De Novo Venom-Gland Transcriptomics of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia—Next-Generation Sequencing, Functional Annotation and Toxinological Correlation
Abstract
:1. Introduction
2. Results and Discussion
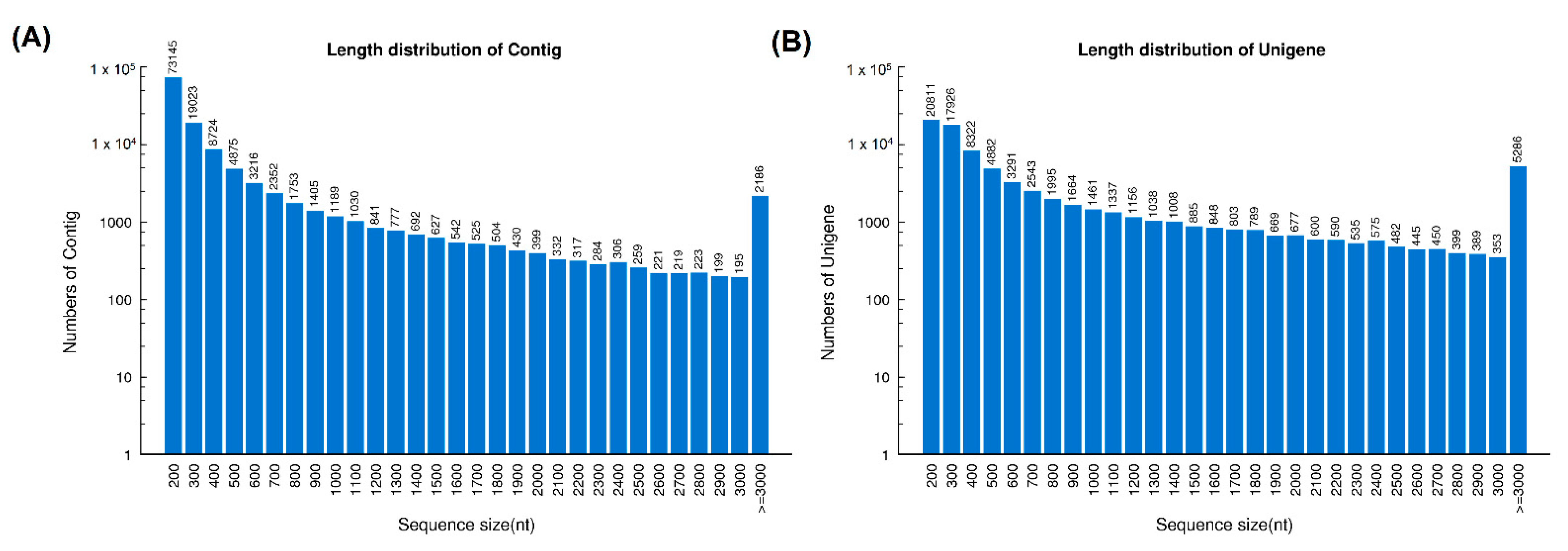
2.1. Sequencing and De Novo Transcriptome Assembly
2.2. Toxin Gene Expression Profile
2.3. Profiling of Toxin Transcripts
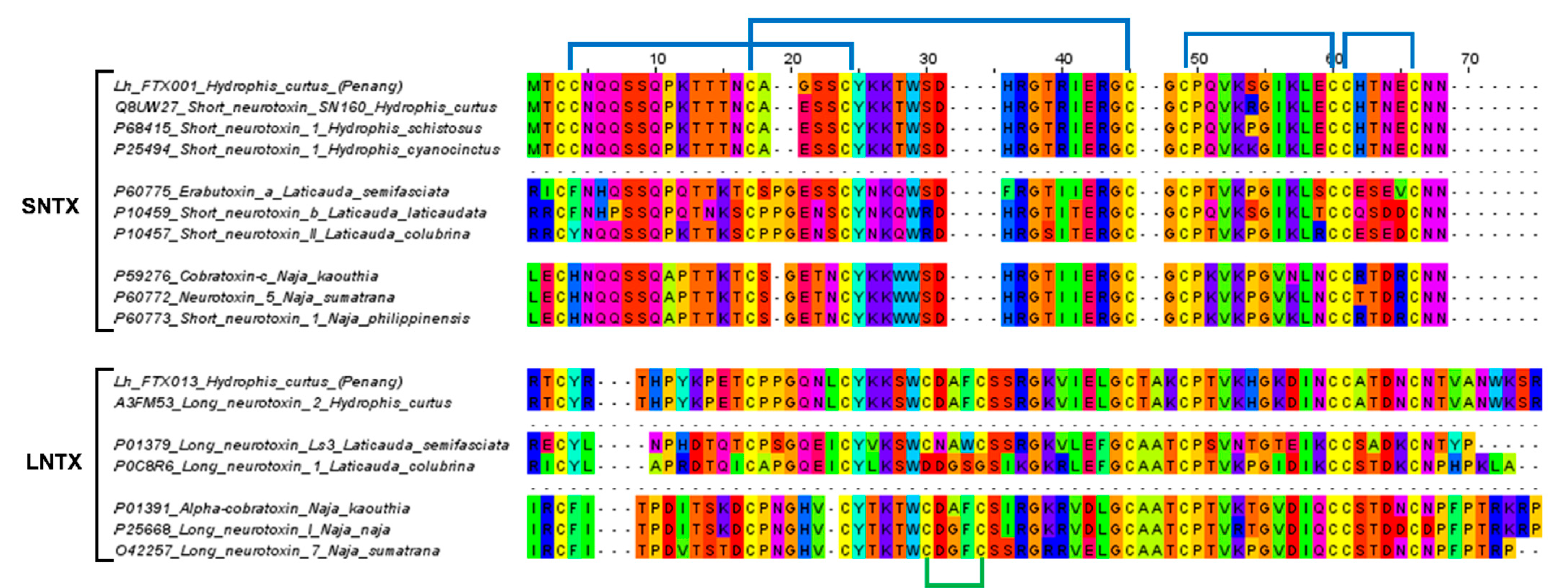
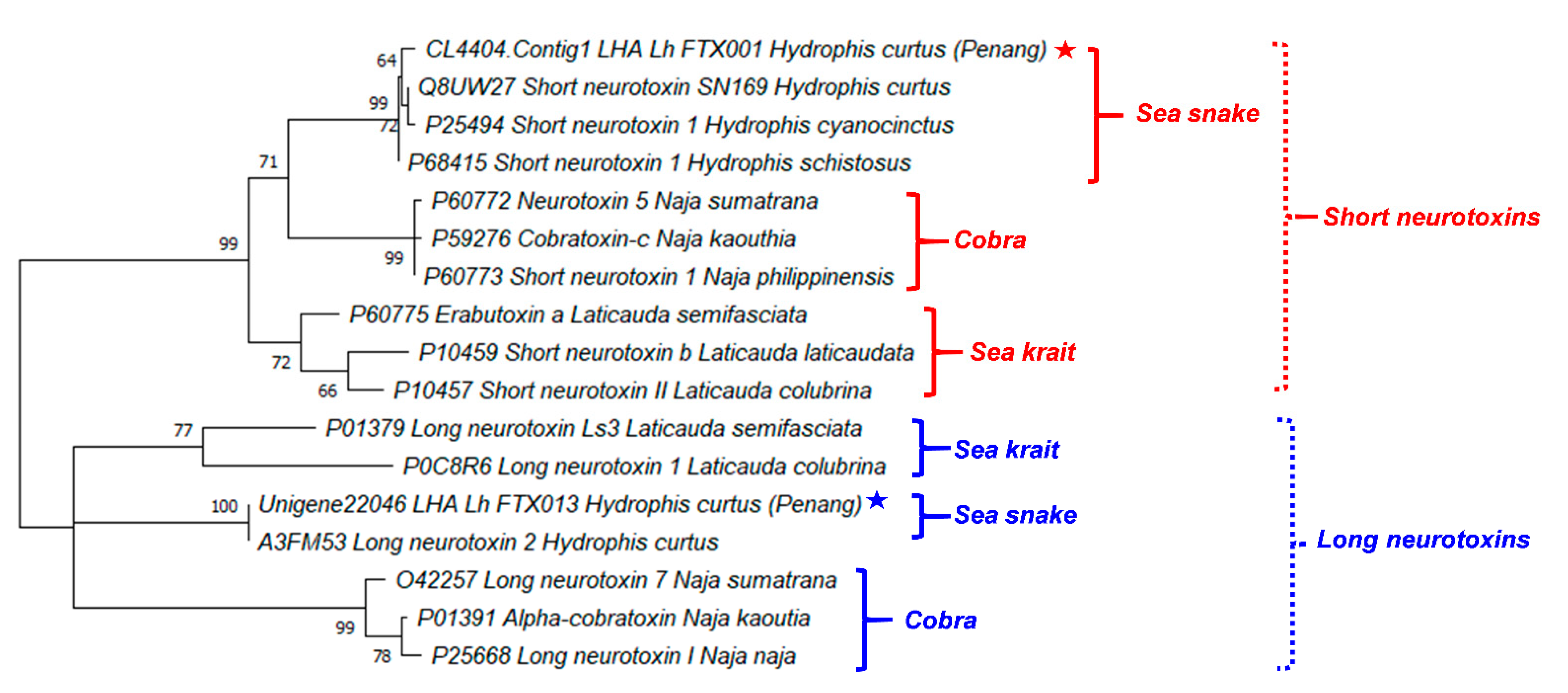
2.4. Sequence Analysis and Phylogenetics of Three-Finger Toxins
2.5. Clinical Relevance and Antigencity of Three-Finger Toxins
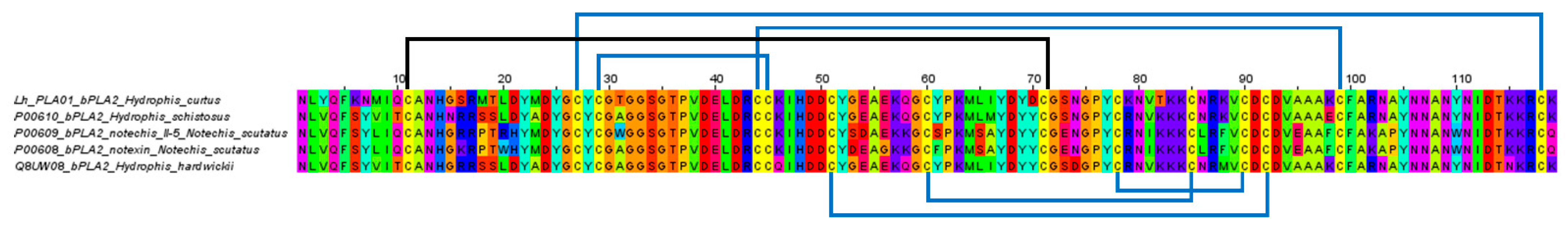
2.6. Phospholipases A2
3. Conclusions
4. Materials and Methods
4.1. Preparation of Snake Venom-Gland Tissue
4.2. RNA Extraction and Purification
4.3. Filtration of Raw Sequenced Reads
4.4. De Novo Transcriptome Assembly
4.5. Clustering and Functional Annotation of Transcripts
4.6. Quantifying Transcript Abundance
4.7. Categorization of Transcripts
4.8. Multiple Sequence Alignment
4.9. Phylogenetic Analysis
4.10. Scale-Based B-Cell Epitope Prediction
4.11. Supporting Data
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar]
- World Health Organization. Guidelines for the Management of Snake-Bites; Regional Office for South-East Asia: New Delhi, India, 2016. [Google Scholar]
- Feola, A.; Marella, G.L.; Carfora, A.; Della Pietra, B.; Zangani, P.; Campobasso, C.P. Snakebite Envenoming a Challenging Diagnosis for the Forensic Pathologist: A Systematic Review. Toxins 2020, 12, 699. [Google Scholar] [CrossRef]
- Jamaiah, I.; Rohela, M.; Ng, T.K.; Ch’ng, K.B.; Teh, Y.S.; Nurulhuda, A.L.; Suhaili, N. Retrospective prevalence of snakebites from Hospital Kuala Lumpur (HKL) (1999–2003). Southeast Asian J. Trop. Med. Public Health 2006, 37, 200–205. [Google Scholar]
- Reid, H.A. Sea-snake bite research. Trans. R. Soc. Trop. Med. Hyg. 1956, 50, 517–538. [Google Scholar] [CrossRef]
- Alirol, E.; Sharma, S.K.; Bawaskar, H.S.; Kuch, U.; Chappuis, F. Snake Bite in South Asia: A Review. PLoS Negl. Trop. Dis. 2010, 4, e603. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.; Tao, N.T.; Moore, A.; Montoya, A.; Rasmussen, A.R.; Broad, K.; Voris, H.K.; Takacs, Z. Sea Snake Harvest in the Gulf of Thailand. Conserv. Biol. 2014, 28, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.K.; Tibballs, J. Australian Animal Toxins: The Creatures, Their Toxins and Care of the Poisoned Patient; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Marsden, A.T.; Reid, H.A. Pathology of sea-snake poisoning. BMJ 1961, 1, 1290–1293. [Google Scholar] [CrossRef] [Green Version]
- Kularatne, S.A.; Hettiarachchi, R.; Dalpathadu, J.; Mendis, A.S.; Appuhamy, P.D.; Zoysa, H.D.; Maduwage, K.; Weerasinghe, V.S.; de Silva, A. Enhydrina schistosa (Elapidae: Hydrophiinae) the most dangerous sea snake in Sri Lanka: Three case studies of severe envenoming. Toxicon 2014, 77, 78–86. [Google Scholar] [CrossRef]
- Sanders, K.L.; Lee, M.S.; Mumpuni; Bertozzi, T.; Rasmussen, A.R. Multilocus phylogeny and recent rapid radiation of the viviparous sea snakes (Elapidae: Hydrophiinae). Mol. Phylogenet. Evol. 2013, 66, 575–591. [Google Scholar] [CrossRef]
- Neale, V.; Sotillo, J.; Seymour, J.E.; Wilson, D. The Venom of the Spine-Bellied Sea Snake (Hydrophis curtus): Proteome, Toxin Diversity and Intraspecific Variation. Int. J. Mol. Sci. 2017, 18, 2695. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.H.; Tan, K.Y.; Ng, T.S.; Sim, S.M.; Tan, N.H. Venom Proteome of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia: Toxicity Correlation, Immunoprofiling and Cross-Neutralization by Sea Snake Antivenom. Toxins 2019, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Lomonte, B.; Pla, D.; Sasa, M.; Tsai, W.C.; Solorzano, A.; Urena-Diaz, J.M.; Fernandez-Montes, M.L.; Mora-Obando, D.; Sanz, L.; Gutierrez, J.M.; et al. Two color morphs of the pelagic yellow-bellied sea snake, Pelamis platura, from different locations of Costa Rica: Snake venomics, toxicity, and neutralization by antivenom. J. Proteom. 2014, 103, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.H.; Tan, N.H.; Tan, K.Y.; Kwong, K.O. Antivenom cross-neutralization of the venoms of Hydrophis schistosus and Hydrophis curtus, two common sea snakes in Malaysian waters. Toxins 2015, 7, 572–581. [Google Scholar] [CrossRef]
- Calvete, J.J.; Ghezellou, P.; Paiva, O.; Matainaho, T.; Ghassempour, A.; Goudarzi, H.; Kraus, F.; Sanz, L.; Williams, D.J. Snake venomics of two poorly known Hydrophiinae: Comparative proteomics of the venoms of terrestrial Toxicocalamus longissimus and marine Hydrophis cyanocinctus. J. Proteom. 2012, 75, 4091–4101. [Google Scholar] [CrossRef]
- Sanders, K.L.; Lee, M.S. Uncoupling ecological innovation and speciation in sea snakes (Elapidae, Hydrophiinae, Hydrophiini). J. Evol. Biol. 2010, 23, 2685–2693. [Google Scholar] [CrossRef]
- Sanders, K.L.; Lee, M.S.; Leys, R.; Foster, R.; Keogh, J.S. Molecular phylogeny and divergence dates for Australasian elapids and sea snakes (hydrophiinae): Evidence from seven genes for rapid evolutionary radiations. J. Evol. Biol. 2008, 21, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Freed, P.; Hošek, J.E. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 15 November 2020).
- Reid, H.A. Epidemiology of sea-snake bites. J. Trop. Med. Hyg. 1975, 78, 106–113. [Google Scholar]
- Chong, H.P.; Tan, K.Y.; Tan, N.H.; Tan, C.H. Exploring the Diversity and Novelty of Toxin Genes in Naja sumatrana, the Equatorial Spitting Cobra from Malaysia through De Novo Venom-Gland Transcriptomics. Toxins 2019, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.Y.; Tan, C.H.; Chanhome, L.; Tan, N.H. Comparative venom gland transcriptomics of Naja kaouthia (monocled cobra) from Malaysia and Thailand: Elucidating geographical venom variation and insights into sequence novelty. PeerJ 2017, 5, e3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.H.; Tan, K.Y.; Fung, S.Y.; Tan, N.H. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah). BMC Genom. 2015, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Netto, C.; Junqueira-de-Azevedo Ide, L.; Silva, D.A.; Ho, P.L.; Leitao-de-Araujo, M.; Alves, M.L.; Sanz, L.; Foguel, D.; Zingali, R.B.; Calvete, J.J. Snake venomics and venom gland transcriptomic analysis of Brazilian coral snakes, Micrurus altirostris and M. corallinus. J. Proteom. 2011, 74, 1795–1809. [Google Scholar] [CrossRef]
- Margres, M.J.; Aronow, K.; Loyacano, J.; Rokyta, D.R. The venom-gland transcriptome of the eastern coral snake (Micrurus fulvius) reveals high venom complexity in the intragenomic evolution of venoms. BMC Genom. 2013, 14, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, A.D.; Swain, M.T.; Hegarty, M.J.; Logan, D.W.; Mulley, J.F. Restriction and recruitment-gene duplication and the origin and evolution of snake venom toxins. Genome Biol. Evol. 2014, 6, 2088–2095. [Google Scholar] [CrossRef] [Green Version]
- Kordis, D.; Gubensek, F. Adaptive evolution of animal toxin multigene families. Gene 2000, 261, 43–52. [Google Scholar] [CrossRef]
- Tsetlin, V. Snake venom alpha-neurotoxins and other ‘three-finger’ proteins. Eur. J. Biochem. 1999, 264, 281–286. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.F.; Peng, L.S.; Wu, W.Y.; Wei, J.W.; Yang, H.; Yang, Y.Z.; Xu, A.L. Identification and Funtional Characterization of Three Postsynaptic Short-chain Neurotoxins from Hydrophiinae, Lapemis hardwickii Gray. Acta Biochim. Biophys. Sin. 2001, 33, 457–462. [Google Scholar] [PubMed]
- Kim, H.S.; Tamiya, N. Amino acid sequences of two novel long-chain neurotoxins from the venom of the sea snake Laticauda colubrina. Biochem. J. 1982, 207, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.H.; Wong, K.Y.; Tan, K.Y.; Tan, N.H. Venom proteome of the yellow-lipped sea krait, Laticauda colubrina from Bali: Insights into subvenomic diversity, venom antigenicity and cross-neutralization by antivenom. J. Proteom. 2017, 166, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Chetty, N.; Du, A.; Hodgson, W.C.; Winkel, K.; Fry, B.G. The in vitro neuromuscular activity of Indo-Pacific sea-snake venoms: Efficacy of two commercially available antivenoms. Toxicon 2004, 44, 193–200. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Neutralization of the Principal Toxins from the Venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into Toxin-Specific Neutralization by Two Different Antivenoms. Toxins 2016, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Lim, S.E.; Tan, N.H. Venomics of the beaked sea snake, Hydrophis schistosus: A minimalist toxin arsenal and its cross-neutralization by heterologous antivenoms. J. Proteom. 2015, 126, 121–130. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutierrez, J.M.; Rasmussen, A.R.; Engmark, M.; Gravlund, P.; Sanders, K.L.; Lohse, B.; Lomonte, B. Danger in the reef: Proteome, toxicity, and neutralization of the venom of the olive sea snake, Aipysurus laevis. Toxicon 2015, 107, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranawaka, U.K.; Lalloo, D.G.; de Silva, H.J. Neurotoxicity in snakebite--the limits of our knowledge. PLoS Negl. Trop. Dis. 2013, 7, e2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Cristofori-Armstrong, B.; Rash, L.D.; Hodgson, W.C.; Isbister, G.K. Defining the role of post-synaptic alpha-neurotoxins in paralysis due to snake envenoming in humans. Cell. Mol. Life Sci. 2018, 75, 4465–4478. [Google Scholar] [CrossRef]
- Wong, K.Y.; Tan, C.H.; Tan, N.H. Venom and Purified Toxins of the Spectacled Cobra (Naja naja) from Pakistan: Insights into Toxicity and Antivenom Neutralization. Am. J. Trop. Med. Hyg. 2016, 94, 1392–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, C.; Laustsen, A.H. Recent Advances in Next Generation Snakebite Antivenoms. Trop. Med. Infect. Dis. 2018, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- de la Rosa, G.; Olvera, F.; Archundia, I.G.; Lomonte, B.; Alagon, A.; Corzo, G. Horse immunization with short-chain consensus alpha-neurotoxin generates antibodies against broad spectrum of elapid venomous species. Nat. Commun. 2019, 10, 3642. [Google Scholar] [CrossRef] [Green Version]
- Ratanabanangkoon, K.; Tan, K.Y.; Pruksaphon, K.; Klinpayom, C.; Gutierrez, J.M.; Quraishi, N.H.; Tan, C.H. A pan-specific antiserum produced by a novel immunization strategy shows a high spectrum of neutralization against neurotoxic snake venoms. Sci. Rep. 2020, 10, 11261. [Google Scholar] [CrossRef] [PubMed]
- Ratanabanangkoon, K.; Tan, K.Y.; Eursakun, S.; Tan, C.H.; Simsiriwong, P.; Pamornsakda, T.; Wiriyarat, W.; Klinpayom, C.; Tan, N.H. A Simple and Novel Strategy for the Production of a Pan-specific Antiserum against Elapid Snakes of Asia. PLoS Negl. Trop. Dis. 2016, 10, e0004565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef] [Green Version]
- Doley, R.; Zhou, X.; Kini, R.M. Snake Venom Phospholipase A2 Enzymes. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Fohlman, J.; Eaker, D. Isolation and characterization of a lethal myotoxic phospholipase A from the venom of the common sea snake Enhydrina schistosa causing myoglobinuria in mice. Toxicon 1977, 15, 385–393. [Google Scholar] [CrossRef]
- Lind, P.; Eaker, D. Amino acid sequence of a lethal myotoxic phospholipase A2 from the venom of the common sea snake (Enhydrina schistosa). Toxicon 1981, 19, 11–24. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Rodrigues, R.S.; Boldrini-Franca, J.; Fonseca, F.P.; de la Torre, P.; Henrique-Silva, F.; Sanz, L.; Calvete, J.J.; Rodrigues, V.M. Combined snake venomics and venom gland transcriptomic analysis of Bothropoides pauloensis. J. Proteom. 2012, 75, 2707–2720. [Google Scholar] [CrossRef]
- Rotenberg, D.; Bamberger, E.S.; Kochva, E. Studies on ribonucleic acid synthesis in the venom glands of Vipera palaestinae (Ophidia, Reptilia). Biochem. J. 1971, 121, 609–612. [Google Scholar] [CrossRef] [Green Version]
- Wery, M.; Descrimes, M.; Thermes, C.; Gautheret, D.; Morillon, A. Zinc-mediated RNA fragmentation allows robust transcript reassembly upon whole transcriptome RNA-Seq. Methods 2013, 63, 25–31. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.G.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; et al. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Dayhoff, M.O.; Schwartz, R.M.; Orcutt, B.C.; Dayhoff, M.O. A Model of Evolutionary Change in Proteins. In Atlas of Protein Sequence and Structure; National Biomedical Research Foundation: Washington, DC, USA, 1978; Volume 5, pp. 345–352. [Google Scholar]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]


| Parameter | Output Statistics |
|---|---|
| Total raw reads | 57,606,566 |
| Total clean reads | 54,140,326 |
| Total clean nucleotides (nt) | 4,872,629,340 |
| Q20 percentage | 98.60% |
| N percentage | <0.01% |
| GC percentage | 44.63% |
| Contigs created | 126,790 |
| Total length (nt) | 51,459,117 |
| Mean length (nt) | 406 |
| N50 | 921 |
| Unigenes/transcripts assembled | 82,209 |
| Total length (nt) | 69,679,280 |
| Mean length (nt) | 848 |
| N50 | 2073 |
| Unigene/transcripts assembled (FPKM > 1) | 70,564 |
| Unidentified | 45,616 (17.97%) |
| -Redundancy (FPKM abundance/number of transcripts) | 3.8 |
| Non-toxin | 24,852 (33.86%) |
| -Redundancy (FPKM abundance/number of transcripts) | 13.16 |
| Toxin | 96 (48.18%) |
| -Redundancy (FPKM abundance/number of transcripts) | 4847.54 |
| Toxin Family/ID | UniProt Accession Code | Species | Expression Abundance (%) |
|---|---|---|---|
| Three-Finger Toxin (3FTx) | 77.26 | ||
| S-3FTX | 56.48 | ||
| Short neurotoxin SN160 | Q8UW27 | H. hardwickii | 56.43 |
| Short neurotoxin homolog NTL4 | Q9YGI8 | B. multicinctus | <0.01 |
| Short neurotoxin OH-35 | Q53B49 | O. hannah | <0.01 |
| 3FTx | C6JUP5 | M. corallinus | <0.01 |
| putative three-finger toxin precursor | F5CPD1 | M. altirostris | <0.01 |
| Short neurotoxin OH-26 | Q53B52 | O. hannah | <0.01 |
| Cytotoxin homolog 5V | Q9W716 | Naja atra | 0.02 |
| Cytotoxin homolog 5 | Q91137 | Naja atra | 0.01 |
| Cytotoxin A5 | P62375 | Naja atra | 0.01 |
| Cardiotoxin-like protein BMLCL | Q9PW19 | B. multicinctus | <0.01 |
| L-3FTX | 20.78 | ||
| Long neurotoxin 2 | A3FM53 | H. hardwickii | 20.74 |
| Alpha-bungarotoxin isoform A31 | P60615 | B. multicinctus | 0.02 |
| Kappa-bungarotoxin | P01398 | B. multicinctus | 0.01 |
| Long neurotoxin homolog NTL2 | Q9YGH9 | B. multicinctus | 0.01 |
| Long chain neurotoxin 6 | U3FYQ0 | M. fulvius | <0.01 |
| Neurotoxin BM10-1-like | Q70WS8 | B. multicinctus | <0.01 |
| Long chain neurotoxin 2 | U3FAC0 | M. fulvius | <0.01 |
| NC-3FTX | <0.01 | ||
| Weak toxin 1 | Q8AY51 | B. candidus | <0.01 |
| Phospholipase A2 | 18.88 | ||
| Basic phospholipase A2 73 | Q8UW30 | H. hardwickii | 18.84 |
| Acidic phospholipase A2 | P00606 | B. multicinctus | 0.03 |
| Phospholipase A2 MALT0035C | F5CPF1 | M. altirostris | 0.01 |
| Phospholipase A2 GL16-1 | Q8JFB2 | L. semifasciata | <0.01 |
| Basic phospholipase A2 beta-bungarotoxin A1 chain | P00617 | B. multicinctus | <0.01 |
| Phospholipase A2 pkP2 | Q8JFG2 | L. semifasciata | <0.01 |
| Cysteine-rich Secretory Protein | 3.34 | ||
| Cysteine-rich venom protein 2 | Q8UW11 | H. hardwickii | 3.34 |
| Cysteine-rich secretory protein Bc-CRPb | F2Q6G2 | B. candidus | 0.01 |
| Phospholipase A2 Inhibitor | 0.13 | ||
| phospholipase A2 inhibitor-like | A0A6J1W4V4 | N. scutatus | 0.13 |
| C-type Lectin | 0.12 | ||
| C-type lectin 1 | A3FM55 | H. hardwickii | 0.07 |
| C-type lectin isoform 1 | H8PG89 | P. nigriceps | 0.04 |
| Venom C-type lectin mannose binding isoform 4 | D2YVK4 | H. stephensii | 0.01 |
| Kunitz-type Protease Inhibitor | 0.09 | ||
| Putative Kunitz-type serine protease inhibitor | B2BS84 | A. labialis | 0.06 |
| Kunitz-type protease inhibitor 1 | V8N7R6 | O. hannah | 0.01 |
| Kunitz-type serine protease inhibitor homolog beta-bungarotoxin B1 chain | Q8AY46 | B. candidus | 0.01 |
| Kunitz-type serine protease inhibitor PILP-2 | B4ESA3 | B. multicinctus | 0.01 |
| Kunitz-type serine protease inhibitor spermatin | C1IC52 | W. aegyptia | <0.01 |
| Kunitz-type serine protease inhibitor 28 | F8J2F3 | D. coronoides | <0.01 |
| Protease inhibitor 4 | C1IC53 | W. aegyptia | <0.01 |
| Kunitz-type serine protease inhibitor vestiginin-2 | A6MFL2 | D. vestigiata | <0.01 |
| Kunitz-type serine protease inhibitor | P20229 | Naja naja | <0.01 |
| Kunitz-type serine protease inhibitor 161 | F8J2F4 | D. coronoides | <0.01 |
| Snake Venom Metalloproteinase | 0.08 | ||
| Zinc metalloproteinase-disintegrin-like NaMP | A8QL59 | N. atra | 0.05 |
| Porphyriacase-1 | B5KFV2 | P. porphyriacus | 0.01 |
| Scutatease-1 | B5KFV7 | N. scutatus | 0.01 |
| Zinc metalloproteinase-disintegrin-like BmMP | A8QL49 | B. fasciatus | <0.01 |
| Zinc metalloproteinase-disintegrin-like MTP9 | F8RKV9 | D. coronoides | <0.01 |
| Carinatease-1 | B5KFV1 | T. carinatus | <0.01 |
| Snake venom metalloproteinase-disintegrin-like mocarhagin | Q10749 | N. mossambica | <0.01 |
| Zinc metalloproteinase-disintegrin-like BfMP | A8QL48 | B. fasciatus | <0.01 |
| Zinc metalloproteinase-disintegrin-like NaMP | A8QL59 | N. atra | <0.01 |
| Stephensease-1 | B5KFV4 | H. stephensii | <0.01 |
| Cystatin | 0.06 | ||
| Cystatin | E3P6N8 | P. australis | 0.03 |
| Cystatin | V8NX38 | O. hannah | 0.02 |
| Cystatin-B | V8P5H9 | O. hannah | 0.01 |
| Dipeptidyl Peptidase IV | 0.02 | ||
| Venom dipeptidylpeptidase IV | A6MJI1 | T. carinatus | 0.02 |
| Snake Venom Serine Protease | 0.01 | ||
| Serine protease harobin | Q5MCS0 | H. hardwickii | 0.01 |
| 5’ Nucleotidase | <0.01 | ||
| 5’ nucleotidase | A6MFL8 | D. vestigiata | <0.01 |
| 5’-nucleotidase domain-containing protein 3 | V8P4R1 | O. hannah | <0.01 |
| 5’-nucleotidase | V8NYW9 | O. hannah | <0.01 |
| Vascular Endothelial Growth Factor | <0.01 | ||
| Vascular endothelial growth factor C | V8NCP7 | O. hannah | <0.01 |
| Hyaluronidase | <0.01 | ||
| Hyaluronidase | V8PHI0 | O. hannah | <0.01 |
| Hyaluronidase | V8PFK9 | O. hannah | <0.01 |
| Hyaluronidase | V8P1Z9 | O. hannah | <0.01 |
| Phosphodiesterase | <0.01 | ||
| 2’,5’-phosphodiesterase 12 | V8PEM5 | O. hannah | <0.01 |
| Waprin | <0.01 | ||
| Supwaprin-a | B5KGY9 | A. superbus | <0.01 |
| Natriuretic Peptide | <0.01 | ||
| Natriuretic peptide Oh-NP | D9IX98 | O. hannah | <0.01 |
| Natriuretic peptide Na-NP | D9IX97 | N. atra | <0.01 |
| Cobra Venom Factor | <0.01 | ||
| A.superbus venom factor 1 | Q0ZZJ6 | A. superbus | <0.01 |
| Nerve Growth Factor | <0.01 | ||
| NGF-Hop-5 | R4G2H9 | H. bungaroides | <0.01 |
| Venom nerve growth factor 1 | Q3HXY6 | N. scutatus | <0.01 |
| Aminopeptidase | <0.01 | ||
| Aminopeptidase N | V8NGF6 | O. hannah | <0.01 |
| Neprilysin | <0.01 | ||
| Neprilysin | V8NQ76 | O. hannah | <0.01 |
| L-amino-acid Oxidase | <0.01 | ||
| L-amino-acid oxidase | A8QL51 | B. multicinctus | <0.01 |
| Acetylcholinesterase | <0.01 | ||
| Acetylcholinesterase | Q92035 | B. fasciatus | <0.01 |
| Transcript ID | Toxin Gene Family/Annotated ID | UniProt Accession Code | Species | Transcript Length (aa) | Annotated ID Length (aa) | Coverage | Coverage to Mature Chain (%) |
|---|---|---|---|---|---|---|---|
| Three-Finger Toxin (3FTx) | |||||||
| Lh_FTX01 | Short neurotoxin SN160 | Q8UW27 | H. hardwickii | 81 | 81 | 1–81 | 100 |
| Lh_FTX02 | Short neurotoxin homolog NTL4 | Q9YGI8 | B. multicinctus | 71 | 86 | 16–86 | 100 |
| Lh_FTX03 | Short neurotoxin OH-35 | Q53B49 | O. hannah | 63 | 86 | 15–85 | 100 |
| Lh_FTX04 | 3FTx | C6JUP5 | M. corallinus | 62 | 79 | 15–78 | 98 |
| Lh_FTX05 | putative three finger toxin precursor | F5CPD1 | M. altirostris | 66 | 82 | 21–82 | 100 |
| Lh_FTX06 | Short neurotoxin OH-26 | Q53B52 | O. hannah | 62 | 78 | 15–77 | 98 |
| Lh_FTX08 | Cytotoxin homolog 5V | Q9W716 | N. atra | 66 | 83 | 15–83 | 10 |
| Lh_FTX10 | Cytotoxin A5 | P62375 | N. atra | 70 | 83 | 7–83 | 100 |
| Lh_FTX11 | Cytotoxin A5 | P62375 | N. atra | 69 | 83 | 7–83 | 100 |
| Lh_FTX12 | Cardiotoxin-like protein BMLCL | Q9PW19 | B. multicinctus | 97 | 103 | 7–103 | 100 |
| Lh_FTX13 | Long neurotoxin 2 | A3FM53 | H. hardwickii | 93 | 93 | 1–93 | 100 |
| Lh_FTX14 | Alpha-bungarotoxin isoform A31 | P60615 | B. multicinctus | 77 | 95 | 15–91 | 95 |
| Lh_FTX15 | Kappa-bungarotoxin | P01398 | B. multicinctus | 72 | 87 | 15–86 | 94 |
| Lh_FTX16 | Long neurotoxin homolog NTL2 | Q9YGH9 | B. multicinctus | 81 | 87 | 8–87 | 100 |
| Lh_FTX17 | Long chain neurotoxin 6 | U3FYQ0 | M. fulvius | 72 | 84 | 14–84 | 100 |
| Lh_FTX18 | Neurotoxin BM10-1-like | Q70WS8 | B. multicinctus | 66 | 84 | 15–84 | 100 |
| Lh_FTX19 | Long chain neurotoxin 2 | U3FAC0 | M. fulvius | 99 | 87 | 4–84 | 96 |
| Lh_FTX20 | Weak toxin 1 | Q8AY51 | B. candidus | 70 | 86 | 17–86 | 100 |
| Phospholipase A2 | |||||||
| Lh_PLA01 | Basic phospholipase A2 73 | Q8UW30 | H. hardwickii | 146 | 146 | 1–146 | 100 |
| Lh_PLA02 | Acidic phospholipase A2 | P00606 | B. multicinctus | 132 | 145 | 14–145 | 100 |
| Cysteine-rich Secretory Protein | |||||||
| Lh_CRP01 | Cysteine-rich venom protein 2 | Q8UW11 | H. hardwickii | 238 | 238 | 1–238 | 100 |
| C-type Lectin | |||||||
| Lh_SCL01 | C-type lectin 1 | A3FM55 | H. hardwickii | 164 | 164 | 1–164 | 100 |
| Lh_SCL02 | C-type lectin isoform 1 | H8PG89 | P. nigriceps | 172 | 157 | 1–157 | 100 |
| Lh_SCL03 | Venom C-type lectin mannose binding isoform 4 | D2YVK4 | H. stephensii | 164 | 165 | 1–164 | 99 |
| Kunitz-type Serine Protease Inhibitor | |||||||
| Lh_KUN01 | Putative Kunitz-type serine protease inhibitor | B2BS84 | A. labialis | 249 | 252 | 1–252 | 100 |
| Lh_KUN02 | Kunitz-type protease inhibitor 1 | V8N7R6 | O. hannah | 515 | 506 | 1–506 | 100 |
| Lh_KUN03 | Kunitz-type serine protease inhibitor homolog beta-bungarotoxin B1 chain | Q8AY46 | B. candidus | 86 | 85 | 1–84 | 98 |
| Lh_KUN04 | Kunitz-type serine protease inhibitor PILP-2 | B4ESA3 | B. multicinctus | 66 | 83 | 1–82 | 98 |
| Lh_KUN05 | Kunitz-type serine protease inhibitor spermatin | C1IC52 | W. aegyptia | 79 | 81 | 1–79 | 98 |
| Lh_KUN06 | Kunitz-type serine protease inhibitor 28 | F8J2F3 | D. coronoides | 66 | 83 | 18–83 | 100 |
| Lh_KUN08 | Kunitz-type serine protease inhibitor vestiginin-2 | A6MFL2 | D. vestigiata | 71 | 83 | 16–81 | 97 |
| Lh_KUN09 | Kunitz-type serine protease inhibitor | P20229 | N. naja | 53 | 57 | 5–57 | 93 |
| Snake Venom Metalloproteinase | |||||||
| Lh_SMP09 | Carinatease-1 | B5KFV1 | T. carinatus | 575 | 608 | 28–596 | 98 |
| Lh_SMP10 | Scutatease-1 | B5KFV7 | N. scutatus | 586 | 608 | 28–608 | 100 |
| Lh_SMP19 | Zinc metalloproteinase-disintegrin-like NaMP | A8QL59 | N. atra | 590 | 621 | 28–618 | 98 |
| Cystatin | |||||||
| Lh_CYS01 | Cystatin | E3P6N8 | P. australis | 141 | 141 | 1–141 | 100 |
| Lh_CYS02 | Cystatin | V8NX38 | O. hannah | 164 | 171 | 8–171 | 96 |
| Dipeptidyl Peptidase IV | |||||||
| Lh_DPP01 | Venom dipeptidylpeptidase IV | A6MJI1 | T. carinatus | 753 | 753 | 1–753 | 100 |
| Snake Venom Serine Protease | |||||||
| Lh_SSP01 | Serine protease harobin | Q5MCS0 | H. hardwickii | 265 | 265 | 1–265 | 100 |
| 5’ Nucleotidase | |||||||
| Lh_NUC01 | 5’ nucleotidase | A6MFL8 | D. vestigiata | 559 | 559 | 1–559 | 100 |
| Vascular Endothelial Growth Factor | |||||||
| Lh_VGF01 | Vascular endothelial growth factor C | V8NCP7 | O. hannah | 421 | 421 | 1–421 | 100 |
| Hyaluronidase | |||||||
| Lh_HYA01 | Hyaluronidase | V8PHI0 | O. hannah | 481 | 469 | 19–469 | 96 |
| Waprin | |||||||
| Lh_WAP01 | Supwaprin-a | B5KGY9 | A. superbus | 64 | 75 | 16–75 | 100 |
| Cobra Venom Factor | |||||||
| Lh_CVF01 | A.superbus venom factor 1 | Q0ZZJ6 | A. superbus | 1652 | 1652 | 1–1652 | 100 |
| Neprilysin | |||||||
| Lh_NEP01 | Neprilysin | V8NQ76 | O. hannah | 750 | 675 | 16–675 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.H.; Tan, K.Y. De Novo Venom-Gland Transcriptomics of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia—Next-Generation Sequencing, Functional Annotation and Toxinological Correlation. Toxins 2021, 13, 127. https://doi.org/10.3390/toxins13020127
Tan CH, Tan KY. De Novo Venom-Gland Transcriptomics of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia—Next-Generation Sequencing, Functional Annotation and Toxinological Correlation. Toxins. 2021; 13(2):127. https://doi.org/10.3390/toxins13020127
Chicago/Turabian StyleTan, Choo Hock, and Kae Yi Tan. 2021. "De Novo Venom-Gland Transcriptomics of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia—Next-Generation Sequencing, Functional Annotation and Toxinological Correlation" Toxins 13, no. 2: 127. https://doi.org/10.3390/toxins13020127






