Novel Cysteine Protease Inhibitor Derived from the Haementeria vizottoi Leech: Recombinant Expression, Purification, and Characterization
Abstract
1. Introduction
2. Results
2.1. Selection and Purification of Cystatin-Hv
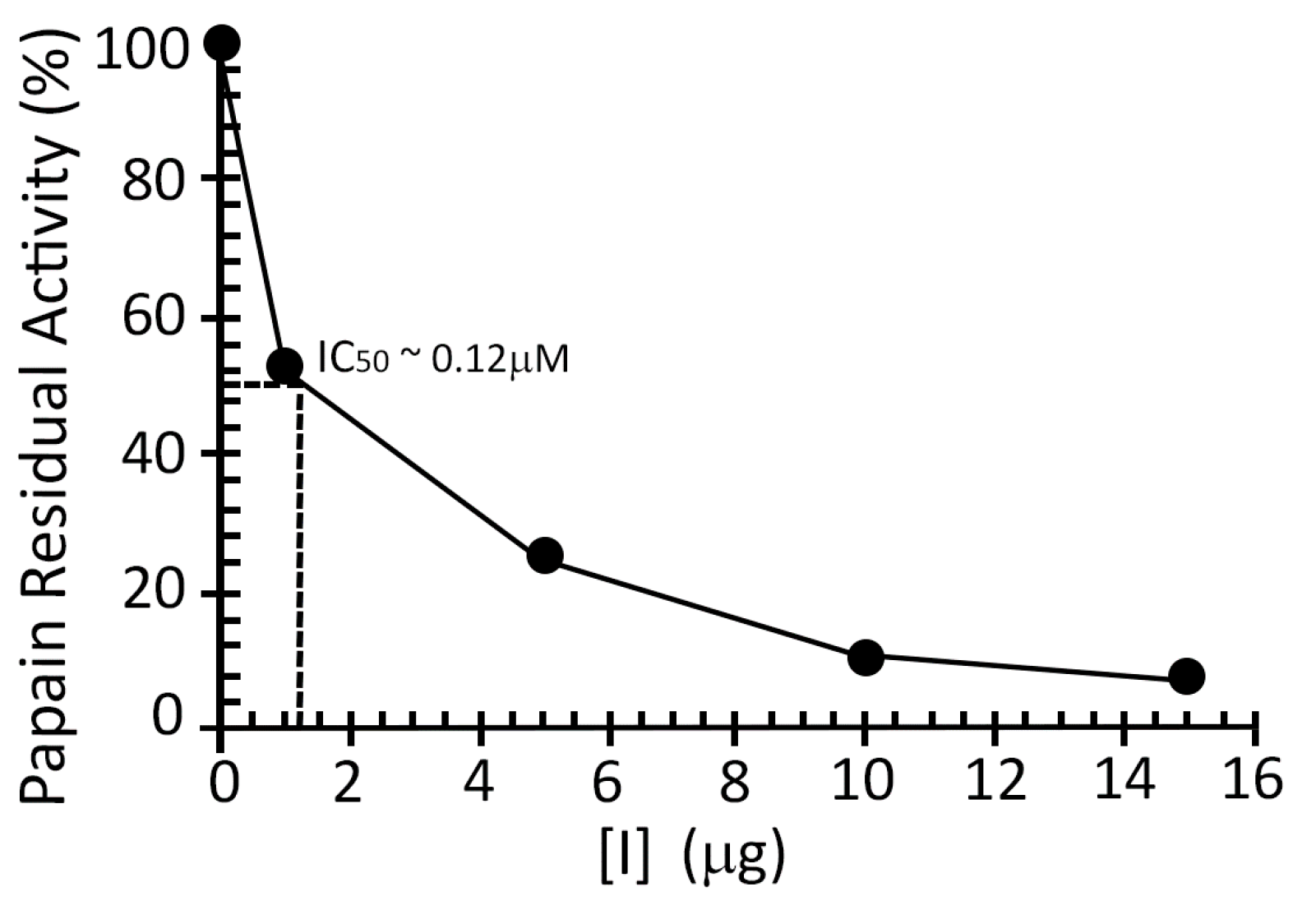
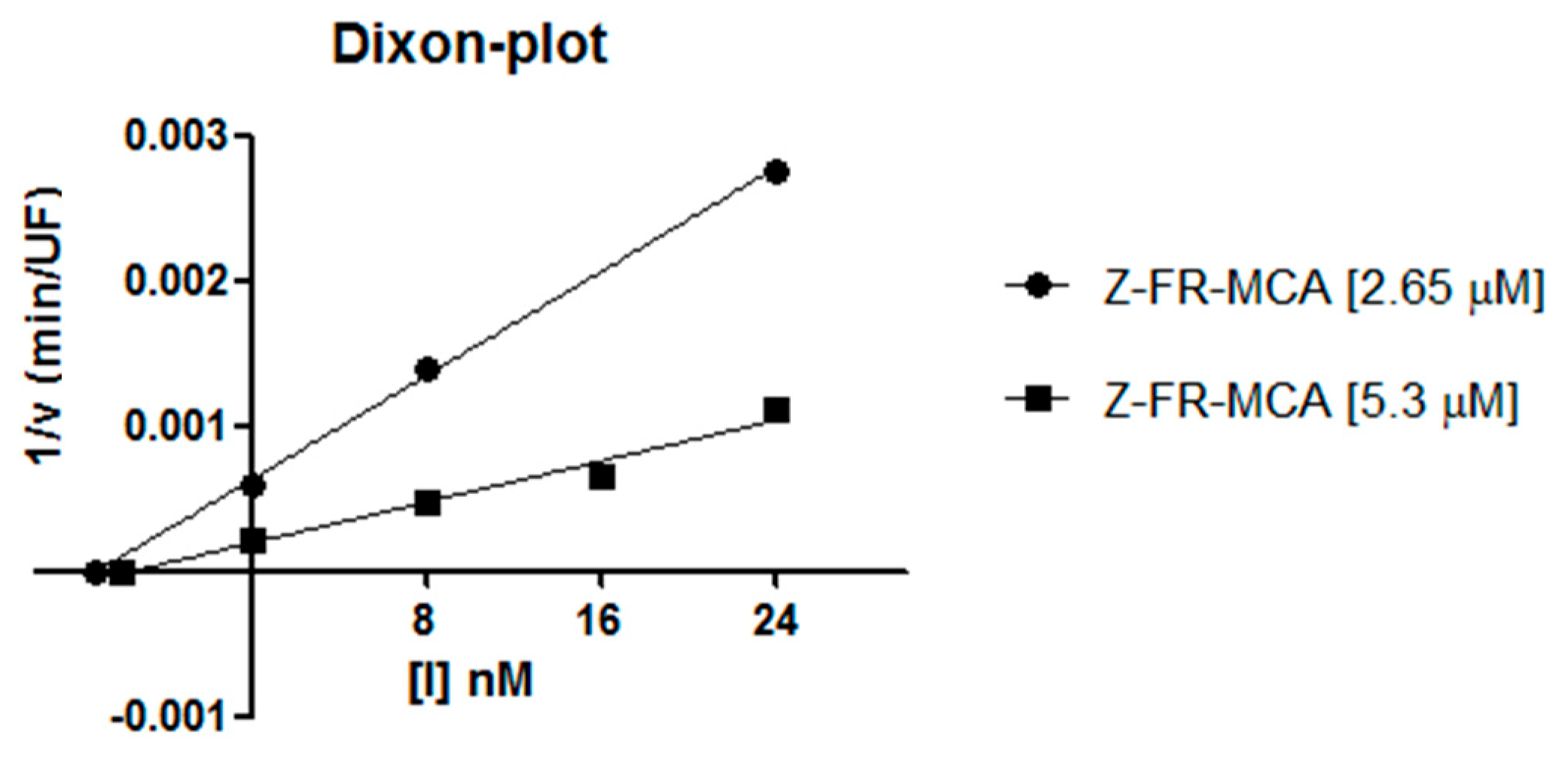
2.2. Inhibition Studies
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strains, Plasmids, Enzymes
5.2. Sequence Source and In Silico Characterization
5.3. Expression and Purification of Recombinant Protein
5.4. Mass Spectrometry for Sequence Confirmation
5.5. Inhibitory Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grzonka, Z.; Jankowska, E.; Kasprzykowski, F.; Kasprzykowska, R.; Lankiewicz, L.; Wiczk, W.; Wieczerzak, E.; Ciarkowski, J.; Drabik, P.; Janowski, R.; et al. Structural studies of cysteine proteases and their inhibitors. Acta Biochim. Pol. 2001, 48, 1–20. [Google Scholar] [CrossRef]
- Sudhan, D.; Siemann, D.W. Cathepsin L targeting in cancer treatment. Pharmacol. Ther. 2015, 155, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Dana, D.; Pathak, S.K. A Review of Small Molecule Inhibitors and Functional Probes of Human Cathepsin L. Molecules 2020, 25, 698. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, M.; Das, P.; Gahlot, G.P.S.; Singh, R.; Roeb, E.; Roderfeld, M.; Gupta, S.D.; Saraya, A.; Pandey, R.M.; Chauhan, S.S. Cathepsin L and B as Potential Markers for Liver Fibrosis: Insights From Patients and Experimental Models. Clin. Transl. Gastroenterol. 2017, 8, e99. [Google Scholar] [CrossRef]
- Liu, C.-L.; Guo, J.; Zhang, X.; Sukhova, G.; Libby, P.; Shi, G.-P. Cysteine protease cathepsins in cardiovascular disease: From basic research to clinical trials. Nat. Rev. Cardiol. 2018, 15, 351–370. [Google Scholar] [CrossRef]
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2021, 1–9. [Google Scholar] [CrossRef]
- Liu, T.; Luo, S.; Libby, P.; Shi, G.-P. Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients. Pharmacol. Ther. 2020, 213, 107587. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Chaudhuri, G. Cystatin Superfamily. J. Health Care Poor Underserved 2010, 21, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Schönemeyer, A.; Lucius, R.; Sonnenburg, B.; Brattig, N.; Sabat, R.; Schilling, K.; Bradley, J.; Hartmann, S. Modulation of Human T Cell Responses and Macrophage Functions by Onchocystatin, a Secreted Protein of the Filarial Nematode Onchocerca volvulus. J. Immunol. 2001, 167, 3207–3215. [Google Scholar] [CrossRef]
- Schwarz, A.; Valdés, J.J.; Kotsyfakis, M. The role of cystatins in tick physiology and blood feeding. Ticks Tick-Borne Dis. 2012, 3, 117–127. [Google Scholar] [CrossRef]
- Lu, S.; da Rocha, L.A.; Torquato, R.J.; Junior, I.D.S.V.; Florin-Christensen, M.; Tanaka, A.S. A novel type 1 cystatin involved in the regulation of Rhipicephalus microplus midgut cysteine proteases. Ticks Tick-Borne Dis. 2020, 11, 101374. [Google Scholar] [CrossRef] [PubMed]
- Kotsyfakis, M.; Sa-Nunes, A.; Francischetti, I.M.B.; Mather, T.N.; Andersen, J.F.; Ribeiro, J. Antiinflammatory and Immunosuppressive Activity of Sialostatin L, a Salivary Cystatin from the Tick Ixodes scapularis. J. Biol. Chem. 2006, 281, 26298–26307. [Google Scholar] [CrossRef]
- Horka, H.; Staudt, V.; Klein, M.; Taube, C.; Reuter, S.; Dehzad, N.; Andersen, J.F.; Kopecky, J.; Schild, H.; Kotsyfakis, M.; et al. The Tick Salivary Protein Sialostatin L Inhibits the Th9-Derived Production of the Asthma-Promoting Cytokine IL-9 and Is Effective in the Prevention of Experimental Asthma. J. Immunol. 2012, 188, 2669–2676. [Google Scholar] [CrossRef]
- Parizi, L.F.; Rangel, C.K.; Sabadin, G.A.; Saggin, B.F.; Kiio, I.; Xavier, M.A.; Matos, R.D.S.; Camargo-Mathias, M.I.; Seixas, A.; Konnai, S.; et al. Rhipicephalus microplus cystatin as a potential cross-protective tick vaccine against Rhipicephalus appendiculatus. Ticks Tick-Borne Dis. 2020, 11, 101378. [Google Scholar] [CrossRef]
- Wei, N.; Lin, Z.; Xu, Z.; Cao, J.; Zhou, Y.; Zhang, H.; Gong, H.; Zhou, J.; Li, G. A Tick Cysteine Protease Inhibitor RHcyst-1 Exhibits Antitumor Potential. Cell. Physiol. Biochem. 2018, 46, 2385–2400. [Google Scholar] [CrossRef]
- Kotál, J.; Stergiou, N.; Buša, M.; Chlastáková, A.; Beránková, Z.; Řezáčová, P.; Langhansová, H.; Schwarz, A.; Calvo, E.; Kopecký, J.; et al. The structure and function of Iristatin, a novel immunosuppressive tick salivary cystatin. Cell. Mol. Life Sci. 2019, 76, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Zavašnik-Bergant, T.; Vidmar, R.; Sekirnik, A.; Fonović, M.; Salat, J.; Grunclová, L.; Kopáček, P.; Turk, B. Salivary Tick Cystatin OmC2 Targets Lysosomal Cathepsins S and C in Human Dendritic Cells. Front. Cell. Infect. Microbiol. 2017, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Faria, F.; Kelen, E.M.A.; Sampaio, C.A.M.; Bon, C.; Duval, N.; Chudzinski-Tavassi, A.M. A New Factor Xa Inhibitor (Lefaxin) from the Haementeria depressa Leech. Thromb. Haemost. 1999, 82, 1469–1473. [Google Scholar] [CrossRef]
- Chudzinski-Tavassi, A.M.; Bermej, E.; Rosenstein, R.E.; Faria, F.; Sarmiento, M.I.K.; Alberto, F.; Sampaio, M.U.; Lazzari, M.A. Nitridergic Platelet Pathway Activation by Hementerin, a Metalloprotease from the Leech Haementeria depressa. Biol. Chem. 2003, 384, 1333–1339. [Google Scholar] [CrossRef]
- Salzet, M.; Chopin, V.; Baert, J.-L.; Matias, I.; Malecha, J. Theromin, a Novel Leech Thrombin Inhibitor. J. Biol. Chem. 2000, 275, 30774–30780. [Google Scholar] [CrossRef]
- Oliveira, D.G.L.; Alvarez-Flores, M.P.; Lopes, A.R.; Chudzinski-Tavassi, A.M. Functional characterisation of Vizottin, the first factor Xa inhibitor purified from the leech Haementeria vizottoi. Thromb. Haemost. 2012, 108, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Cocquerelle, C.; Vandenbulcke, F.; Hot, D.; Huot, L.; Lemoine, Y.; Salzet, M. Transcriptomic analysis in the leech Theromyzon tessulatum: Involvement of cystatin B in innate immunity. Biochem. J. 2004, 380, 617–625. [Google Scholar] [CrossRef]
- Amorim, A.M.X.P.; De Oliveira, U.C.; Faria, F.; Pasqualoto, K.F.M.; Junqueira-De-Azevedo, I.D.L.; Chudzinski-Tavassi, A.M. Transcripts involved in hemostasis: Exploring salivary complexes from Haementeria vizottoi leeches through transcriptomics, phylogenetic studies and structural features. Toxicon 2015, 106, 20–29. [Google Scholar] [CrossRef]
- Zi, M.; Xu, Y. Involvement of cystatin C in immunity and apoptosis. Immunol. Lett. 2018, 196, 80–90. [Google Scholar] [CrossRef]
- Lefebvre, C.; Vandenbulcke, F.; Bocquet, B.; Tasiemski, A.; Desmons, A.; Verstraete, M.; Salzet, M.; Cocquerelle, C. Cathepsin L and cystatin B gene expression discriminates immune cœlomic cells in the leech Theromyzon tessulatum. Dev. Comp. Immunol. 2008, 32, 795–807. [Google Scholar] [CrossRef][Green Version]
- Valenzuela, J.G.; Francischetti, I.M.B.; Pham, V.M.; Garfield, M.K.; Mather, T.N.; Ribeiro, J.M.C. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 2002, 205, 2843–2864. [Google Scholar] [CrossRef]
- Salát, J.; Paesen, G.C.; Řezáčová, P.; Kotsyfakis, M.; Kovářová, Z.; Šanda, M.; Majtán, J.; Grunclová, L.; Horká, H.; Andersen, J.F.; et al. Crystal structure and functional characterization of an immunomodulatory salivary cystatin from the soft tick Ornithodoros moubata. Biochem. J. 2010, 429, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Parizi, L.F.; Githaka, N.W.; Acevedo, C.; Benavides, U.; Seixas, A.; Logullo, C.; Konnai, S.; Ohashi, K.; Masuda, A.; da Silva Vaz, I., Jr. Sequence characterization and immunogenicity of cystatins from the cattle tick Rhipicephalus (Boophilus) microplus. Ticks Tick-Borne Dis. 2013, 4, 492–499. [Google Scholar] [CrossRef]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; Viley-VCH: New York, NY, USA, 2000. [Google Scholar]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Silver, A.; Graf, J. Innate and procured immunity inside the digestive tract of the medicinal leech. Invertebr. Surviv. J. ISJ 2011, 8, 173–178. [Google Scholar]
- Chmelař, J.; Kotál, J.; Langhansová, H.; Kotsyfakis, M. Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction. Front. Cell. Infect. Microbiol. 2017, 7, 216. [Google Scholar] [CrossRef]
- Reiser, J.; Adair, B.; Reinheckel, T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Investig. 2010, 120, 3421–3431. [Google Scholar] [CrossRef] [PubMed]
- Goulet, B.; Sansregret, L.; Leduy, L.; Bogyo, M.; Weber, E.; Chauhan, S.S.; Nepveu, A. Increased Expression and Activity of Nuclear Cathepsin L in Cancer Cells Suggests a Novel Mechanism of Cell Transformation. Mol. Cancer Res. 2007, 5, 899–907. [Google Scholar] [CrossRef]
- Berdowska, I. Cysteine proteases as disease markers. Clin. Chim. Acta 2004, 342, 41–69. [Google Scholar] [CrossRef]
- Tamhane, T.; Lllukkumbura, R.; Lu, S.; Maelandsmo, G.M.; Haugen, M.H.; Brix, K. Nuclear cathepsin L activity is required for cell cycle progression of colorectal carcinoma cells. Biochimie 2016, 122, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Cardoso, T.H.; Lu, S.; Gonzalez, B.R.; Torquato, R.J.; Tanaka, A.S. Characterization of a novel cystatin type 2 from Rhipicephalus microplus midgut. Biochimie 2017, 140, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Botella, M. Ángel; Subramanian, L.; Niu, X.; Nielsen, S.S.; Bressan, R.A.; Hasegawa, P.M. Two Wound-Inducible Soybean Cysteine Proteinase Inhibitors Have Greater Insect Digestive Proteinase Inhibitory Activities than a Constitutive Homolog. Plant Physiol. 1996, 111, 1299–1306. [Google Scholar] [CrossRef]
- Abe, M.; Abe, K.; Iwabuchi, K.; Domoto, C.; Arai, S. Corn Cystatin I Expressed in Escherichia coli: Investigation of Its Inhibitory Profile and Occurrence in Corn Kernels. J. Biochem. 1994, 116, 488–492. [Google Scholar] [CrossRef]
- Pernas, M.; Sánchez-Monge, R.; Gómez, L.; Salcedo, G. A chestnut seed cystatin differentially effective against cysteine proteinases from closely related pests. Plant Mol. Biol. 1998, 38, 1235–1242. [Google Scholar] [CrossRef]
- Eliyahu, E.; Shtraizent, N.; He, X.; Chen, D.; Shalgi, R.; Schuchman, E.H. Identification of Cystatin SA as a Novel Inhibitor of Acid Ceramidase. J. Biol. Chem. 2011, 286, 35624–35633. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; López, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Wessel, D.; Flügge, U. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Portaro, F.C.V.; Santos, A.B.F.; Cezari, M.H.S.; Juliano, M.A.; Juliano, L.; Carmona, E. Probing the specificity of cysteine proteinases at subsites remote from the active site: Analysis of P4, P3, P2′ and P3′ variations in extended substrates. Biochem. J. 2000, 347, 123–129. [Google Scholar] [CrossRef]
- Kodama, R.T.; Kuniyoshi, A.K.; Da Silva, C.C.F.; Cajado-Carvalho, D.; Duzzi, B.; Mariano, D.C.; Pimenta, D.C.; Borges, R.; Da Silva, W.D.; Portaro, F.C.V. A Kunitz-type peptide from Dendroaspis polylepis venom as a simultaneous inhibitor of serine and cysteine proteases. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Portaro, F.C.V.; Cezari, M.H.S.; Juliano, M.A.; Juliano, L.; Walmsley, A.R.; Prado, E.S. Design of kallidin-releasing tissue kallikrein inhibitors based on the specificities of the enzyme’s binding subsites. Biochem. J. 1997, 323, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Segel, I. Enzyme Kinetics Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems; John Wiley: New York, NY, USA, 1976. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linhares, D.d.C.; Faria, F.; Kodama, R.T.; Amorim, A.M.X.P.; Portaro, F.C.V.; Trevisan-Silva, D.; Ferraz, K.F.; Chudzinski-Tavassi, A.M. Novel Cysteine Protease Inhibitor Derived from the Haementeria vizottoi Leech: Recombinant Expression, Purification, and Characterization. Toxins 2021, 13, 857. https://doi.org/10.3390/toxins13120857
Linhares DdC, Faria F, Kodama RT, Amorim AMXP, Portaro FCV, Trevisan-Silva D, Ferraz KF, Chudzinski-Tavassi AM. Novel Cysteine Protease Inhibitor Derived from the Haementeria vizottoi Leech: Recombinant Expression, Purification, and Characterization. Toxins. 2021; 13(12):857. https://doi.org/10.3390/toxins13120857
Chicago/Turabian StyleLinhares, Débora do Carmo, Fernanda Faria, Roberto Tadashi Kodama, Adriane Michele Xavier Prado Amorim, Fernanda Calheta Vieira Portaro, Dilza Trevisan-Silva, Karla Fernanda Ferraz, and Ana Marisa Chudzinski-Tavassi. 2021. "Novel Cysteine Protease Inhibitor Derived from the Haementeria vizottoi Leech: Recombinant Expression, Purification, and Characterization" Toxins 13, no. 12: 857. https://doi.org/10.3390/toxins13120857
APA StyleLinhares, D. d. C., Faria, F., Kodama, R. T., Amorim, A. M. X. P., Portaro, F. C. V., Trevisan-Silva, D., Ferraz, K. F., & Chudzinski-Tavassi, A. M. (2021). Novel Cysteine Protease Inhibitor Derived from the Haementeria vizottoi Leech: Recombinant Expression, Purification, and Characterization. Toxins, 13(12), 857. https://doi.org/10.3390/toxins13120857





