The Antagonistic Effect of Glutamine on Zearalenone-Induced Apoptosis via PI3K/Akt Signaling Pathway in IPEC-J2 Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of ZEN and Gln on Cell Viability
2.2. Effects of ZEN and Gln on the Activities of Enzymes
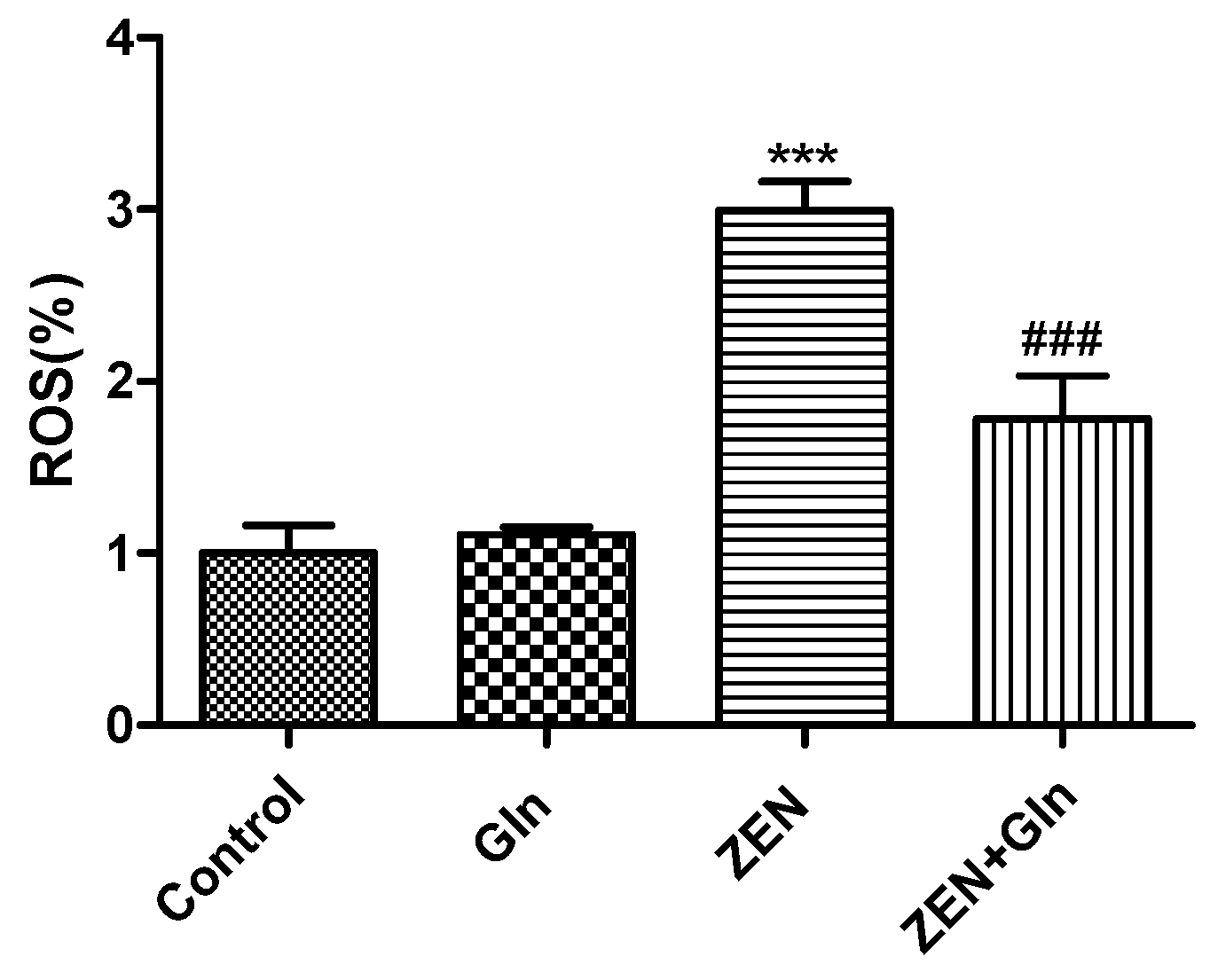
2.3. Intracellular ROS Generation
2.4. Intracellular Ca2+
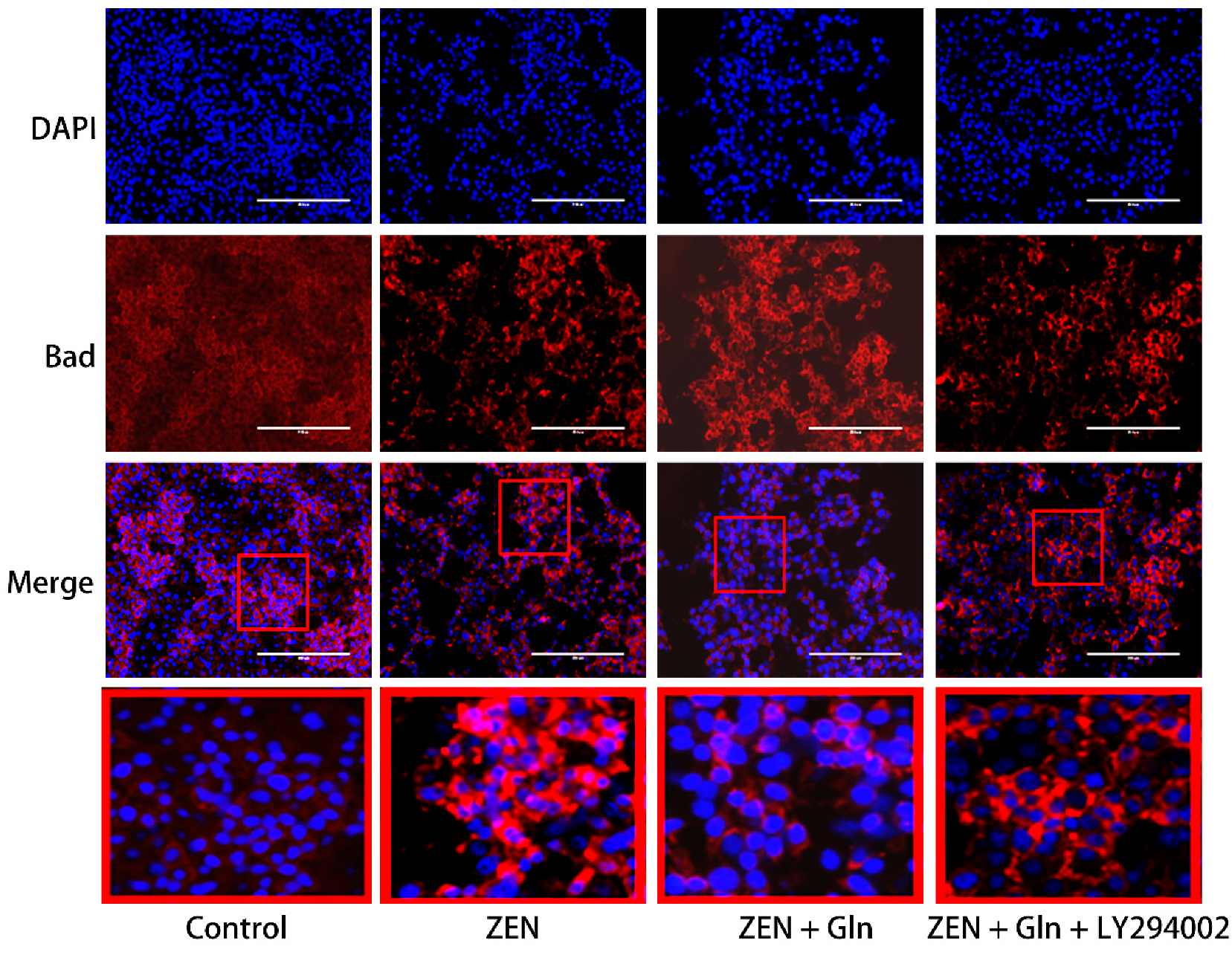
2.5. Immunofluorescence Staining of Cells
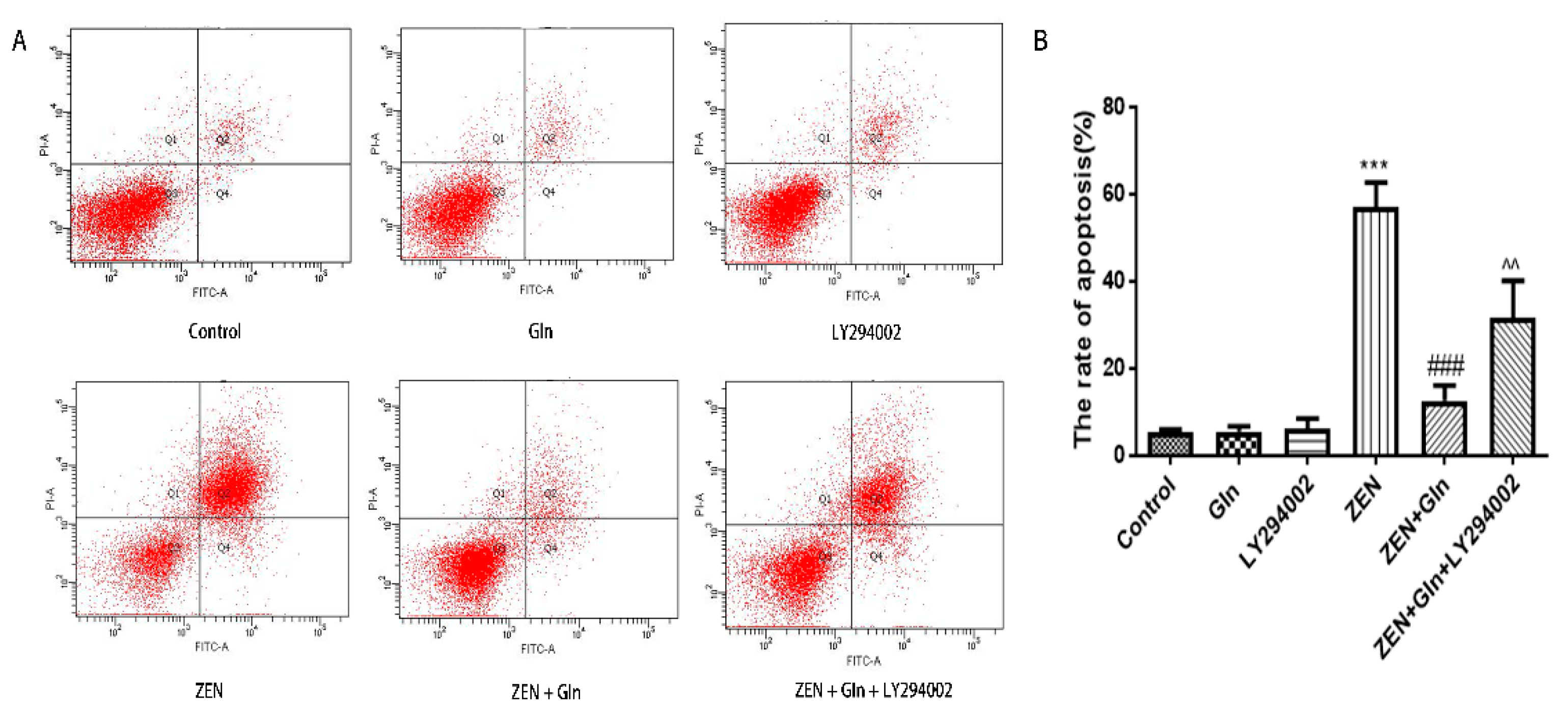
2.6. Apoptosis Rate in IPEC-J2 Cells
2.7. The mRNA Expression of Apoptosis-Related Genes
2.8. Immunofluorescence
2.9. Western Blotting
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cell and Cell Culture
5.3. Cell Viability Assay
5.4. Determination of IPEC-J2 Cellular the Activities of Enzymes
5.5. Detection of ROS Generation
5.6. Measurement of Intracellular Calcium (Ca2+) Levels
5.7. Hoechst-33258 Staining
5.8. Apoptosis Detection
5.9. Realtime PCR (RT-PCR) Assay
5.10. Western Blotting Analyses
5.11. Immunofluorescence Staining of Cells
5.12. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lei, Y.; Zhao, L.; Ma, Q.; Zhang, J.Y.; Zhou, T.; Gao, C.; Ji, C. Degradation of zearalenone in swine feed and feed ingredients by Bacillus subtilis ANSB01G. World Mycotoxin J. 2014, 7, 143–151. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, B.; Li, X.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Bai, J.; Bian, J.; et al. Zearalenone Promotes Cell Proliferation or Causes Cell Death? Toxins 2018, 10, 184. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wu, W.; Pan, J.; Long, M. Detoxification Strategies for Zearalenone Using Microorganisms: A Review. Microorganisms 2019, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Deng, J.L.; Xu, S.W.; Peng, X.; Zuo, Z.C.; Cui, H.M.; Wang, Y.; Ren, Z.H. Effects of Zearalenone on IL-2, IL-6, and IFN-γmRNA Levels in the Splenic Lymphocytes of Chickens. Sci. World J. 2012, 2012, 567327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, S.; Zhang, M.; Yang, L.; Cheng, B.; Li, J.; Shan, A. Individual and combined effects of Fusarium toxins on apoptosis in PK15 cells and the protective role of N-acetylcysteine. Food Chem. Toxicol. 2018, 111, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Zhang, W.; Gu, A.; Dong, J.; Li, J.; Shan, A. Protective Effect of N-Acetylcysteine against Oxidative Stress Induced by Zearalenone via Mitochondrial Apoptosis Pathway in SIEC02 Cells. Toxins 2018, 10, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, D.C.; Lima, F.D.S.; Sartori, T.; Santos, A.C.A.; Rogero, M.M.; Fock, R.A. Glutamine metabolism and its effects on immune response: Molecular mechanism and gene expression. Nutrire 2016, 41, 14. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.A.Q.A.; Braga-Neto, M.B.; Oliveira, M.R.; Freire, R.S.; Barros, E.B.; Santiago, T.M.; Rebelo, L.M.; Mermelstein, C.; Warren, C.A.; Guerrant, R.L.; et al. Glutamine and Alanyl-Glutamine Increase RhoA Expression and Reduce Clostridium difficile Toxin-A-Induced Intestinal Epithelial Cell Damage. BioMed Res. Int. 2012, 2013, 567327. [Google Scholar] [CrossRef] [Green Version]
- Xing, S.; Zhang, B.; Lin, M.; Zhou, P.; Li, J.; Zhang, L.; Gao, F.; Zhou, G. Effects of alanyl-glutamine supplementation on the small intestinal mucosa barrier in weaned piglets. Asian-Australas. J. Anim. Sci. 2016, 30, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, C.; Wu, G.; Sun, Y.; Wang, B.; He, B.; Dai, Z.; Wu, Z. Glutamine Enhances Tight Junction Protein Expression and Modulates Corticotropin-Releasing Factor Signaling in the Jejunum of Weanling Piglets. J. Nutr. 2014, 145, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xia, Y.; Zhu, G.; Yan, J.; Tan, C.; Deng, B.; Deng, J.; Yin, Y.; Ren, W. Glutamine supplementation improves intestinal cell proliferation and stem cell differentiation in weanling mice. Food Nutr. Res. 2018, 62, 1439. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, J.; Liu, S.; Liu, G.; Yao, K.; Yin, Y. l-Glutamine Attenuates Apoptosis Induced by Endoplasmic Reticulum Stress by Activating the IRE1α-XBP1 Axis in IPEC-J2: A Novel Mechanism of l-Glutamine in Promoting Intestinal Health. Int. J. Mol. Sci. 2017, 18, 2617. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, Z.; Ji, Y.; Sun, K.; Dai, Z.; Wu, G. L-Glutamine Enhances Tight Junction Integrity by Activating CaMK Kinase 2–AMP-Activated Protein Kinase Signaling in Intestinal Porcine Epithelial Cells. J. Nutr. 2016, 146, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, B.A.; Fujii, J.; Brito, G.A.C.; Alcantara, C.; Oriá, R.; Lima, A.; Obrig, T.; Guerrant, R.L. Caspase and Bid Involvement in Clostridium difficile Toxin A-Induced Apoptosis and Modulation of Toxin A Effects by Glutamine and Alanyl-Glutamine In Vivo and In Vitro. Infect. Immun. 2006, 74, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluske, J.R.; Miller, D.W.; Sterndale, S.O.; Turpin, D. Associations between gastrointestinal-tract function and the stress response after weaning in pigs. Anim. Prod. Sci. 2019, 59, 2015. [Google Scholar] [CrossRef]
- Goossens, J.; Pasmans, F.; Verbrugghe, E.; Vandenbroucke, V.; De Baere, S.; Meyer, E.; Haesebrouck, F.; De Backer, P.; Croubels, S. Porcine intestinal epithelial barrier disruption by the Fusarium mycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Veter.-Res. 2012, 8, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Fan, Y.; Jia, Y.; Sun, L.; Ma, S.; Ji, C.; Ma, Q.; Zhang, J. Occurrence of mycotoxins in feed ingredients and complete feeds obtained from the Beijing region of China. J. Anim. Sci. Biotechnol. 2014, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Lei, Y.; Bao, Y.; Jia, R.; Ma, Q.; Zhang, J.; Chen, J.; Ji, C. Ameliorative effects of Bacillus subtilisANSB01G on zearalenone toxicosis in pre-pubertal female gilts. Food Addit. Contam. Part A 2014, 32, 617–625. [Google Scholar] [CrossRef]
- Braicu, C.; Selicean, S.; Cojocneanu-Petric, R.; Lajos, R.; Balacescu, O.; Taranu, I.; Marin, D.E.; Motiu, M.; Jurj, A.; Achimas-Cadariu, P.; et al. Evaluation of cellular and molecular impact of zearalenone and E. coli co-exposure on IPEC-1 cells using microarray technology. BMC Genom. 2016, 17, 576. [Google Scholar] [CrossRef] [Green Version]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Crocin and Quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones 2015, 20, 927–938. [Google Scholar] [CrossRef] [Green Version]
- Long, M.; Yang, S.; Zhang, Y.; Li, P.; Han, J.; Dong, S.; Chen, X.; He, J. Proanthocyanidin protects against acute zearalenone-induced testicular oxidative damage in male mice. Environ. Sci. Pollut. Res. 2016, 24, 938–946. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Jiao, R.; Peng, Z.; Li, Y.; Bai, W. Toxic effects of zearalenone on gametogenesis and embryonic development: A molecular point of review. Food Chem. Toxicol. 2018, 119, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, M.; Nayaka, S.C.; Anand, T.; Rajesh, R.; Aiyaz, M.; Divakara, S.; Murali, H.; Prakash, H.; Rao, P.L. Zearalenone induced toxicity in SHSY-5Y cells: The role of oxidative stress evidenced by N-acetyl cysteine. Food Chem. Toxicol. 2014, 65, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Mano, J.; Tanaka, K.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S. High level of reduced glutathione contributes to detoxification of lipid peroxide-derived reactive carbonyl species in transgenic Arabidopsis overexpressing glutathione reductase under aluminum stress. Physiol. Plant. 2017, 161, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Kopp, T.I.; Vogel, U.; Dragsted, L.O.; Tjonneland, A.; Ravn-Haren, G. Association between single nucleotide polymorphisms in the antioxidant genes CAT, GR and SOD1, erythrocyte enzyme activities, dietary and life style factors and breast cancer risk in a Danish, prospective cohort study. Oncotarget 2017, 8, 62984–62997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvamme, E.; Roberg, B.; Torgner, I.A. Glutamine transport in brain mitochondria. Neurochem. Int. 2000, 37, 131–138. [Google Scholar] [CrossRef]
- Marques, C.; Mauriz, J.L.; Simonetto, D.; Marroni, C.A.; Tuñon, M.J.; González-Gallego, J.; Marroni, N.P. Glutamine prevents gastric oxidative stress in an animal model of portal hypertension gastropathy. Ann. Hepatol. 2011, 10, 531–539. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [Green Version]
- Curi, R.; Newsholme, P.; Procopio, J.; Lagranha, C.; Gorjão, R.; Pithon-Curi, T.C. Glutamine, gene expression, and cell function. Front. Biosci. 2007, 12, 344–357. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Guo, W.; Zhao, Y.; Liu, G.; Wu, J.; Chang, C. Deoxynivalenol-Induced Cytotoxicity and Apoptosis in IPEC-J2 Cells through the Activation of Autophagy by Inhibiting PI3K-AKT-mTOR Signaling Pathway. ACS Omega 2019, 4, 18478–18486. [Google Scholar] [CrossRef]
- Marone, R.; Erhart, D.; Mertz, A.C.; Bohnacker, T.; Schnell, C.; Cmiljanovic, V.; Stauffer, F.; Garcia-Echeverria, C.; Giese, B.; Maira, S.-M.; et al. Targeting Melanoma with Dual Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Inhibitors. Mol. Cancer Res. 2009, 7, 601–613. [Google Scholar] [CrossRef] [Green Version]
- Gharbi, S.I.; Zvelebil, M.J.; Shuttleworth, S.J.; Hancox, T.; Saghir, N.; Timms, J.F.; Waterfield, M.D. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem. J. 2007, 404, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.T.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.S.; Geng, Y.; Zhao, M.; Su, L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 11497. [Google Scholar] [CrossRef] [Green Version]
- Hyun, D.-H.; Hunt, N.D.; Emerson, S.S.; Hernandez, J.O.; Mattson, M.P.; de Cabo, R. Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria: Plasma Membrane-Associated Redox Activities in Neuronal Cells. J. Neurochem. 2006, 100, 1364–1374. [Google Scholar] [CrossRef]
- Hempel, N.; Trebak, M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium 2017, 63, 70–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-J.; Oh, S.-Y.; Jo, I. Zearalenone Induces Endothelial Cell Apoptosis through Activation of a Cytosolic Ca2+/ERK1/2/p53/Caspase 3 Signaling Pathway. Toxins 2021, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Saemann, M.D. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67, iii70–iii74. [Google Scholar] [CrossRef]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2013, 343, 179–189. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Hou, X.; Li, D.; He, S.; Wan, C.; Yin, P.; Liu, M.; Liu, F.; Xu, J. Punicalagin Induces Nrf2/HO-1 Expression via Upregulation of PI3K/AKT Pathway and Inhibits LPS-Induced Oxidative Stress in RAW264.7 Macrophages. Mediat. Inflamm. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, M.; Liu, J.; Hong, G.; Lin, Q. The preventative effect of Akt knockout on liver cancer through modulating NF-?B-regulated inflammation and Bad-related apoptosis signaling pathway. Int. J. Oncol. 2016, 48, 1467–1476. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Q.; Wang, Y.; Li, J.; Lu, G.; Liu, Z. Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environ. Health Prev. Med. 2019, 24, 4. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Nandeesha, H.; Dorairajan, L.N.; Ganesh, R.N. Over expression of PI3K-AkT reduces apoptosis and increases prostate size in benign prostatic hyperplasia. Aging Male 2018, 23, 440–446. [Google Scholar] [CrossRef]
- Xuan, F.; Jian, J.; Qin, F.; Huang, R. Bauhinia championii flavone inhibits apoptosis and autophagy via the PI3K/Akt pathway in myocardial ischemia/reperfusion injury in rats. Drug Des. Dev. Ther. 2015, 9, 5933–5945. [Google Scholar] [CrossRef] [Green Version]
- Plas, D.R.; Talapatra, S.; Edinger, A.L.; Rathmell, J.C.; Thompson, C.B. Akt and Bcl-xL Promote Growth Factor-independent Survival through Distinct Effects on Mitochondrial Physiology. J. Biol. Chem. 2001, 276, 12041–12048. [Google Scholar] [CrossRef] [Green Version]
- Stiles, B.L. PI-3-K and AKT: Onto the mitochondria. Adv. Drug Deliv. Rev. 2009, 61, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Harada, H.; Yang, E.; Jockel, J.; Korsmeyer, S.J. Serine Phosphorylation of Death Agonist BAD in Response to Survival Factor Results in Binding to 14-3-3 Not BCL-XL. Cell 1996, 87, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xie, L.-P.; Zheng, X.-Y.; Wang, Y.-B.; Bai, Y.; Shen, H.-F.; Li, L.-C.; Dahiya, R. A component of green tea, (−)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2007, 354, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Y.; Shi, Z.; Lu, D.; Li, T.; Ding, Y.; Ruan, Y.; Xu, A.-D. The Neuroprotection of Liraglutide Against Ischaemia-induced Apoptosis through the Activation of the PI3K/AKT and MAPK Pathways. Sci. Rep. 2016, 6, 26859. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qu, X.; Hou, K.; Zhang, Y.; Dong, Q.; Teng, Y.; Zhang, J.; Liu, Y. PI3K/Akt is involved in bufalin-induced apoptosis in gastric cancer cells. Anti-Cancer Drugs 2009, 20, 59–64. [Google Scholar] [CrossRef] [PubMed]






| Genes | Accession Number | Orientation | Sequence (5′-3′) | Fragments Size (bp) | Tm (°C) |
|---|---|---|---|---|---|
| GAPDH | NM_ | Forward | GATGGTGAAGGTCGGAGTGAAC | 153 | 60.9 |
| 001206359.1 | Reversed | TGGGTGGAATCATACTGGAACA | |||
| Caspase-3 | NM_ | Forward | GACACTCGCTCAACTTCTTGG | 121 | 54.5 |
| 214131.1 | Reversed | TTGGACTGTGGGATTGAGAC | |||
| Caspase-9 | XM_ | Forward | GGACATTGGTTCTGGAGGATT | 116 | 52.3 |
| 013998997.1 | Reversed | TGTTGATGATGAGGCAGTGG | |||
| Cyto-c | NM_ | Forward | CTCTTACACAGATGCCAACAA | 139 | 56.1 |
| 001129970.1 | Reversed | TTCCCTTTCTCCCTTCTTCT | |||
| Bax | XM_ | Forward | TTTGCTTCAGGGTTTCATCC | 113 | 54.4 |
| 003127290.3 | Reversed | GACACTCGCTCAACTTCTTGG | |||
| Bcl-2 | AB | Forward | GCGACTTTGCCGAGATGT | 116 | 55.9 |
| 271960.1 | Reversed | CACAATCCTCCCCCAGTTC | |||
| Bcl-xl | XM_ | Forward | GCAGGTAGTGAACGAACTCTTCCG | 140 | 60.08 |
| 021077298.1 | Reversed | CCATCCAAGTTGCGATCCGACTC | |||
| Bad | XM_ | Forward | CTTACCCAGAGGGGACCGAG | 153 | 58.39 |
| 021082883.1 | Reversed | AGGAACCCTGGAACTCGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, J.; Zhang, T.; Gu, A.; Li, J.; Shan, A. The Antagonistic Effect of Glutamine on Zearalenone-Induced Apoptosis via PI3K/Akt Signaling Pathway in IPEC-J2 Cells. Toxins 2021, 13, 891. https://doi.org/10.3390/toxins13120891
Wang T, Wang J, Zhang T, Gu A, Li J, Shan A. The Antagonistic Effect of Glutamine on Zearalenone-Induced Apoptosis via PI3K/Akt Signaling Pathway in IPEC-J2 Cells. Toxins. 2021; 13(12):891. https://doi.org/10.3390/toxins13120891
Chicago/Turabian StyleWang, Tianhu, Jingjing Wang, Tong Zhang, Aixin Gu, Jianping Li, and Anshan Shan. 2021. "The Antagonistic Effect of Glutamine on Zearalenone-Induced Apoptosis via PI3K/Akt Signaling Pathway in IPEC-J2 Cells" Toxins 13, no. 12: 891. https://doi.org/10.3390/toxins13120891
APA StyleWang, T., Wang, J., Zhang, T., Gu, A., Li, J., & Shan, A. (2021). The Antagonistic Effect of Glutamine on Zearalenone-Induced Apoptosis via PI3K/Akt Signaling Pathway in IPEC-J2 Cells. Toxins, 13(12), 891. https://doi.org/10.3390/toxins13120891





