Metabolism of Zearalenone in the Rumen of Dairy Cows with and without Application of a Zearalenone-Degrading Enzyme
Abstract
:1. Introduction
2. Results
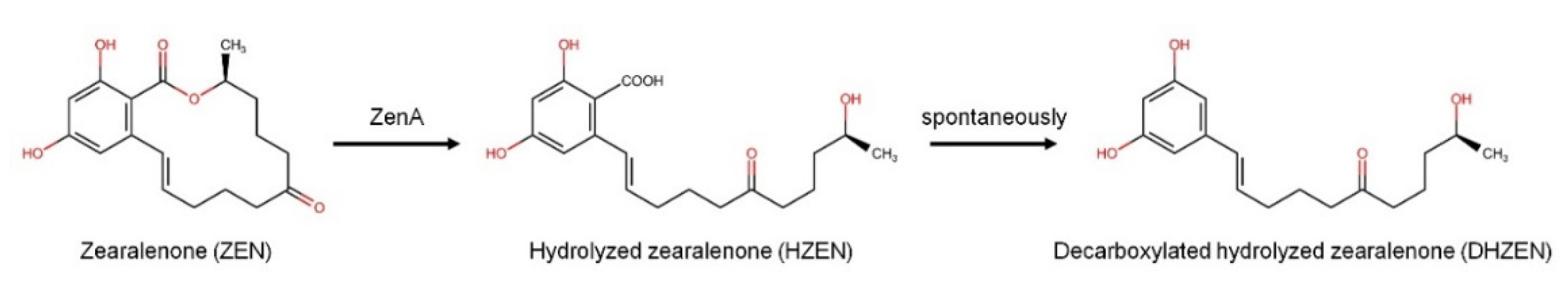
2.1. Degradation of ZEN by ZenA in a Simulated Rumen Environment In Vitro
2.2. Metabolism of ZEN in the Reticulorumen of Dairy Cows
2.3. Effect of ZenA on the Metabolism of ZEN in the Reticulorumen of Dairy Cows
3. Discussion
3.1. Degradation of ZEN by ZenA in a Simulated Rumen Environment In Vitro
3.2. Metabolism of ZEN in the Reticulorumen of Dairy Cows
3.3. ZEN-Degrading Enzyme ZenA as a New Strategy for Mycotoxin Inactivation in Feed
4. Conclusions
5. Materials and Methods
5.1. In Vitro Rumen Fermentation Experiment
5.1.1. Analysis of ZEN and Its Metabolites
5.1.2. Statistical Analysis (In Vitro Trial)
5.2. Feeding Trial in Rumen-Fistulated Dairy Cows
5.2.1. Experimental Setup
5.2.2. Preparation of ZEN-Contaminated and ZenA-Supplemented Feed
5.2.3. Analysis of Mycotoxin Concentrations in Feed
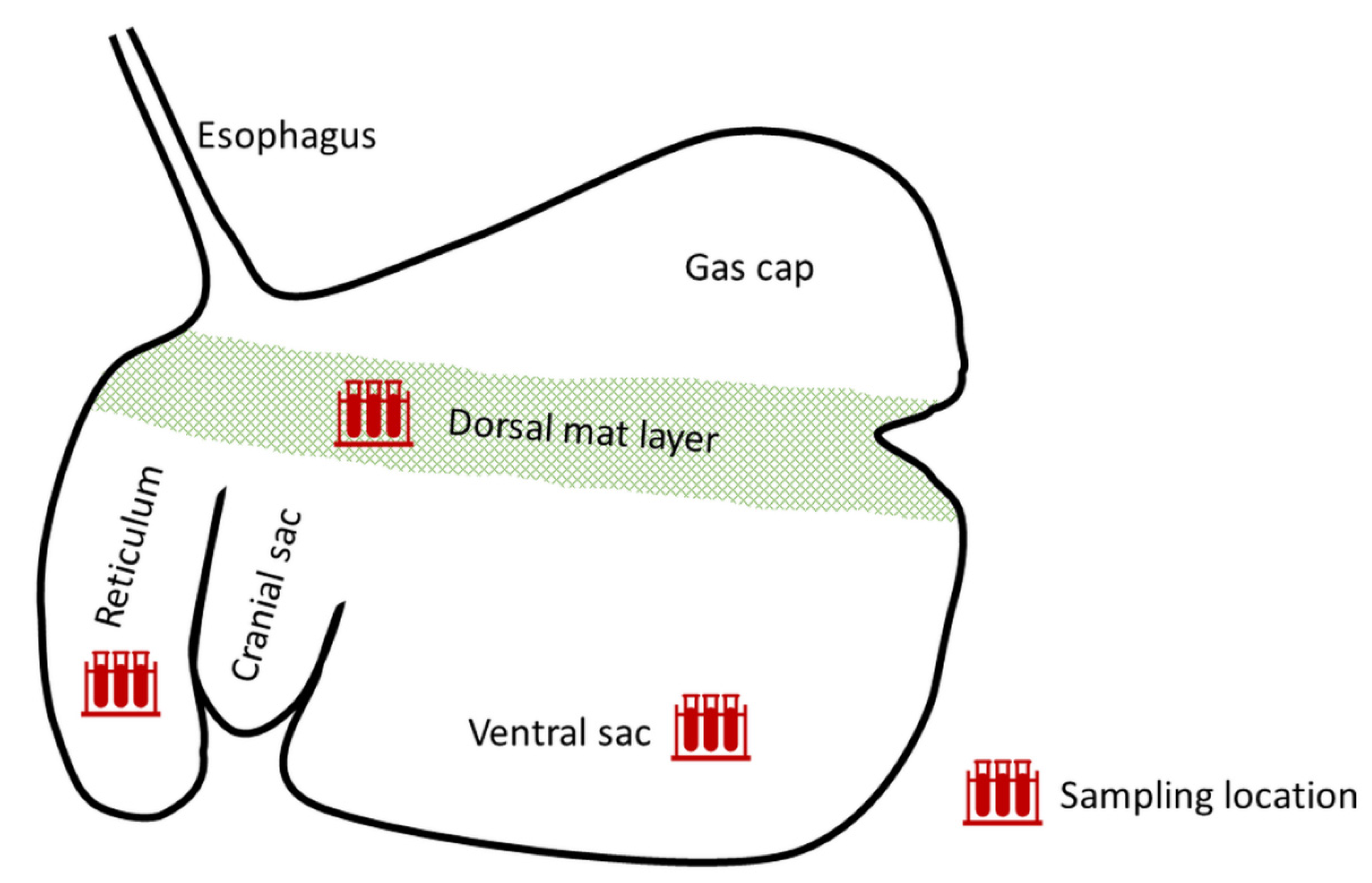
5.2.4. Sampling
5.2.5. Analysis of ZEN and Its Metabolites in Rumen Fluid and Feces
5.2.6. Statistical Analysis (Feeding Trial)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited FAO estimate of 25. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Tech. 2007, 137, 326–341. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Hildebrand, A.A. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin J. 2010, 3, 385–401. [Google Scholar] [CrossRef]
- Weaver, G.A.; Kurtz, H.J.; Behrens, J.C.; Robison, T.S.; Seguin, B.E.; Bates, F.Y.; Mirocha, C.J. Effect of zearalenone on the fertility of virgin dairy heifers. Am. J. Vet. Res. 1986, 47, 1395–1397. [Google Scholar]
- Coppock, R.W.; Mostrom, M.S.; Sparling, C.G.; Jacobsen, B.; Ross, S.C. Apparent zearalenone intoxication in a dairy herd from feeding spoiled acid-treated corn. Vet. Hum. Toxicol. 1990, 32, 246–248. [Google Scholar]
- Smith, J.F.; di Menna, M.E.; McGowan, L.T. Reproductive performance of Coopworth ewes following oral doses of zearalenone before and after mating. J. Reprod. Fertil. 1990, 89, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Fushimi, Y.; Takagi, M.; Monniaux, D.; Uno, S.; Kokushi, E.; Shinya, U.; Kawashima, C.; Otoi, T.; Deguchi, E.; Fink-Gremmels, J. Effects of dietary contamination by zearalenone and its metabolites on serum anti-Müllerian hormone: Impact on the reproductive performance of breeding cows. Reprod. Domest. Anim. 2015, 50, 834–839. [Google Scholar] [CrossRef]
- Seeling, K.; Danicke, S.; Valenta, H.; Van Egmond, H.P.; Schothorst, R.C.; Jekel, A.A.; Lebzien, P.; Schollenberger, M.; Razzazi-Fazeli, E.; Flachowsky, G. Effects of Fusarium toxin-contaminated wheat and feed intake level on the biotransformation and carry-over of deoxynivalenol in dairy cows. Food Addit. Contam. 2006, 23, 1008–1020. [Google Scholar] [CrossRef] [Green Version]
- Valgaeren, B.; Theron, L.; Croubels, S.; Devreese, M.; De Baere, S.; Van Pamel, E.; Daeseleire, E.; De Boevre, M.; De Saeger, S.; Vidal, A.; et al. The role of roughage provision on the absorption and disposition of the mycotoxin deoxynivalenol and its acetylated derivatives in calves: From field observations to toxicokinetics. Arch. Toxicol. 2019, 93, 293–310. [Google Scholar] [CrossRef]
- Kiessling, K.H.; Pettersson, H.; Sandholm, K.; Olsen, M. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 1984, 47, 1070–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debevere, S.; Cools, A.; Baere, S.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. In vitro rumen simulations show a reduced disappearance of deoxynivalenol, nivalenol and enniatin B at conditions of rumen acidosis and lower microbial activity. Toxins 2020, 12, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeling, K.; Boguhn, J.; Strobel, E.; Dänicke, S.; Valenta, H.; Ueberschar, K.H.; Rodehutscord, M. On the effects of Fusarium toxin contaminated wheat and wheat chaff on nutrient utilisation and turnover of deoxynivalenol and zearalenone in vitro (Rusitec). Toxicol. In Vitro 2006, 20, 703–711. [Google Scholar] [CrossRef]
- Seeling, K.; Dänicke, S.; Lebzien, P.; Valenta, H.; Ueberschar, K.H.; Flachowsky, G. On the effects of Fusarium-contaminated wheat and the feed intake level on ruminal fermentation and toxin-turnover of cows. Mycotoxin Res. 2005, 21, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Matthaus, K.; Lebzien, P.; Valenta, H.; Stemme, K.; Ueberschar, K.H.; Razzazi-Fazeli, E.; Böhm, J.; Flachowsky, G. Effects of Fusarium toxin-contaminated wheat grain on nutrient turnover, microbial protein synthesis and metabolism of deoxynivalenol and zearalenone in the rumen of dairy cows. J. Anim. Physiol. Anim. Nutr. 2005, 89, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Seeling, K.; Dänicke, S.; Ueberschar, K.H.; Lebzien, P.; Flachowsky, G. On the effects of Fusarium toxin-contaminated wheat and the feed intake level on the metabolism and carry over of zearalenone in dairy cows. Food Addit. Contam. 2005, 22, 847–855. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar] [CrossRef]
- Neubauer, V.; Humer, E.; Mann, E.; Kroger, I.; Reisinger, N.; Wagner, M.; Zebeli, Q.; Petri, R.M. Effects of clay mineral supplementation on particle-associated and epimural microbiota, and gene expression in the rumen of cows fed high-concentrate diet. Anaerobe 2019, 59, 38–48. [Google Scholar] [CrossRef]
- Neubauer, V.; Humer, E.; Kroger, I.; Braid, T.; Wagner, M.; Zebeli, Q. Differences between pH of indwelling sensors and the pH of fluid and solid phase in the rumen of dairy cows fed varying concentrate levels. J. Anim. Physiol. Anim. Nutr. 2018, 102, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Kakeya, H.; Takahashi-Ando, N.; Kimura, M.; Onose, R.; Yamaguchi, I.; Osada, H. Biotransformation of the mycotoxin, zearalenone, to a non-estrogenic compound by a fungal strain of Clonostachys sp. Biosci. Biotechnol. Biochem. 2002, 66, 2723–2726. [Google Scholar] [CrossRef] [Green Version]
- Fruhauf, S.; Novak, B.; Nagl, V.; Hackl, M.; Hartinger, D.; Rainer, V.; Labudova, S.; Adam, G.; Aleschko, M.; Moll, W.D.; et al. Biotransformation of the mycotoxin zearalenone to its metabolites hydrolyzed zearalenone (HZEN) and decarboxylated hydrolyzed zearalenone (DHZEN) diminishes its estrogenicity in vitro and in vivo. Toxins 2019, 11, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vekiru, E.; Fruhauf, S.; Hametner, C.; Schatzmayr, G.; Krska, R.; Moll, W.D.; Schuhmacher, R. Isolation and characterisation of enzymatic zearalenone hydrolysis reaction products. World Mycotoxin J. 2016, 9, 353–363. [Google Scholar] [CrossRef]
- Debevere, S.; Schatzmayr, D.; Reisinger, N.; Aleschko, M.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. Evaluation of the efficacy of mycotoxin modifiers and mycotoxin binders by using an in vitro rumen model as a first screening tool. Toxins 2020, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Tafaj, M.; Junck, B.; Maulbetsch, A.; Steingass, H.; Piepho, H.P.; Drochner, W. Digesta characteristics of dorsal, middle and ventral rumen of cows fed with different hay qualities and concentrate levels. Arch. Anim. Nutr. 2004, 58, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Zebeli, Q.; Aschenbach, J.R.; Tafaj, M.; Boguhn, J.; Ametaj, B.N.; Drochner, W. Invited review: Role of physically effective fiber and estimation of dietary fiber adequacy in high-producing dairy cattle. J. Dairy Sci. 2012, 95, 1041–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storm, A.C.; Kristensen, N.B. Effects of particle size and dry matter content of a total mixed ration on intraruminal equilibration and net portal flux of volatile fatty acids in lactating dairy cows. J. Dairy Sci. 2010, 93, 4223–4238. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Pathre, S.V.; Robison, T.S. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet. Toxicol. 1981, 19, 25–30. [Google Scholar] [CrossRef]
- Kleinova, M.; Zollner, P.; Kahlbacher, H.; Hochsteiner, W.; Lindner, W. Metabolic profiles of the mycotoxin zearalenone and of the growth promoter zeranol in urine, liver, and muscle of heifers. J. Agric. Food Chem. 2002, 50, 4769–4776. [Google Scholar] [CrossRef]
- Dänicke, S.; Keese, C.; Meyer, U.; Starke, A.; Kinoshita, A.; Rehage, J. Zearalenone (ZEN) metabolism and residue concentrations in physiological specimens of dairy cows exposed long-term to ZEN-contaminated diets differing in concentrate feed proportions. Arch. Anim. Nutr. 2014, 68, 492–506. [Google Scholar] [CrossRef]
- Dänicke, S.; Gadeken, D.; Ueberschar, K.H.; Meyer, U.; Scholz, H. Effects of Fusarium toxin contaminated wheat and of a detoxifying agent on performance of growing bulls, on nutrient digestibility in wethers and on the carry over of zearalenone. Arch. Tierernähr. 2002, 56, 245–261. [Google Scholar] [CrossRef]
- Winkler, J.; Kersten, S.; Meyer, U.; Stinshoff, H.; Locher, L.; Rehage, J.; Wrenzycki, C.; Engelhardt, U.H.; Dänicke, S. Diagnostic opportunities for evaluation of the exposure of dairy cows to the mycotoxins deoxynivalenol (DON) and zearalenone (ZEN): Reliability of blood plasma, bile and follicular fluid as indicators. J. Anim. Physiol. Anim. Nutr. 2015, 99, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Winkler, J. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals and transfer of its residues into edible tissues (carry over). Food. Chem. Toxicol. 2015, 84, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Di Gregorio, M.C.; De Nee, D.V.; Jager, A.V.; Corassin, C.H.; Carão, Á.C.D.P.; De Albuquerque, R.; De Azevedo, A.C.; Oliveira, C.A.F. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Rev. 2014, 33, 1–11. [Google Scholar] [CrossRef]
- Phillips, T.D.; Wang, M.; Elmore, S.E.; Hearon, S.; Wang, J.-S. NovaSil clay for the protection of humans and animals from aflatoxins and other contaminants. Clay. Clay. Miner 2019, 67, 99–110. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Elliott, C.T.; Connolly, L.; Kolawole, O. Potential adverse effects on animal health and performance caused by the addition of mineral adsorbents to feeds to reduce mycotoxin exposure. Mycotoxin Res. 2020, 36, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Loi, M.; Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Mule, G. Mycotoxin biotransformation by native and commercial enzymes: Present and future perspectives. Toxins 2017, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Masching, S.; Naehrer, K.; Schwartz-Zimmermann, H.E.; Sarandan, M.; Schaumberger, S.; Dohnal, I.; Nagl, V.; Schatzmayr, D. Gastrointestinal degradation of fumonisin B1 by carboxylesterase FumD prevents fumonisin induced alteration of sphingolipid metabolism in turkey and swine. Toxins 2016, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Grenier, B.; Schwartz-Zimmermann, H.E.; Gruber-Dorninger, C.; Dohnal, I.; Aleschko, M.; Schatzmayr, G.; Moll, W.D.; Applegate, T.J. Enzymatic hydrolysis of fumonisins in the gastrointestinal tract of broiler chickens. Poult. Sci. 2017, 96, 4342–4351. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Zimmermann, H.E.; Hartinger, D.; Doupovec, B.; Gruber-Dorninger, C.; Aleschko, M.; Schaumberger, S.; Nagl, V.; Hahn, I.; Berthiller, F.; Schatzmayr, D.; et al. Application of biomarker methods to investigate FUMzyme mediated gastrointestinal hydrolysis of fumonisins in pigs. World Mycotoxin J. 2018, 11, 201–214. [Google Scholar] [CrossRef]
- Yu, X.; Tu, T.; Luo, H.; Huang, H.; Su, X.; Wang, Y.; Zhang, J.; Bai, Y.; Yao, B. Biochemical characterization and mutational analysis of a lactone hydrolase from Phialophora americana. J. Agric. Food Chem. 2020, 68, 2570–2577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, L.; Chen, J.; Wang, W.; Zhang, R.; Li, Y.; Zhang, Q. Degradation mechanism for zearalenone ring-cleavage by zearalenone hydrolase RmZHD: A QM/MM study. Sci. Total Environ. 2020, 709, 135897–135906. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ko, T.-P.; Yang, Y.; Zheng, Y.; Chen, C.-C.; Zhu, Z.; Huang, C.-H.; Zeng, Y.-F.; Huang, J.-W.; Wang, A.H.-J.; et al. Crystal structure and substrate-binding mode of the mycoestrogen-detoxifying lactonase ZHD from Clonostachys rosea. RSC Adv. 2014, 4, 62321–62325. [Google Scholar] [CrossRef]
- Igawa, T.; Takahashi-Ando, N.; Ochiai, N.; Ohsato, S.; Shimizu, T.; Kudo, T.; Yamaguchi, I.; Kimura, M. Reduced contamination by the Fusarium mycotoxin zearalenone in maize kernels through genetic modification with a detoxification gene. Appl. Environ. Microbiol. 2007, 73, 1622–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higa-Nishiyama, A.; Takahashi-Ando, N.; Shimizu, T.; Kudo, T.; Yamaguchi, I.; Kimura, M. A model transgenic cereal plant with detoxification activity for the estrogenic mycotoxin zearalenone. Transgenic Res. 2005, 14, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, H.; Sun, J.; Wang, J.; Zhao, C.; Zhang, W.; Zhang, J.; Sun, C. Zearalenone removal from corn oil by an enzymatic strategy. Toxins 2020, 12, 117. [Google Scholar] [CrossRef] [Green Version]
- Teorell, T.; Stenhagen, E. Ein Universalpuffer für den pH-Bereich 2.0 bis 12.0. Biochem. Z. 1938, 299, 416–419. [Google Scholar]
- European Commission. Directive 2010/63/eu of the European Parliament and of the council of 22 september 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L 276, 33–79. [Google Scholar]
- European Commission (EC). Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). Off. J. Eur. Union 2006, L 229, 7–9. [Google Scholar]
- Zebeli, Q.; Tafaj, M.; Junck, B.; Mansmann, D.; Steingass, H.; Drochner, W. Evaluation of the effects of dietary particle fractions on fermentation profile and concentration of microbiota in the rumen of dairy cows fed grass silage-based diets. Arch. Anim. Nutr. 2008, 62, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmay, H.; Adam, G.; Berthiller, F. Metabolism of zearalenone and its major modified forms in pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| ZEN [%] 1 | α-ZEL [%] 1 | β-ZEL [%] 1 | HZEN [%] 1 | DHZEN [%] 1 | |
|---|---|---|---|---|---|
| Reticulorumen 2 ZEN treatment | |||||
| 15 min | 100.0 | 0.0 (traces) | 0.0 (traces) | 0.0 | 0.0 |
| 1 h | 84.8 | 7.6 | 7.6 | 0.0 | 0.0 |
| 4 h | 84.1 | 15.9 | 0.0 (traces) | 0.0 | 0.0 |
| 7 h | 78.7 | 21.3 | 0.0 (traces) | 0.0 | 0.0 |
| 10 h | 74.4 | 25.6 | 0.0 | 0.0 | 0.0 |
| 24 h | 50.2 | 49.8 | 0.0 | 0.0 | 0.0 |
| Reticulorumen 2 ZEN+ZenA treatment | |||||
| 15 min | 5.9 | 0.0 (traces) | 0.0 | 94.1 | 0.0 |
| 1 h | 0.0 (traces) | 0.0 | 0.0 | 100.0 | 0.0 |
| 4 h | 0.0 (traces) | 0.0 | 0.0 | 100.0 | 0.0 (traces) |
| 7 h | 0.0 (traces) | 0.0 | 0.0 | 100.0 | 0.0 (traces) |
| 10 h | 0.0 (traces) | 0.0 (traces) | 0.0 | 100.0 | 0.0 (traces) |
| Feces 3 ZEN treatment | |||||
| 10 h | 11.0 | 10.9 | 78.1 | 0.0 | 0.0 |
| Feces 3 ZEN+ZenA treatment | |||||
| 0 h | 15.6 | 15.5 | 69.0 | 0.0 | 0.0 |
| 10 h | 0.0 (traces) | 9.0 | 59.1 | 31.9 | 0.0 |
| Analyte 1 | Q1 Mass (Da) | Q3 Mass (Da) 2 | Declustering Potential (V) | Entrance Potential (V) | Collision Energy (V) 2 | Collision Cell Exit Potential (V) 2 |
|---|---|---|---|---|---|---|
| ZEN | 317.1 | 131.0/175.0 | −100 | −10 | −42/−34 | −9/−7 |
| α-ZEL | 319.1 | 275.1/160.0 | −105 | −10 | −30/−42 | −7/−9 |
| β-ZEL | 319.1 | 275.1/160.0 | −105 | −10 | −30/−42 | −7/−9 |
| HZEN | 335.0 | 149.0/161.0 | −80 | −10 | −34/−34 | −1/−9 |
| DHZEN | 291.1 | 149.0/161.0 | −80 | −10 | −25/−25 | −8/−8 |
| Analyte 1 | Spike Concentration (µg/L) | Recovery (% of Spike Concentration) |
|---|---|---|
| ZEN | 3 | 81 |
| 30 | 91 | |
| α-ZEL | 3 | 105 |
| 30 | 85 | |
| β-ZEL | 6 | 69 |
| 30 | 78 | |
| HZEN | 3 | 82 |
| 30 | 103 | |
| DHZEN | 3 | 65 |
| 30 | 80 |
| Roughage (% of Dry Matter) | |
| Grass silage | 50.0 |
| Hay | 50.0 |
| Concentrate (Control; % of Dry Matter) | |
| Corn | 50.0 |
| Wheat | 33.3 |
| Barley grain | 8.3 |
| Mineral-vitamin premix 1 | 5.0 |
| CaCO3 | 3.3 |
| Roughage | Concentrate (Control) | |
|---|---|---|
| Dry matter (DM; % in fresh matter) | 54.6 | 89.6 |
| Ash (% of DM) | 7.5 | 7.9 |
| Crude Protein (% of DM) | 13.2 | 11.0 |
| Ether extract (% of DM) | 1.7 | 2.7 |
| Neutral detergent fiber (% of DM) | 57.5 | 13.7 |
| Acid detergent fiber (% of DM) | 37.3 | 4.3 |
| Non fiber carbohydrates (NFC) 1 | 20.0 | 64.4 |
| Day | Treatment | Diet 1 | Sampling |
|---|---|---|---|
| 1 | ZEN | Roughage provided ad libitum. Manual feeding of 500 g ZEN-contaminated (10 mg/kg) 3 concentrate at 08:30 a.m. Manual feeding of 500 g control concentrate at 11:30 a.m., 2:30 p.m. and 5:30 p.m., respectively. | Rumen fluid: 2 immediately before ZEN-contaminated concentrate feeding (0 h), 6 sampling time points 15 min–10 h after ZEN-contaminated concentrate feeding (15 min, 1 h, 2 h, 4 h, 7 h, 10 h) Feces: immediately before ZEN-contaminated concentrate feeding (0 h), 10 h after ZEN-contaminated concentrate feeding (10 h) |
| 2 | No treatment (ZEN washout) | Roughage provided ad libitum. Manual feeding of 500 g control concentrate at 08:30 a.m., 11:30 a.m., 2:30 p.m. and 5:30 p.m., respectively. | Rumen fluid: 2 immediately before first concentrate feeding (24 h), 10 h after first concentrate feeding (34 h) |
| 3 | ZEN+ZenA | Roughage provided ad libitum. Manual feeding of 500 g ZEN-contaminated (10 mg/kg) 4 concentrate supplemented with ZenA (128 U/kg) at 08:30 a.m. Manual feeding of 500 g control concentrate at 11:30 a.m., 2:30 p.m. and 5:30 p.m., respectively. | Rumen fluid: 2 immediately before ZEN+ZenA concentrate feeding (0 h), 6 sampling time points 15 min–10 h after ZEN+ZenA concentrate feeding (15 min, 1 h, 2 h, 4 h, 7 h, 10 h) Feces: immediately before ZEN+ZenA concentrate feeding (0 h), 10 h after ZEN+ZenA concentrate feeding (10 h) |
| 4 | No treatment (ZEN+ZenA washout) | Roughage provided ad libitum. Manual feeding of 500 g control concentrate at 08:30 a.m., 11:30 a.m., 2:30 p.m. and 5:30 p.m., respectively. | Rumen fluid: 2 immediately before first concentrate feeding (24 h), 10 h after first concentrate feeding (34 h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber-Dorninger, C.; Faas, J.; Doupovec, B.; Aleschko, M.; Stoiber, C.; Höbartner-Gußl, A.; Schöndorfer, K.; Killinger, M.; Zebeli, Q.; Schatzmayr, D. Metabolism of Zearalenone in the Rumen of Dairy Cows with and without Application of a Zearalenone-Degrading Enzyme. Toxins 2021, 13, 84. https://doi.org/10.3390/toxins13020084
Gruber-Dorninger C, Faas J, Doupovec B, Aleschko M, Stoiber C, Höbartner-Gußl A, Schöndorfer K, Killinger M, Zebeli Q, Schatzmayr D. Metabolism of Zearalenone in the Rumen of Dairy Cows with and without Application of a Zearalenone-Degrading Enzyme. Toxins. 2021; 13(2):84. https://doi.org/10.3390/toxins13020084
Chicago/Turabian StyleGruber-Dorninger, Christiane, Johannes Faas, Barbara Doupovec, Markus Aleschko, Christian Stoiber, Andreas Höbartner-Gußl, Karin Schöndorfer, Manuela Killinger, Qendrim Zebeli, and Dian Schatzmayr. 2021. "Metabolism of Zearalenone in the Rumen of Dairy Cows with and without Application of a Zearalenone-Degrading Enzyme" Toxins 13, no. 2: 84. https://doi.org/10.3390/toxins13020084





