Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars
Abstract
1. Introduction
2. Results
2.1. Occurrence of Deoxynivalenol and Zearalenone and Their Modified Forms in Forage Maize Samples
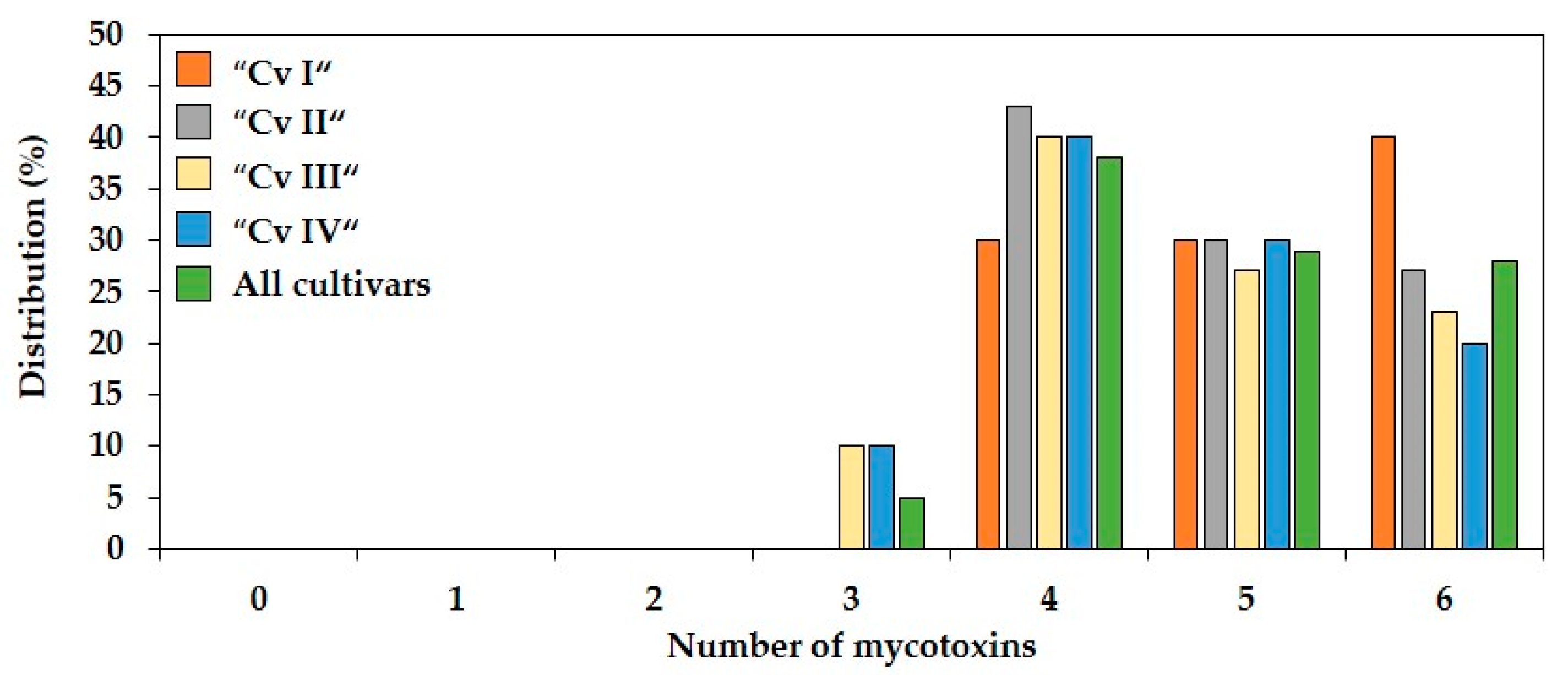
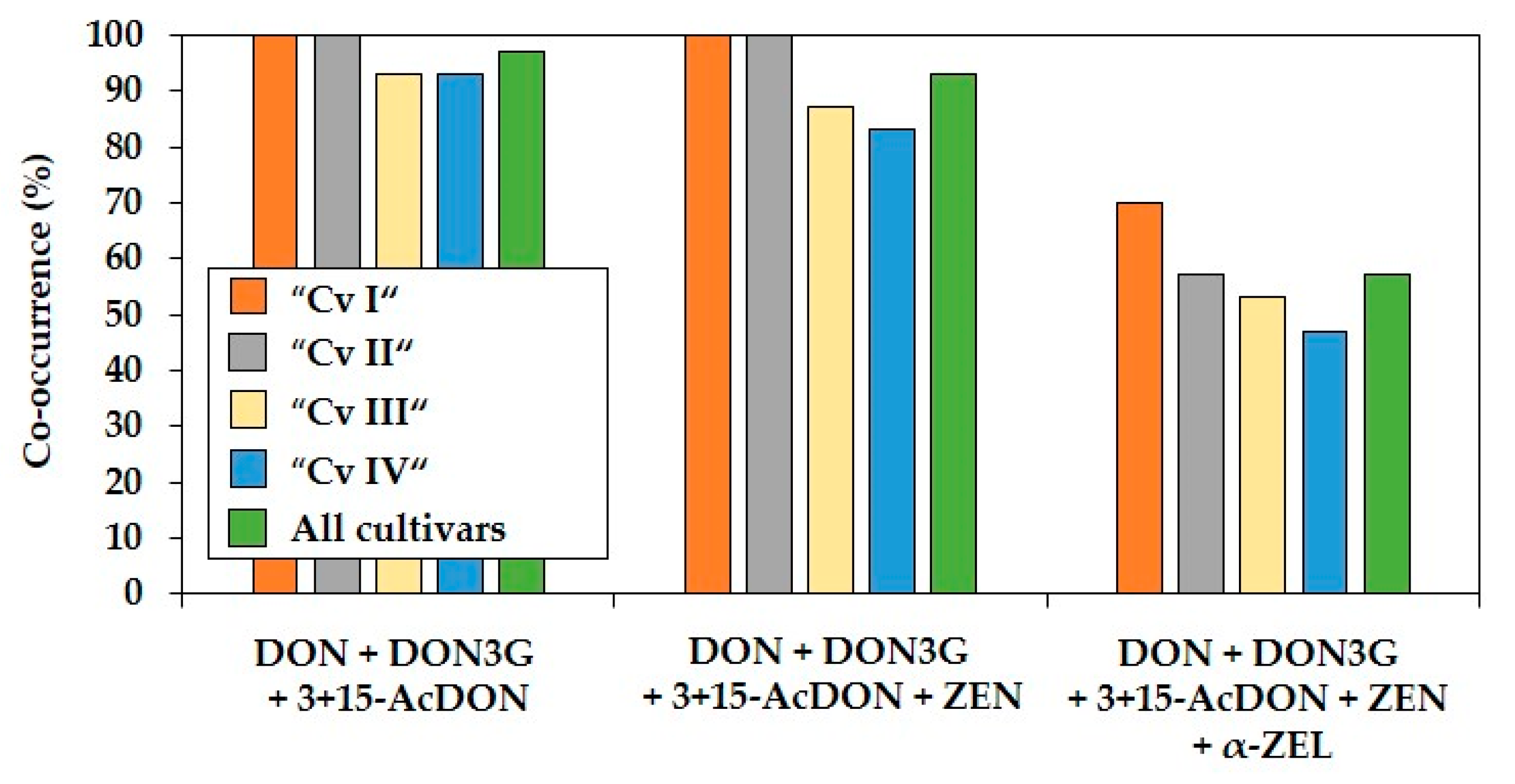
2.2. Co-Occurrence and Correlations between Fusarium Mycotoxins
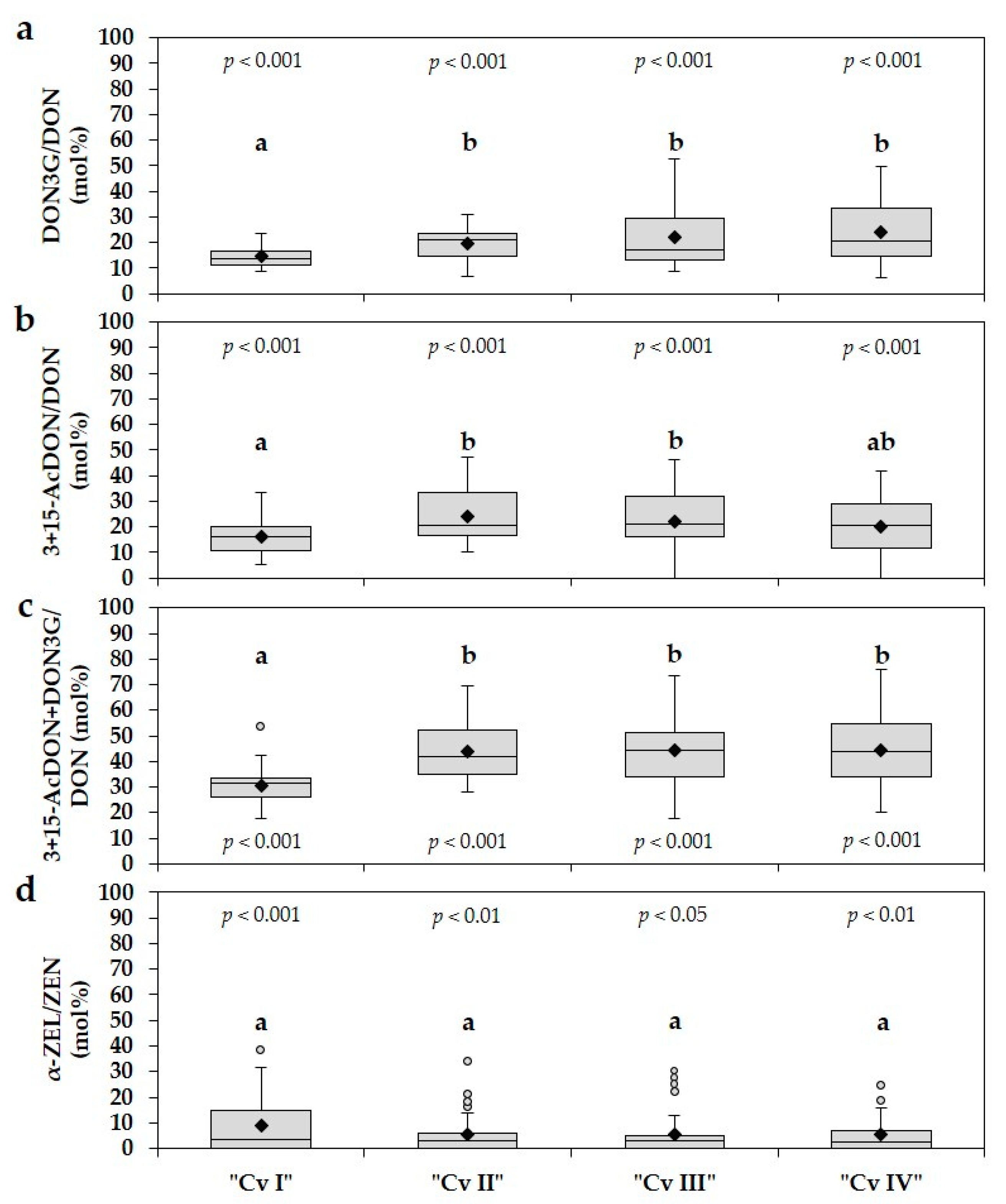
2.3. Ratios between DON3G and DON, 3+15-AcDON and DON, α-ZEL and ZEN
2.4. Total Concentrations of DON and ZEN by Considering DON3G, 3+15-AcDON and α-ZEL
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Forage Maize Sampling
5.2. Analysis of Mycotoxins
5.3. Determination of Ratios between DON3G and DON, 3+15-AcDON and DON, α-ZEL and ZEN
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Grain Maize and Corn-Cob-Mix by Area, Production and Humidity. Available online: https://ec.europa.eu/eurostat/databrowser/view/tag00093/default/table (accessed on 13 July 2020).
- Eurostat. Green Maize by Area, Production and Humidity. Available online: https://ec.europa.eu/eurostat/databrowser/view/tag00101/default/table (accessed on 13 July 2020).
- Schollenberger, M.; Müller, H.-M.; Ernst, K.; Sondermann, S.; Liebscher, M.; Schlecker, C.; Wischer, G.; Drochner, W.; Hartung, K.; Piepho, H.-P. Occurrence and distribution of 13 trichothecene toxins in naturally contaminated maize plants in Germany. Toxins 2012, 4, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Taube, F.; Vogeler, I.; Kluß, C.; Herrmann, A.; Hasler, M.; Rath, J.; Loges, R.; Malisch, C.S. Yield progress in forage maize in NW Europe–Breeding progress or climate change effects? Front. Plant Sci. 2020, 11, 1214. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Dorn, B.; Forrer, H.-R.; Schürch, S.; Vogelgsang, S. Fusarium species complex on maize in Switzerland: Occurrence, prevalence, impact and mycotoxins in commercial hybrids under natural infection. Eur. J. Plant Pathol. 2009, 125, 51–61. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef]
- Scauflaire, J.; Mahieu, O.; Louvieaux, J.; Foucart, G.; Renard, F.; Munaut, F. Biodiversity of Fusarium species in ears and stalks of maize plants in Belgium. Eur. J. Plant Pathol. 2011, 131, 59–66. [Google Scholar] [CrossRef]
- Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in Flanders’ fields: Occurrence and correlations with Fusarium species in whole-plant harvested maize. Microorganisms 2019, 7, 571. [Google Scholar] [CrossRef]
- Pfordt, A.; Ramos Romero, L.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Impact of environmental conditions and agronomic practices on the prevalence of Fusarium species associated with ear- and stalk rot in maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- Oldenburg, E.; Höppner, F.; Ellner, F.; Weinert, J. Fusarium diseases of maize associated with mycotoxin contamination of agricultural products intended to be used for food and feed. Mycotoxin Res. 2017, 33, 167–182. [Google Scholar] [CrossRef]
- Oldenburg, E.; Ellner, F. Fusarium mycotoxins in forage maize–Detection and evaluation. Mycotoxin Res. 2005, 21, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, M.; Müller, H.-M.; Rüfle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Te Giffel, M.C. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam. Part B 2008, 1, 41–50. [Google Scholar] [CrossRef]
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; De Vries, I.; Oerke, E.C. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar] [CrossRef]
- Dorn, B.; Forrer, H.R.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Vogelgsang, S. Fusarium species complex and mycotoxins in grain maize from maize hybrid trials and from grower’s fields. J. Appl. Microbiol. 2011, 111, 693–706. [Google Scholar] [CrossRef]
- Van Asselt, E.D.; Azambuja, W.; Moretti, A.; Kastelein, P.; De Rijk, T.C.; Stratakou, I.; Van Der Fels-Klerx, H.J. A Dutch field survey on fungal infection and mycotoxin concentrations in maize. Food Addit. Contam. Part A 2012, 29, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Landschoot, S.; Audenaert, K.; Maene, P.; Di Mavungu, D.; Eeckhout, M.; Haesaert, G.; De Saeger, S. Occurrence and within field variability of Fusarium mycotoxins and their masked forms in maize crops in Belgium. World Mycotoxin J. 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Lešić, T.; Zadravec, M.; Vahčić, N.; Malenica Staver, M.; Markov, K. Deoxynivalenol and zearalenone in unprocessed cereals and soybean from different cultivation regions in Croatia. Food Addit. Contam. Part B 2017, 10, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Pfeiffer, E.; Hildebrand, A. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin J. 2010, 3, 385–401. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium mycotoxins in cereals and their products–Metabolism, occurrence, and toxicity: An updated review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- European Commision. Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L255, 14–17. [Google Scholar]
- European Commission. Commission Recommendation (2006/576/EC) of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT- 2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Berthiller, F.; Schuhmacher, R.; Adam, G.; Krska, R. Formation, determination and significance of masked and other conjugated mycotoxins. Anal. Bioanal. Chem. 2009, 395, 1243–1252. [Google Scholar] [CrossRef]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. Part A 2012, 29, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Gratz, S. Do plant-bound masked mycotoxins contribute to toxicity? Toxins 2017, 9, 85. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; De, B.S.; De, B.P.; Croubels, S. Modified Fusarium mycotoxins unmasked: From occurrence in cereals to animal and human excretion. Food Chem. Toxicol. 2015, 80, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, N.; Devreese, M.; Demeyere, K.; Berthiller, F.; Michlmayr, H.; Varga, E.; Adam, G.; Meyer, E.; Croubels, S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016, 95, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC–MS–MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Graniczkowska, K.; De Saeger, S. Metabolism of modified mycotoxins studied through in vitro and in vivo models: An overview. Toxicol. Lett. 2015, 233, 24–28. [Google Scholar] [CrossRef]
- Panasiuk, Ł.; Jedziniak, P.; Pietruszka, K.; Posyniak, A. Simultaneous determination of deoxynivalenol, its modified forms, nivalenol and fusarenone-X in feedstuffs by the liquid chromatography–tandem mass spectrometry method. Toxins 2020, 12, 362. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’asta, C.; Corradini, R.; Marchelli, R.; Sulyok, M.; Krska, R.; Adam, G.; Schuhmacher, R. Occurrence of deoxynivalenol and its 3-β-D-glucoside in wheat and maize. Food Addit. Contam. Part A 2009, 26, 507–511. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Ozsisli, B.; Anderson, J.; Whitney, K.; Ohm, J.-B.; Simsek, S. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum. Toxins 2013, 5, 2522–2532. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Simsek, S.; Brûlé-Babel, A.; Fernando, W.G.D. Analysis of deoxynivalenol and deoxynivalenol-3-glucosides content in Canadian spring wheat cultivars inoculated with Fusarium graminearum. Food Addit. Contam. Part A 2016, 33, 1254–1264. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Dong, F.; Wang, S.; Yu, M.; Sun, Y.; Xu, J.; Shi, J. Natural occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in various wheat cultivars grown in Jiangsu province, China. World Mycotoxin J. 2017, 10, 285–293. [Google Scholar] [CrossRef]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.T.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Yoshinari, T.; Waśkiewicz, A.; Szymczyk, K. Contamination of wheat cultivated in various regions of Poland during 2017 and 2018 agricultural seasons with selected trichothecenes and their modified forms. Toxins 2019, 11, 88. [Google Scholar] [CrossRef]
- Tucker, J.R.; Badea, A.; Blagden, R.; Pleskach, K.; Tittlemier, S.A.; Fernando, W.G.D. Deoxynivalenol-3-glucoside content is highly associated with deoxynivalenol levels in two-row barley genotypes of importance to Canadian barley breeding programs. Toxins 2019, 11, 319. [Google Scholar] [CrossRef]
- Wang, W.; Ma, J.-J.; Yu, C.-C.; Lin, X.-H.; Jiang, H.-R.; Shao, B.; Li, F.-Q. Simultaneous determination of masked deoxynivalenol and some important type B trichothecenes in Chinese corn kernels and corn-based products by ultra-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2012, 60, 11638–11646. [Google Scholar] [CrossRef]
- Mitsuhashi, S.; Nakagawa, H.; Matsuo, Y.; Kikawada, T.; Tamaki, H.; Sato, H. Varietal differences in deoxynivalenol and its glucoside (modified mycotoxin) content in forage corn inoculated with Fusarium graminearum. JSM Mycotoxins 2019, 69, 1–8. [Google Scholar] [CrossRef]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Driehuis, F. Silage and the safety and quality of dairy foods: A review. Agric. Food Sci. 2013, 22, 16–34. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- Döll, S.; Dänicke, S. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, E.; Höppner, F. Fusarium mycotoxins in forage maize—Occurrence, risk assessment, minimization. Mycotoxin Res. 2003, 19, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Eckard, S.; Wettstein, F.E.; Forrer, H.-R.; Vogelgsang, S. Incidence of Fusarium species and mycotoxins in silage maize. Toxins 2011, 3, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twarużek, M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant. Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Lauren, D.R.; Jensen, D.J.; Smith, W.A.; Dow, B.W.; Sayer, S.T. Mycotoxins in New Zealand maize: A study of some factors influencing contamination levels in grain. N. Z. J. Crop. Hortic. Sci. 1996, 24, 13–20. [Google Scholar] [CrossRef]
- Bundessortenamt. Beschreibende Sortenliste 2020. Available online: https://www.bundessortenamt.de/bsa/media/Files/BSL/bsl_getreide_2020.pdf (accessed on 10 September 2020).
- Dill-Macky, R.; Jones, R.K. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant. Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Champeil, A.; Doré, T.; Fourbet, J.F. Fusarium head blight: Epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Sci. 2004, 166, 1389–1415. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Tamburic-Ilincic, L.; Hooker, D.C. Effect of previous crop, tillage, field size, adjacent crop, and sampling direction on airborne propagules of Gibberella zeae/Fusarium graminearum, fusarium head blight severity, and deoxynivalenol accumulation in winter wheat. Can. J. Plant Pathol. 2005, 27, 217–224. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Liu, S.; Yao, L.; Liu, Y.; Wu, Y.; Gong, Z. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Barthel, J.; Engelhardt, G.; Bauer, J.; Meyer, K. Simultaneous determination of type A, B and D trichothecenes and their occurrence in cereals and cereal products. Food Addit. Contam. Part A 2009, 26, 1273–1289. [Google Scholar] [CrossRef]
- Speijers, G.J.; Speijers, M.H. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Oswald, I.P. Mycotoxin co-contamination of food and feed: Meta-Analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar] [CrossRef]
- Wang, W. (Ed.) Transformation of trichothecene acetyldeoxynivalenol to deoxynivalenol by bacterial acetyltransferase. In Medicine Sciences and Bioengineering; CRC Press: London, UK, 2015; pp. 775–781. [Google Scholar]
- Jensen, T.; De Boevre, M.; De Saeger, S.; Preußke, N.; Sönnichsen, F.D.; Kramer, E.; Klink, H.; Verreet, J.-A.; Birr, T. Effect of ensiling duration on the fate of deoxynivalenol, zearalenone and their derivatives in maize silage. Mycotoxin Res. 2020, 36, 127–136. [Google Scholar] [CrossRef]
- Driehuis, F.; Oude Elferink, S.J.W.H. The impact of the quality of silage on animal health and food safety: A review. Vet. Q. 2000, 22, 212–216. [Google Scholar] [CrossRef]
- Richard, E.; Heutte, N.; Sage, L.; Pottier, D.; Bouchart, V.; Lebailly, P.; Garon, D. Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol. 2007, 45, 2420–2425. [Google Scholar] [CrossRef]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sørlie, M.; Ropstad, E.; Muller, M.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef]
- González Pereyra, M.L.; Sulyok, M.; Baralla, V.; Dalcero, A.M.; Krska, R.; Chulze, S.; Cavaglieri, L.R. Evaluation of zearalenone, α-zearalenol, β-zearalenol, zearalenone 4-sulfate and β-zearalenol 4-glucoside levels during the ensiling process. World Mycotoxin J. 2014, 7, 291–295. [Google Scholar] [CrossRef]
- Deutscher Wetterdienst (DWD). Klimareport Schleswig-Holstein. Available online: https://www.dwd.de/DE/leistungen/klimareport_sh/download_report_2017.pdf?__blob=publicationFile&v=5 (accessed on 10 March 2020).
- Statistisches Amt für Hamburg und Schleswig-Holstein. Die Bodennutzung in Schleswig-Holstein 2017. Available online: http://epub.sub.uni-hamburg.de/epub/volltexte/2018/78253/pdf/C_I_1_j_17_SH_e.pdf (accessed on 10 March 2020).
- Jensen, T.; De Boevre, M.; Preußke, N.; De Saeger, S.; Birr, T.; Verreet, J.-A.; Sönnichsen, F.D. Evaluation of high-resolution mass spectrometry for the quantitative analysis of mycotoxins in complex feed matrices. Toxins 2019, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 22 June 2020).
- Laird, N.M.; Ware, J.H. Random-effects models for longitudinal data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.J.; Ruppert, D. Transformation and Weighting in Regression; CRC Press: London, UK, 1988. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar]
- Box, G.E.P.; Jenkins, G.M.; Reinsel, G.C.; Ljung, G.M. Time Series Analysis; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Bretz, F.; Hothorn, T.; Westfall, P.H. Multiple Comparisons Using R; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
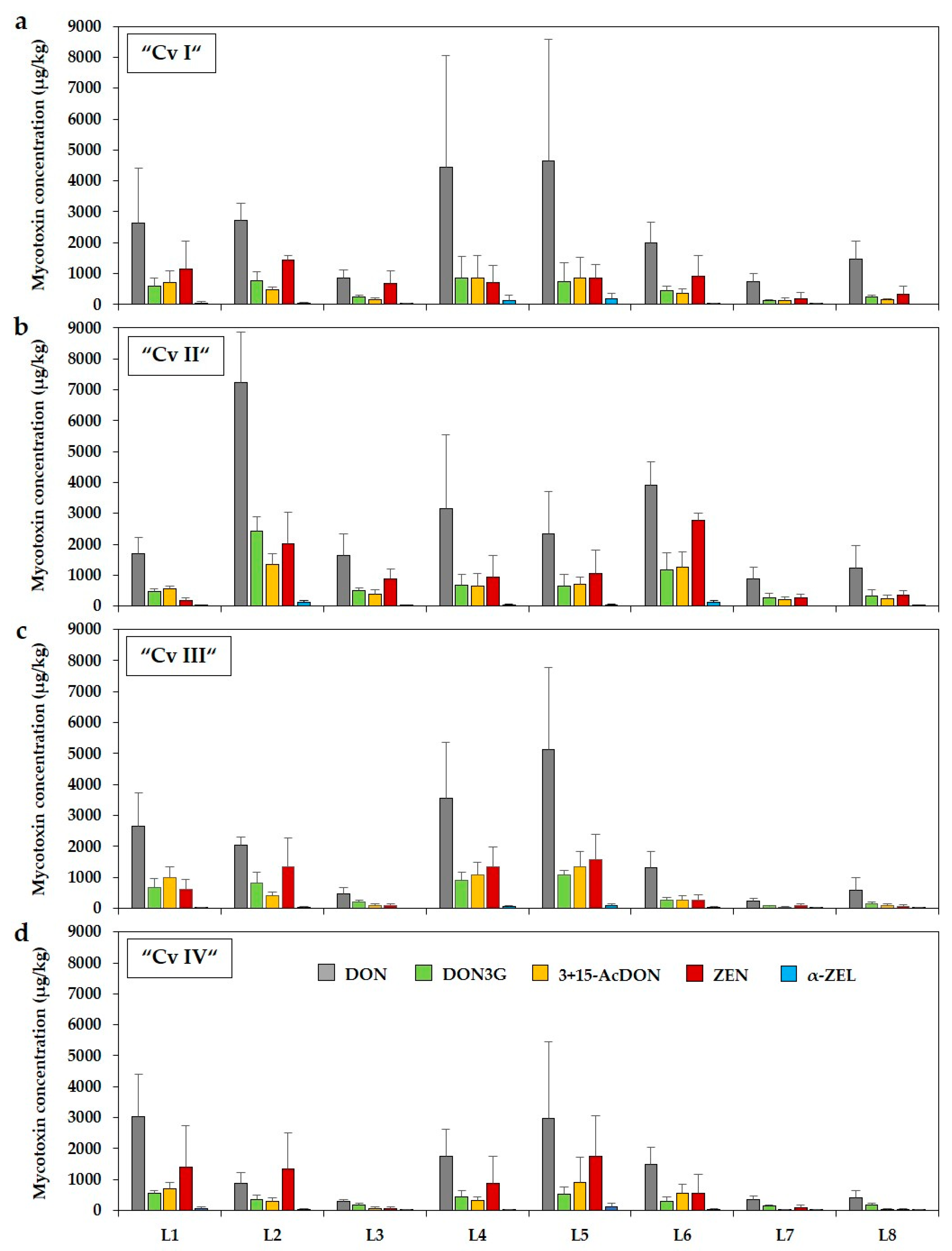

| Mycotoxin | Incidence (%) a | Mean (µg/kg) b | Mean Positive (µg/kg) c | Median (µg/kg) b | Min–Max (µg/kg) | Samples ≥Guidance Value (%) d |
|---|---|---|---|---|---|---|
| DON | 100 | 2165 | 2165 | 1391 | <CCβ–10,972 | 9 (11/120) |
| DON3G | 100 | 537 | 537 | 348 | 95–3038 | − f |
| 3+15-AcDON | 97 | 516 | 534 | 346 | <CCα–2237 | − f |
| ZEN | 96 | 819 | 862 | 426 | <CCα–3910 | 46 (55/120) |
| α-ZEL | 59 | 44 | 74 | 26 | <CCα–423 | − f |
| β-ZEL | 32 | n.a e | n.a e | n.a e | <CCα–203 | − f |
| Cv a | Mycotoxin | Incidence (%) b | Mean (µg/kg) c | Mean Positive (µg/kg) d | Median (µg/kg) c | Min–Max (µg/kg) | Samples ≥Guidance Value (%) e |
|---|---|---|---|---|---|---|---|
| “Cv I” (n = 30) | DON | 100 | 2486 Aa | 2486 | 1651 | 466–10,972 | 13 (4/30) |
| DON3G | 100 | 507 ABb | 507 | 300 | 119–1911 | − g | |
| 3+15-AcDON | 100 | 472 ABb | 472 | 281 | 29–1837 | − g | |
| ZEN | 100 | 785 Ab | 785 | 621 | <CCβ–2334 | 53 (16/30) | |
| α-ZEL | 70 | 64 Ac | 92 | 26 | <CCα–423 | − g | |
| β-ZEL | 53 | n.a f | n.a f | n.a f | <CCα–163 | − g | |
| “Cv II” (n = 30) | DON | 100 | 2673 Aa | 2673 | 1778 | 467–9305 | 13 (4/30) |
| DON3G | 100 | 776 Ab | 776 | 511 | 112–3038 | − g | |
| 3+15-AcDON | 100 | 655 Ab | 655 | 524 | 112–2049 | − g | |
| ZEN | 100 | 1055 Ab | 1055 | 558 | 124–3076 | 53 (16/30) | |
| α-ZEL | 57 | 44 Ac | 78 | 26 | <CCα–188 | − g | |
| β-ZEL | 30 | n.a f | n.a f | n.a f | <CCα–118 | − g | |
| “Cv III” (n = 30) | DON | 100 | 2054 ABa | 2054 | 1414 | <CCβ–9638 | 7 (2/30) |
| DON3G | 100 | 526 ABb | 526 | 328 | 95–1286 | − g | |
| 3+15-AcDON | 93 | 559 ABb | 599 | 378 | <CCα–2087 | − g | |
| ZEN | 93 | 666 Ab | 714 | 397 | <CCα–2881 | 43 (13/30) | |
| α-ZEL | 57 | 31 Ac | 55 | 26 | <CCα–199 | − g | |
| β-ZEL | 23 | n.a f | n.a f | n.a f | <CCα–119 | − g | |
| “Cv IV” (n = 30) | DON | 100 | 1449 Ba | 1449 | 991 | <CCβ–7104 | 3 (1/30) |
| DON3G | 100 | 339 Bb | 339 | 253 | 100–795 | − g | |
| 3+15-AcDON | 93 | 378 Bb | 405 | 267 | <CCα–2237 | − g | |
| ZEN | 90 | 768 Ab | 886 | 225 | <CCα–3910 | 33 (10/30) | |
| α-ZEL | 53 | 36 Ac | 68 | 26 | <CCα–324 | − g | |
| β-ZEL | 20 | n.a f | n.a f | n.a f | <CCα–203 | − g |
| Correlation | Cultivar | ||||
|---|---|---|---|---|---|
| “Cv I” | “Cv II” | “Cv III” | “Cv IV” | All Cultivars | |
| DON vs. DON3G | 0.907 | 0.877 | 0.926 | 0.833 | 0.866 |
| DON vs. 3+15-AcDON | 0.892 | 0.888 | 0.929 | 0.952 | 0.915 |
| DON3G vs. 3+15-AcDON | 0.883 | 0.799 | 0.866 | 0.815 | 0.841 |
| DON vs. ZEN | 0.704 | 0.667 | 0.788 | 0.696 | 0.714 |
| DON3G vs. ZEN | 0.686 | 0.579 | 0.751 | 0.633 | 0.662 |
| 3+15-AcDON vs. ZEN | 0.712 | 0.631 | 0.689 | 0.640 | 0.668 |
| DON vs. α-ZEL | 0.610 | 0.600 | 0.615 | 0.443 | 0.567 |
| DON3G vs. α-ZEL | 0.627 | 0.537 | 0.517 | 0.334 | 0.504 |
| 3+15-AcDON vs. α-ZEL | 0.661 | 0.582 | 0.647 | 0.471 | 0.590 |
| ZEN vs. α-ZEL | 0.663 | 0.593 | 0.635 | 0.584 | 0.619 |
| Location (Abbreviation) | Coordinates (EPSG 3857) | Crop Rotation a | Previous Crop a | Soil Cultivation | |
|---|---|---|---|---|---|
| x | y | ||||
| Barkhorn (L1) | 1,073,958 | 7,211,748 | Continuous FM | FM | Reduced tillage |
| Futterkamp (L2) | 1,183,896 | 7,225,601 | OR-WW-FM-FM | FM | Plough |
| Leezen (L3) | 1,139,549 | 7,146,520 | WR-FM | WR | Plough |
| Medelby 1 b (L4) | 1,022,547 | 7,325,312 | Continuous FM | FM | Plough |
| Medelby 2 b (L5) | 1,022,547 | 7,325,312 | Continuous FM | FM | Reduced tillage |
| Scholderup (L6) | 1,076,900 | 7,278,022 | Continuous FM | FM | Plough |
| Schuby (L7) | 1,051,158 | 7,269,157 | Potato-WR-FM | WR | Plough |
| Wallsbüll (L8) | 1,030,547 | 7,318,384 | WW-FM-WR | WW | Plough |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birr, T.; Jensen, T.; Preußke, N.; Sönnichsen, F.D.; De Boevre, M.; De Saeger, S.; Hasler, M.; Verreet, J.-A.; Klink, H. Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars. Toxins 2021, 13, 110. https://doi.org/10.3390/toxins13020110
Birr T, Jensen T, Preußke N, Sönnichsen FD, De Boevre M, De Saeger S, Hasler M, Verreet J-A, Klink H. Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars. Toxins. 2021; 13(2):110. https://doi.org/10.3390/toxins13020110
Chicago/Turabian StyleBirr, Tim, Tolke Jensen, Nils Preußke, Frank D. Sönnichsen, Marthe De Boevre, Sarah De Saeger, Mario Hasler, Joseph-Alexander Verreet, and Holger Klink. 2021. "Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars" Toxins 13, no. 2: 110. https://doi.org/10.3390/toxins13020110
APA StyleBirr, T., Jensen, T., Preußke, N., Sönnichsen, F. D., De Boevre, M., De Saeger, S., Hasler, M., Verreet, J.-A., & Klink, H. (2021). Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars. Toxins, 13(2), 110. https://doi.org/10.3390/toxins13020110







