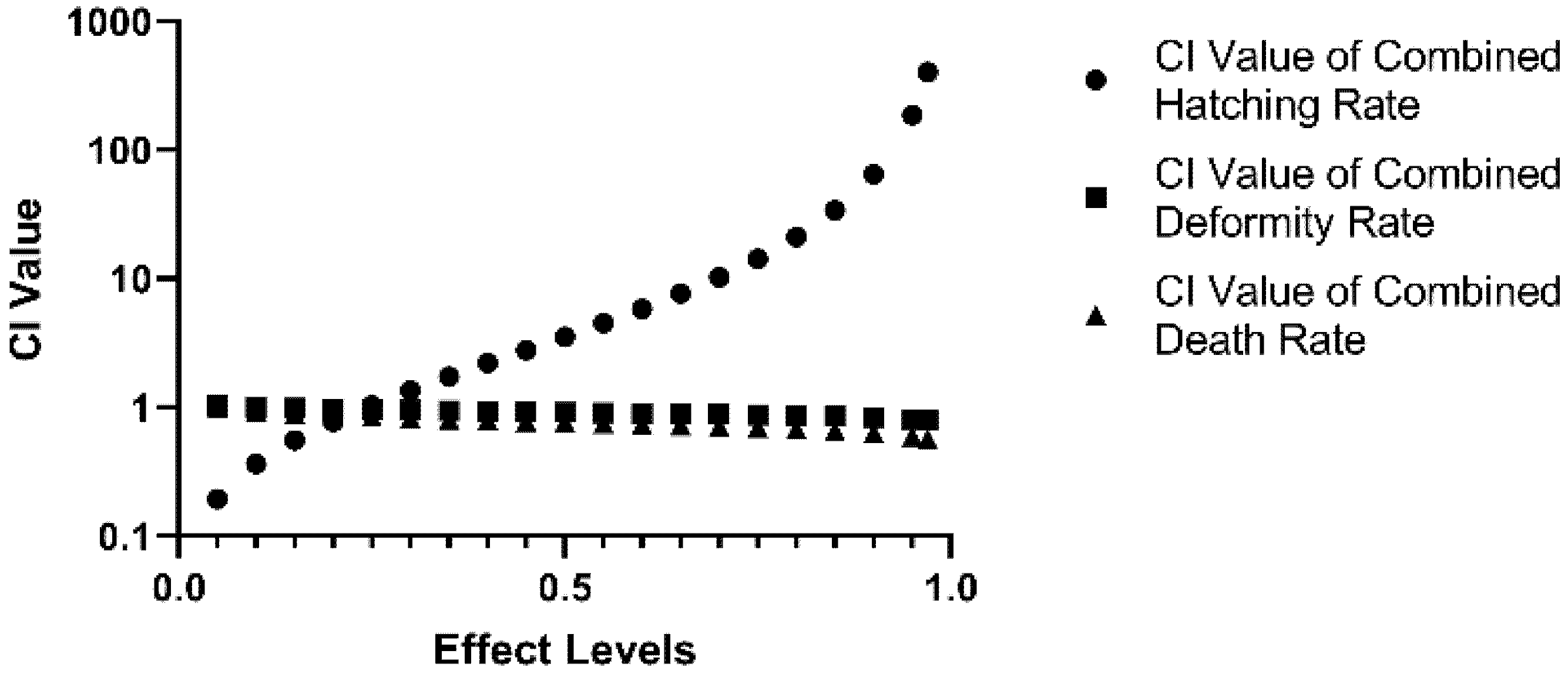
2.4. Synergistic Effects of Combined DIF and DIM on Developmental Parameters
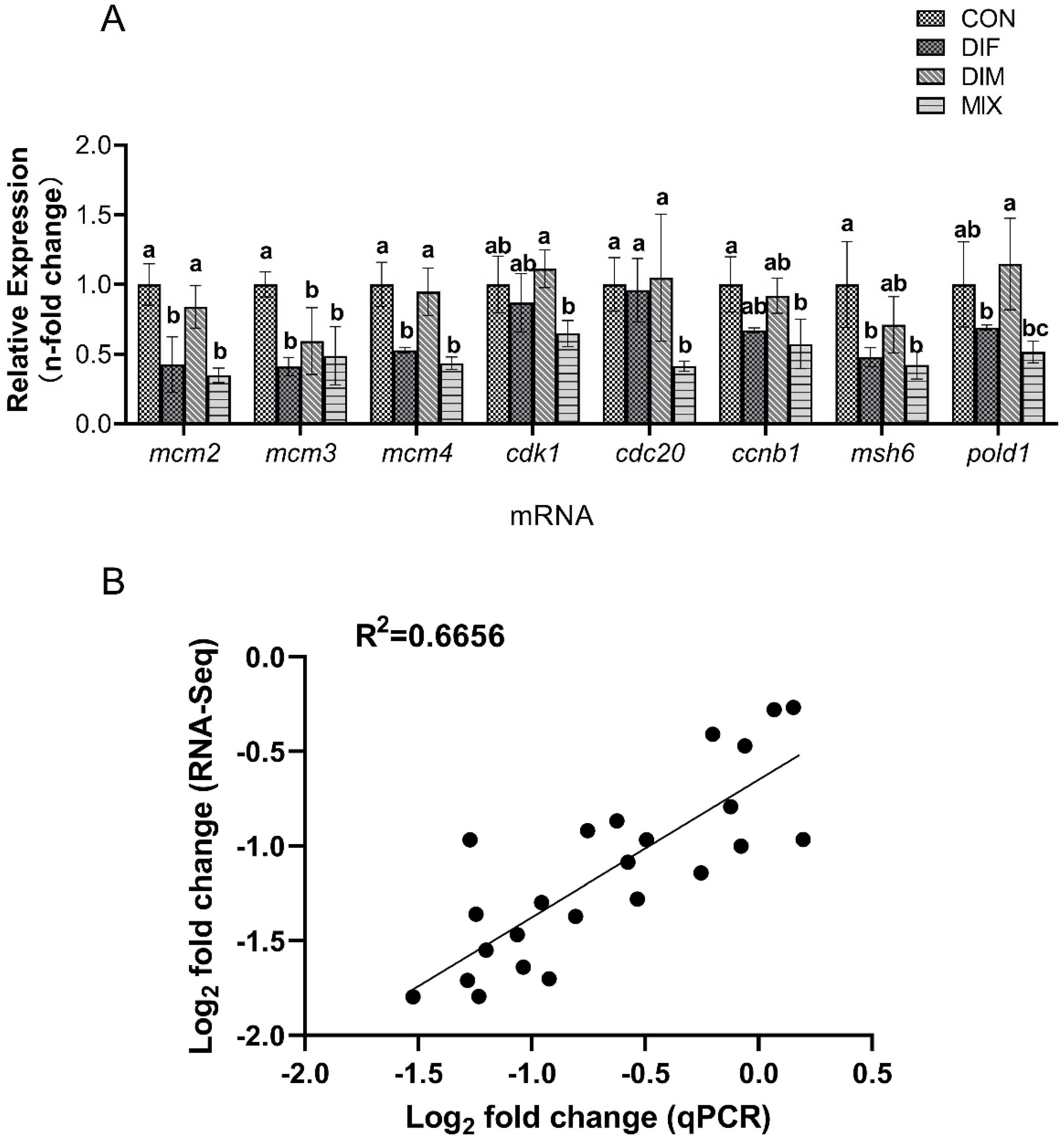
2.5. Transcriptomic and qPCR Analyses of Differentially Expressed Genes under DIF, DIM, and Combined Treatment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Maintenance and Breeding of Zebrafish
5.3. Pesticide Exposure
5.4. Measurement of Developmental Parameters
5.5. Transcriptomics
5.6. Real-Time Quantitative Reverse Transcription PCR
5.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Chen, A.; Jiang, G.; Zhuo, L.; Luo, X.; Zhang, S.; Zhang, D.; Liu, Y. Acute toxicity of difenoconazole on aquatic organism. Agrochemicals 2014, 53, 900–903. [Google Scholar]
- Zhang, G.; Li, B.; Wang, J.; Xia, X. Acute toxicity of technical material and different formulations of difenoconazole and azoxystrobin to Ze-brafish. J. Agro-Environ. Sci. 2014, 33, 2125–2130. [Google Scholar]
- Ge, J.; Jiang, J.; Cai, L. Study on toxicity of three kinds of triazole fungicides on zebrafish (Danio rerio). Acta Agric. Zhejiangensis 2018, 30, 744–755. [Google Scholar]
- Mu, X.Y.; Chai, T.T.; Wang, K.; Zhu, L.Z.; Huang, Y.; Shen, G.M.; Li, Y.R.; Li, X.F.; Wang, C.J. The developmental effect of difenoconazole on zebrafish embryos: A mechanism research. Environ. Pollut. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Zhu, J.S.; Liu, C.L.; Wang, J.Y.; Liang, Y.Y.; Gong, X.; You, L.H.; Ji, C.B.; Wang, S.L.; Wang, C.; Chi, X. Difenoconazole induces cardiovascular toxicity through oxidative stress-mediated apoptosis in early life stages of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 216, 9. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, X.; Lv, D.; Xie, D. Residue analysis of difenoconazole in soil for mango. J. Henan Agric. Univ. 2017, 51, 115–119. [Google Scholar]
- Lucini, L.; Magistrati, P.; Molinari, G.P. Residues of a triazole fungicide in soil after 4 years of application to sugar beet. Water Air Soil Pollut. 2009, 202, 13–18. [Google Scholar] [CrossRef]
- Brancato, A.; Brocca, D.; De Lentdecker, C.; Erdos, Z.; Ferreira, L.; Greco, L.; Janossy, J.; Jarrah, S.; Kardassi, D.; Leuschner, R.; et al. Modification of the existing maximum residue levels for difenoconazole in various crops. Efsa J. 2018, 16, e05143. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Zhang, Q.T.; Yu, Y.R.; Chen, Y.; Zeng, S.; Lu, P.; Hu, D.Y. Residues, dissipation kinetics, and dietary intake risk assessment of two fungicides in grape and soil. Regul. Toxicol. Pharmacol. 2018, 100, 72–79. [Google Scholar] [CrossRef]
- Sun, L.; Huang, J.; Deng, Y.; Luo, C.; Ye, Q.; Liang, Y. Risk assessment of dietary exposure to pesticide residues in staple leaf vegetables in Guangdong Province. Food Sci. 2017, 38, 223–227. [Google Scholar]
- Vargas, T.d.S.; Salustriano, N.d.A.; Klein, B.; Romao, W.; Campos da Silva, S.R.; Wagner, R.; Scherer, R. Fungicides in red wines produced in South America. Food Addit. Contam. Part A-Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 2135–2144. [Google Scholar] [CrossRef]
- Abd-Alrahman, S.H.; Elhalwagy, M.E.A.; Kotb, G.A.; Farid, H.; Farag, A.A.G.; Draz, H.M.; Isa, A.M.; Sabico, S. Exposure to difenoconazole, diclofop-methyl alone and combination alters oxidative stress and biochemical parameters in albino rats. Int. J. Clin. Exp. Med. 2014, 7, 3637–3646. [Google Scholar]
- Chen, H.; Chen, K.; Qiu, X.C.; Xu, H.; Mao, G.H.; Zhao, T.; Feng, W.W.; Okeke, E.S.; Wu, X.Y.; Yang, L.Q. The reproductive toxicity and potential mechanisms of combined exposure to dibutyl phthalate and diisobutyl phthalate in male zebrafish (Danio rerio). Chemosphere 2020, 258, 13. [Google Scholar] [CrossRef]
- Li, Y.N.; Zhang, Q.N.; Fang, J.; Ma, N.; Geng, X.; Xu, M.; Yang, H.; Jia, X.D. Hepatotoxicity study of combined exposure of DEHP and ethanol: A comprehensive analysis of transcriptomics and metabolomics. Food Chem. Toxicol. 2020, 141, 9. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Das, S.; Bhattacharyya, A.; Pal, S. Dissipation study of difenoconazole in/on chili fruit and soil in India. Bull. Environ. Contam. Toxicol. 2011, 87, 54–57. [Google Scholar] [CrossRef]
- Telo, G.M.; Marchesan, E.; Zanella, R.; de Oliveira, M.L.; Coelho, L.L.; Martins, M.L. Residues of fungicides and insecticides in rice field. Agron. J. 2015, 107, 851–863. [Google Scholar] [CrossRef]
- Wang, K.; Wu, J.X.; Zhang, H.Y. Dissipation of difenoconazole in rice, paddy soil, and paddy water under field conditions. Ecotoxicol. Environ. Saf. 2012, 86, 111–115. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wan, K.; Huang, J.X.; Wang, Y.C.; Wang, F.H. Behavior of mixed formulation of metalaxyl and dimethomorph in grape and soil under field conditions. Ecotoxicol. Environ. Saf. 2012, 84, 112–116. [Google Scholar] [CrossRef]
- Liang, H.W.; Li, L.; Li, W.; Wu, Y.J.; Zhou, Z.Q.; Liu, F.M. Dissipation and residue of dimethomorph in pepper and soil under field conditions. Ecotoxicol. Environ. Saf. 2011, 74, 1331–1335. [Google Scholar] [CrossRef]
- Zambito Marsala, R.; Capri, E.; Russo, E.; Bisagni, M.; Colla, R.; Lucini, L.; Gallo, A.; Suciu, N.A. First evaluation of pesticides occurrence in groundwater of Tidone Valley, an area with intensive viticulture. Sci. Total Environ. 2020, 736, 139730. [Google Scholar] [CrossRef]
- Zilliacus, J.; Beronius, A.; Hanberg, A.; Luijten, M.; van der Voet, H.; van Klaveren, J. EuroMix handbook for mixture risk assessment. Toxicol. Lett. 2019, 314, S263. [Google Scholar]
- Zoupa, M.; Zwart, E.P.; Gremmer, E.R.; Nugraha, A.; Compeer, S.; Slob, W.; van der Ven, L.T.M. Dose addition in chemical mixtures inducing craniofacial malformations in zebrafish (Danio rerio) embryos. Food Chem. Toxicol. 2020, 137, 111117. [Google Scholar] [CrossRef]
- Teng, M.; Zhu, W.; Wang, D.; Qi, S.; Wang, Y.; Yan, J.; Dong, K.; Zheng, M.; Wang, C. Metabolomics and transcriptomics reveal the toxicity of difenoconazole to the early life stages of zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 112–120. [Google Scholar] [CrossRef]
- Teng, M.; Qi, S.; Zhu, W.; Wang, Y.; Wang, D.; Dong, K.; Wang, C. Effects of the bioconcentration and parental transfer of environmentally relevant concentrations of difenoconazole on endocrine disruption in zebrafish (Danio rerio). Environ. Pollut. 2018, 233, 208–217. [Google Scholar] [CrossRef]
- Regueiro, J.; Olguín, N.; Simal-Gándara, J.; Suñol, C. Toxicity evaluation of new agricultural fungicides in primary cultured cortical neurons. Environ. Res. 2015, 140, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Kuriya, K.; Higashiyama, E.; Avşar-Ban, E.; Okochi, N.; Hattori, K.; Ogata, S.; Takebayashi, S.-i.; Ogata, M.; Tamaru, Y.; Okumura, K. Direct visualization of replication dynamics in early zebrafish embryos. Biosci. Biotechnol. Biochem. 2016, 80, 945–948. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.S.; Bong, J.; Choi, J.S.; Henry, T.B.; Park, J.W. Differentially transcriptional regulation on cell cycle pathway by silver nanoparticles from ionic silver in larval zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2016, 479, 753–758. [Google Scholar] [CrossRef]
- Cortez, D.; Glick, G.; Elledge, S.J. Minichromosome maintenance proteins are direct targets of the ATM and ATR checkpoint kinases. Proc. Natl. Acad. Sci. USA 2004, 101, 10078–10083. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Driever, W. Minichromosome maintenance proteins as markers for proliferation zones during embryogenesis. Cell Cycle 2006, 5, 1140–1142. [Google Scholar] [CrossRef]
- Hou, J.; Liu, H.Q.; Zhang, S.Y.; Liu, X.H.; Hayat, T.; Alsaedi, A.; Wang, X.K. Mechanism of toxic effects of Nano-ZnO on cell cycle of zebrafish (Danio rerio). Chemosphere 2019, 229, 206–213. [Google Scholar] [CrossRef]
- Saint, R.; Wigley, P.L. Developmental regulation of the cell cycle. Curr. Opin. Genet. Dev. 1992, 2, 614–620. [Google Scholar] [CrossRef]
- Pellegrin, P.; Menard, C.; Mery, J.; Lory, P.; Charnet, P.; Bennes, R. Cell cycle dependent toxicity of an amphiphilic synthetic peptide. FEBS Lett. 1997, 418, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Prevo, R.; Pirovano, G.; Puliyadi, R.; Herbert, K.J.; Rodriguez-Berriguete, G.; O’Docherty, A.; Greaves, W.; McKenna, W.G.; Higgins, G.S. CDK1 inhibition sensitizes normal cells to DNA damage in a cell cycle dependent manner. Cell Cycle 2018, 17, 1513–1523. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.-L.; Zhang, F.-L.; Tian, Y.; Zhu, M.; Meng, L.-Y.; Dyce, P.W.; Shen, W.; Li, L. Whole-transcriptome analysis of the toxic effects of zearalenone exposure on ceRNA networks in porcine granulosa cells. Environ. Pollut. 2020, 261, 114007. [Google Scholar] [CrossRef]
- Marc, J.; Mulner-Lorillon, O.; Durand, G.; Belle, R. Embryonic cell cycle for risk assessment of pesticides at the molecular level. Environ. Chem. Lett. 2003, 1, 8–12. [Google Scholar] [CrossRef]
- Mansur, A.; Adir, M.; Racowsky, C.; Combelles, C.M.H.; Landa, N.; Machtinger, R. Susceptibility of human cumulus cells to bisphenol a In vitro. Reprod. Toxicol. 2017, 74, 189–194. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Chang, M.-Y.; Wang, M.-J.; Liu, H.-C.; Chang, S.-J.; Harnod, T.; Hung, C.-H.; Lee, H.-T.; Shen, C.-C.; Chung, J.-G. Phenethyl Isothiocyanate Alters the Gene Expression and the Levels of Protein Associated with Cell Cycle Regulation in Human Glioblastoma GBM 8401 Cells. Environ. Toxicol. 2017, 32, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Li, X.; Qiu, Y.; Zeng, X.; Yu, X.; Wang, W.; Yi, X.; Huang, L. Low-dose effects on thyroid disruption in zebrafish by long-term exposure to oxytetracycline. Aquat. Toxicol. 2020, 227, 105608. [Google Scholar] [CrossRef]
- Maharaj, S.; El Ahmadie, N.; Rheingold, S.; El Chehouri, J.; Yang, L.; Souders, C.L., II; Martyniuk, C.J. Sub-lethal toxicity assessment of the phenylurea herbicide linuron in developing zebrafish (Danio rerio) embryo/larvae. Neurotoxicology Teratol. 2020, 81, 106917. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.-Y.; Lim, W.; Song, G. Assessment of the in vivo genotoxicity of pendimethalin via mitochondrial bioenergetics and transcriptional profiles during embryogenesis in zebrafish: Implication of electron transport chain activity and developmental defects. J. Hazard. Mater. 2021, 411, 125153. [Google Scholar] [CrossRef]
- Davis, J.A.; Gift, J.S.; Zhao, Q.J. Introduction to benchmark dose methods and US EPA’s benchmark dose software (BMDS) version 2.1.1. Toxicol. Appl. Pharmacol. 2011, 254, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Preclinical versus clinical drug combination studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Zhang, W.; Jia, L.; Li, L.; Zhao, J.; Zhao, Z.; Peng, S.; Chen, Y.; Yuan, X. Combined Developmental Toxicity of the Pesticides Difenoconazole and Dimethomorph on Embryonic Zebrafish. Toxins 2021, 13, 854. https://doi.org/10.3390/toxins13120854
Fan R, Zhang W, Jia L, Li L, Zhao J, Zhao Z, Peng S, Chen Y, Yuan X. Combined Developmental Toxicity of the Pesticides Difenoconazole and Dimethomorph on Embryonic Zebrafish. Toxins. 2021; 13(12):854. https://doi.org/10.3390/toxins13120854
Chicago/Turabian StyleFan, Ruiqi, Wanjun Zhang, Li Jia, Lizhong Li, Jun Zhao, Zengming Zhao, Shuangqing Peng, Yiqiang Chen, and Xiaoyan Yuan. 2021. "Combined Developmental Toxicity of the Pesticides Difenoconazole and Dimethomorph on Embryonic Zebrafish" Toxins 13, no. 12: 854. https://doi.org/10.3390/toxins13120854




