Abstract
Bacillus cereus is a ubiquitous soil bacterium responsible for two types of food-associated gastrointestinal diseases. While the emetic type, a food intoxication, manifests in nausea and vomiting, food infections with enteropathogenic strains cause diarrhea and abdominal pain. Causative toxins are the cyclic dodecadepsipeptide cereulide, and the proteinaceous enterotoxins hemolysin BL (Hbl), nonhemolytic enterotoxin (Nhe) and cytotoxin K (CytK), respectively. This review covers the current knowledge on distribution and genetic organization of the toxin genes, as well as mechanisms of enterotoxin gene regulation and toxin secretion. In this context, the exceptionally high variability of toxin production between single strains is highlighted. In addition, the mode of action of the pore-forming enterotoxins and their effect on target cells is described in detail. The main focus of this review are the two tripartite enterotoxin complexes Hbl and Nhe, but the latest findings on cereulide and CytK are also presented, as well as methods for toxin detection, and the contribution of further putative virulence factors to the diarrheal disease.
Keywords:
Bacillus cereus; hemolysin BL; non-hemolytic enterotoxin; cytotoxin K; cereulide; pore formation; cytotoxicity; food poisoning Key Contribution:
This review summarizes 30 years of work on the food poisoning toxins of B. cereus with special focus on the latest findings regarding genetic distribution, organization, evolution and regulation, secretion, detection and mode of action of the tripartite enterotoxins Hbl and Nhe.
1. Introduction
Bacillus cereus is estimated to be responsible for 1.4%–12% of all food poisoning outbreaks worldwide [1]. In the European Union, bacterial toxins (Clostridium, Staphylococcus and B. cereus) accounted for 17.7% (2016) and 15.9% (2017) of all registered food- and water-borne outbreaks, which ranked them second behind Salmonella [2,3]. With 98 registered outbreaks in the EU in 2018, B. cereus toxins ranked in fifth place behind Salmonella, Campylobacter, the norovirus and Staphylococcus toxins. Among these was also one large food poisoning outbreak with more than 100 affected persons. Furthermore, six fatal cases were attributed to bacterial toxins (Clostridium botulinum, Clostridium perfringens and B. cereus) [4].
Basically, B. cereus is responsible for two types of gastrointestinal diseases. The emetic kind of illness is mainly characterized by nausea and emesis, which appear as soon as half an hour after consumption of the contaminated food and are clinically indistinguishable from intoxications with Staphylococcus aureus enterotoxins [5]. In this classical food intoxication, the emetic toxin cereulide is pre-formed during vegetative growth of B. cereus in foodstuffs and the consumption of the bacteria is not necessary [6]. Indeed, there are several reports of outbreaks where only the cereulide toxin was detected in the food, but no bacteria could be isolated [7]. Nevertheless, it is generally thought that at least 103–105 B. cereus per g food are needed to produce cereulide in disease-provoking concentrations [5,6,7,8,9]. Cereulide is a cyclic dodecadepsipeptide with a molecular weight of 1.2 kDa. The basic repeated amino acid sequence [D-O-Leu D-Ala L-O-Val D-Val]3 is extremely stable towards heat, acid or digestive enzymes and, thus, the toxin can hardly be removed or inactivated [10,11,12]. Usually, the emetic form of disease is self-limiting and symptoms disappear after 6–24 h. Nevertheless, some severe and fatal outbreaks mostly related to liver failure are reported [10,13,14,15,16,17,18,19,20,21,22,23]. Due to the ubiquitous nature of the pathogen and its production of highly resistant spores, B. cereus is frequently found in various kinds of food [24,25,26]. Historically, starchy foodstuffs such as rice or pasta are connected to food intoxications with emetic B. cereus, but more recently evidence is growing that emetic B. cereus are much more volatile than once thought. The comprehensive analysis of a total of 3654 food samples obtained from suspected food-borne illnesses with a preliminary report of vomiting, collected over a period of seven years, revealed that emetic B. cereus strains were detected in a broad diversity of foods, including vegetables, fruit products, sauces, soups, and salads as well as milk and meat products [7].
The second, diarrheal form of food poisoning is also associated with a variety of different foodstuffs [27]. This form of disease manifests mainly in diarrhea and abdominal cramps, similar to food poisoning by Clostridium perfringens type A [5]. Symptoms occur after approximately 8–16 h. This incubation time is typical for toxico-infections, in which the toxins are produced by viable bacteria inside the human intestine [5,28,29]. Unlike cereulide, enterotoxins pre-formed in foods most likely do not contribute to the disease, as they are considered sensitive towards heat, acids or proteases. Thus, vegetative B. cereus and, especially, spores must be consumed. The infective dose is estimated between 105–108 cfu/g [11,30] or 104–109 cfu/g [9,29] vegetative cells or spores. The course of disease is mainly mild and—after approximately 12–24 h—self-limiting. Fatal outbreaks are only very rarely reported [31]. A food infection with enteropathogenic B. cereus can be seen as a multifactorial process, as a number of individual steps have to be considered before the onset of the disease, including prevalence and survival of B. cereus in different foodstuffs, survival of the stomach passage, germination of spores, active movement towards and adhesion to the intestinal epithelium, enterotoxin production under intestinal conditions, as well as the influence of consumed foods and the intestinal microbiota on these processes. We recently summarized these steps in a separate review [27]. Nevertheless, production and action of the enterotoxins are of the upmost relevance for the course of the diarrheal disease. Three main, pore-forming protein enterotoxins are known, which are the tripartite hemolysin BL (Hbl) [32] and non-hemolytic enterotoxin (Nhe) [33], as well as the single protein cytotoxin K (CytK) [31]. Progress made in studies from the early 1990s until today on highly variable, strain-specific enterotoxin production (distribution, genetic organization, gene expression and toxin secretion), as well as on the mode of action and the effects on target cells of these pore-forming enterotoxins, is depicted in detail in the present review. The two three-component enterotoxin complexes Hbl and Nhe are the focus of attention. In addition, the latest findings regarding cereulide and CytK are also summarized, as well as known methods for toxin detection and prevention of illness, and the possible contribution of further secreted virulence factors to the diarrheal form of disease.
2. Distribution of Toxin Genes
2.1. Prevalence among Isolates from Environment, Foods and Outbreaks
B. cereus is a ubiquitous soil bacterium and can thus be found worldwide in the ground, in dust, or on different foods. Early studies pointed to an occurrence of diarrheal or emetic outbreaks according to country-specific dietary habits, with the emetic form manifesting in Great Britain or Japan, and the diarrheal form rather in Northern Europe or the USA [34,35]. Lately, both syndromes have been reported from all over the world. Basically, emetic strains are found less frequently in foods as well as in the environment than enteropathogenic strains [27,36,37]. In a multitude of studies, new isolates were screened for the presence of the toxin genes nhe (ABC), hbl (CDAB), cytK (1,2), entFM, and ces. In some studies, the presence of bceT (enterotoxin T) was also assessed; however, its enterotoxic capacity is disproven [38,39,40]. Virulence/enterotoxin gene patterns are compiled for B. cereus which has been mainly isolated from foods, but also from clinical, soil and environmental samples worldwide. Generally, those patterns are highly diverse [41,42,43,44,45,46,47].
Common distribution of the toxin genes is approximately 85%–100% nhe (ABC), approximately 40%–70% hbl (CDA), approximately 40%–70% cytK-2, very few ces+, typically no cytK-1+, and—if tested—approximately 60%–100% entFM, which has been detected in studies from Europe [44,48,49,50,51,52,53,54], South America [55,56], North America [41,57], Asia [58,59,60,61,62,63,64,65] and Africa [66,67,68]. Nevertheless, in some studies, a connection was established between toxin gene patterns and geographical location of the isolates. Drewnowska et al. found that strains possessing nheA, hblA and cytK-2 were predominant in regions with arid hot climate, and were comparably rare in continental cold climates [69]. This is supported by other studies suggesting that geographic origin might have an impact on the conservation of hblA among B. cereus populations [70,71,72]. Zhang et al. also claim a “regional feature for toxin gene distribution” [73].
Besides geographical location, toxin gene patterns seem to be also influenced by the kind of foodstuffs analyzed. For instance, Berthold-Pluta et al. found higher prevalence of nhe+ and hbl+, but lower prevalence of ces+ strains in food products of animal than of plant origin [74]. Rossi et al. showed that strains from dairy products had significantly lower cytK-2 and hblCDA prevalence than strains from equipment or raw milk [75], and Hwang and Park found hbl in >95% of tested ready-to-eat (RTE) foods, but only in 30% of infant formulas. Furthermore, the prevalence of cytK-2 was comparably low in the latter food [76].
Studies were also conducted comparing food related and food poisoning related strains. Santos et al. showed that food poisoning strains had a higher occurrence and higher genetic diversity of plcR-papR, nheA, cytK-2, plcA, and gyrB genes than strains isolated from soil or foods [77]. CytK and the combination hbl-nhe-cytK were more often found among food poisoning related than among food related strains [51,52,78].
Generally, all B. cereus isolates can be categorized into seven different toxin profiles: A (nhe+, hbl+, cytK+), B (nhe+, cytK+, ces+), C (nhe+, hbl+), D (nhe+, cytK+), E (nhe+, ces+), F (nhe+), and G (cytK+) [48]. In fact, the hbl genes alone or a combination of ces and hbl have only been reported for the very few emetic Bacillus weihenstephanensis isolates described so far [79]. There are further studies showing “unusual” results, particularly low or no prevalence of nhe [45,74,80,81,82,83,84] or extraordinarily high prevalence of hbl [76,85,86,87,88] or ces [89], which must be interpreted cautiously, especially as nhe is well known for its molecular heterogeneity [48,51,52]. Thus, the choice of detection methods, especially primer pairs for nhe, can have a crucial influence on the results.
However, it has to be mentioned that the presence of enterotoxin genes or a certain toxin gene profile does not necessarily allow conclusions on the toxic activity of a B. cereus isolate [53,90]. In our own studies, we chose pairs of strains with an identical toxin gene profile, but one strain exhibited high and the other low toxic activity both under routine laboratory and simulated intestinal growth conditions [91,92]. The reasons for this are so far not completely understood, but it is believed that highly variable and strain-specific mechanisms in toxin gene transcription, posttranscriptional and posttranslational modification and protein secretion are involved, which are summarized in Section 4.1.2.
2.2. Presence within the B. cereus Group
In many of the studies mentioned in Section 2.1, often only B. cereus sensu lato (s. l.) strains are investigated, meaning there is no differentiation between the members of the B. cereus group. In routine microbiological diagnostics, only “presumptive” B. cereus are detected on selective culture media according to international standards (ISO 7932:2005-03) [93,94]. The B. cereus group comprises at least eight species: B. anthracis, B. cereus sensu stricto (s. s.), B. thuringiensis, B. mycoides, B. pseudomycoides, B. weihenstephanensis, B. cytotoxicus and B. toyonensis [95,96,97,98]. Additionally, more and more species such as B. wiedmannii, B. bingmayongensis, B. gaemokensis, B. manliponensis, and others are described [99,100,101,102,103]. Generally, they exhibit high genetic similarities and, thus, it has been suggested that they be considered as one species [5,104,105] or to completely change the taxonomic nomenclature of the B. cereus group [106]. Species definition is historically based on phenotypes or clinical and economical relevance. While the unique characteristics of B. anthracis, emetic B. cereus and B. thuringiensis are located on plasmids [105], the enterotoxins are chromosome-coded and can thus be present throughout the B. cereus group. This is particularly problematic for the assessment of B. thuringiensis, which is frequently used as biopesticide worldwide [107,108,109]. B. thuringiensis has been isolated from a variety of foodstuffs and the presence of the enterotoxin genes nhe, hbl and cytK-2 has been shown, with similar percentages as for B. cereus [57,60,72,90,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125], while ces genes have not been found [126,127]. Enterotoxin production and cytotoxic activity have also been shown [57,113,114,116,117,123,128,129,130,131], and B. thuringiensis could therefore be involved in food poisoning outbreaks [132]. Consequently, it was debated whether the B. thuringiensis-associated biopesticides represent a risk for public health. To clarify this question, there is a demand for simple methods enabling a clear discrimination between B. cereus and B. thuringiensis in routine food and clinical diagnostics as well as for unequivocal identification of the strains used as biopesticides [126].
Next to B. cereus and B. thuringiensis, further species of the B. cereus group were isolated from foods and the presence of enterotoxin genes was proven, such as B. anthracis [48], B. mycoides [42,43,48,70,71,133,134], B. pseudomycoides [42,71], B. toyonensis [135], and B. weihenstephanensis [42,48,71,136,137]. It has also been shown that Bacillus spp. outside the B. cereus group can harbor one or more enterotoxin genes [138,139]. For instance, Mäntynen and Lindström found hblA+ B. pasteurii DSM 33, B. smithii DSM 459, and Bacillus sp. DSM 466 [70]. Nhe and/or hbl genes were also detected in B. amyloliquefaciens, B. circulans, B. lentimorbis, and B. pasteurii [140]. On the other hand, From et al. found no enterotoxin genes outside the B. cereus group in the strains analyzed [141].
According to MLST (multi-locus sequence typing), AFLP (amplified fragment length polymorphism) and whole genome sequencing, the B. cereus group was first assigned to three phylogenetic groups (clades) [142], then seven (panC types) [96], and later nine [120], which do not correlate with species definition [105]. Prevalence of enterotoxin genes and their profiles were also compared to phylogenetic groups. B. cereus isolates from dairy products in Brazil with approximately 50% cytK-2 and hbl, and approximately 85% nhe were mostly assigned to phylogenetic group III. Group IV and V showed significantly higher prevalence of hblCDA and group IV showed additionally higher prevalence of cytK-2 [75,96]. In another study on dairy isolates, strains of clade IIIc had no hblCDA operon, while strains of clade IV carried it and produced the Hbl toxin, whereas strains of clade VI carried the gene but did not produce the toxin [120]. Furthermore, a broad distribution of enterotoxin genes among seven phylogenetic clades, in which dairy-associated isolates were divided, was shown [90]. Okutani et al. investigated the genomes of 44 B. cereus group isolates from soil, animal and food poisoning cases in Japan. Strains were assigned to four different panC types and five different clades. The nhe operon was found in all strains tested, while ces was detected only in the food poisoning strains. When the presence or absence of virulence-associated genes was statistically analyzed, the majority of soil and animal isolates was part of overlapping clusters, while three of the four food poisoning isolates formed a distinct cluster [143]. Furthermore, the hbl and the ces genes were significantly correlated with the phylogenetic group [143,144]. Several further studies suggested that the toxic potential of B. cereus s. l. strains depends rather on the phylogenetic group than on the species [96,120,145].
3. The Emetic Toxin Cereulide
In this section, key features of the highly bioactive depsipeptide toxin cereulide are presented, its biosynthesis and mode of action are briefly discussed, and an overview of diagnostic methods is given. For a more detailed overview from the perspective of the food industry, it is referred to a recent review of Rouzeau-Szynalski et al. [146] and in-depth insights into genetics and regulatory circuits directing cereulide biosynthesis are provided by Ehling-Schulz et al. [37].
3.1. Characteristics of Cereulide and Its Assembly via the Non-Ribosomal Peptide Synthetase Ces-NRPS
Cereulide, which was originally described by Agata et al. in 1994 [147], is a potassium binding depsipeptide that structurally resembles the ionophore valinomycin [148,149,150,151]. Due to its structure, which is characterized by alternating peptide and ester bounds (see Figure 1), it is highly lipophilic and extremely heat resistant and stable from pH 2 to 11. It does not lose activity even after two hours at 121 °C [12]. Because of its small size, it cannot be removed by normal hygienic procedures in food processing, such as bactofugation or filtration, nor will it be inactivated by heat treatments. Because of its resistance to cleavage by pepsin and trypsin, it will not be inactivated during stomach passage in the host [10]. Thus, it is of the utmost importance to prevent its synthesis in food production and processing. There are some indications that certain food additives, such as polyphosphates, have a specific inhibiting effect on cereulide synthesis while other food ingredients stimulate it [7,152] (see also Section 3.2).
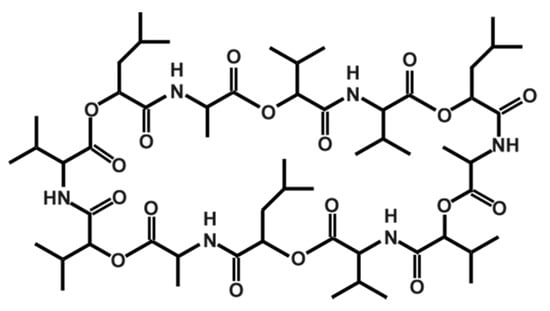
Figure 1.
Structure of the depsipeptide toxin cereulide, the causative agent for the emetic type of B. cereus food-borne intoxications.
More recently, several isoforms of cereulide have been described [153], which vary significantly in their cytotoxic potential. For instance, isocereulide A shows about 10-fold in vitro cytotoxicity compared to wild type cereulide, while isocereulide B does not show any cytotoxicity at all. These differences in cytotoxicity might be explained by different membrane activity of the isocereulides, as revealed by in vitro lipid bilayer studies [153]. The latter results foster the hypothesis that the ionophoric membrane activity of cereulide is one of the key features of its bioactivity (for more details see Section 3.3).
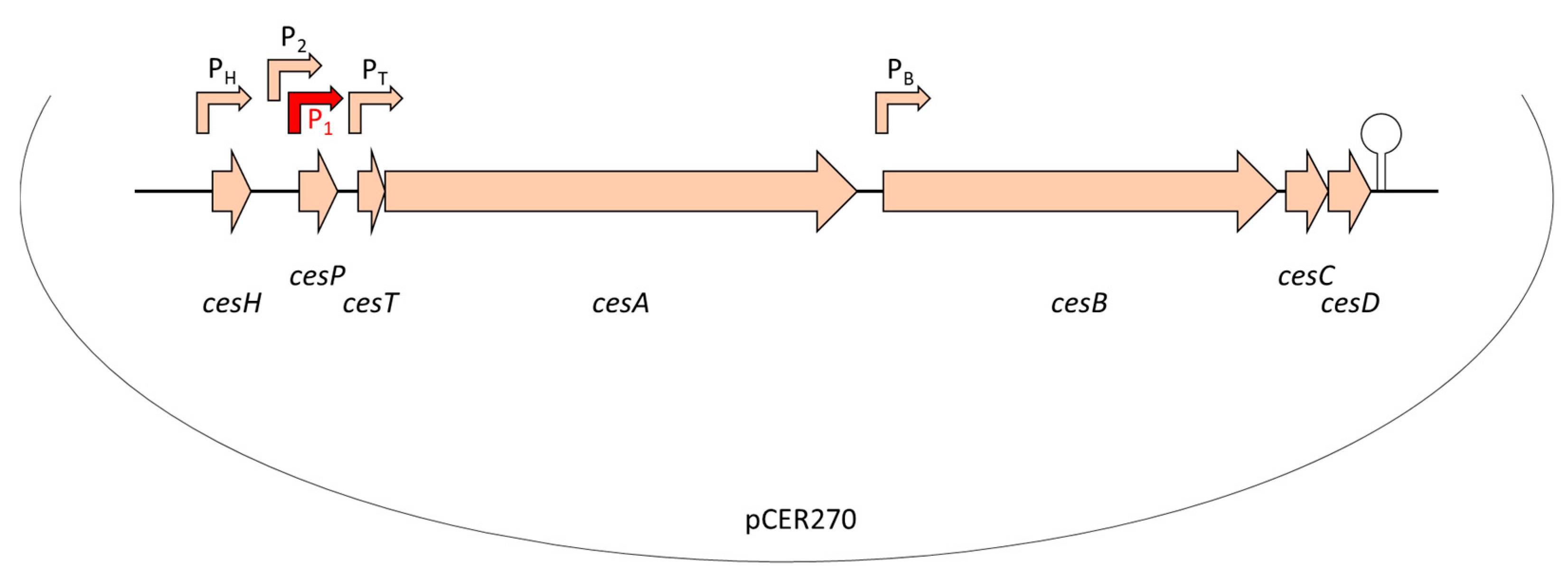
Similar to valinomycin and other peptide antibiotics, cereulide is assembled by a non-ribosomal peptide synthetase (NRPS), called Ces-NRPS [148,154,155]. NRPSs are huge multienzyme intracellular machineries, which are often genetically organized into gene clusters [156]. Apart from the structural synthetase genes, the corresponding genetic loci often contain genes involved in the activation of the NRPS as well as putative ABC transporters, which are thought to be involved in peptide export and may confer self-resistance [157]. In addition, NRPS genetic loci may contain or be flanked by accessory genes encoding enzymes responsible for monomer modifications [158] or type II thioesterases ensuring correct peptide assembly [159]. The ces genes are encoded on the pCER270 mega-plasmid, which shares it backbone with the pX01 toxin plasmid from B. anthracis [154,160]. The 24kb ces locus comprises a total of seven coding sequences (CDSs) [154] (see also Figure 2): cesA and cesB, the structural cereulide synthetase genes, which are flanked on the 5′ end by cesH, a putative hydrolase; cesP, a phosphopantetheinyl transferase (PPtase) involved in the activation of the cereulide synthetase; and cesT, a type II thioesterase thought to be involved in removal of mis-primed monomers. On the 3′ end of the ces locus two additional CDSs, designated cesC and cesD, are located that encode an ABC transporter. Very recently, it was shown that this ABC transporter is not only responsible for toxin export but also plays an essential and direct role in cereulide biosynthesis, by tethering the CesAB synthetase to the cell membrane [161]. In vivo studies revealed that CesAB colocalizes with CesCD on the cell membrane and BACTH (bacterial adenylate cyclase-based two-hybrid system) and mutation studies provided evidence that specific domains within the CesAB synthetase are interacting with CesC. Complementary in vitro as well as in silico studies suggested that this novel role of an ABC transporter, beyond the canonical function, discovered in emetic B. cereus, might represent a conserved mechanism involved in the biosynthesis of microbial natural products. This may facilitate the discovery of new bioactive metabolites [161].
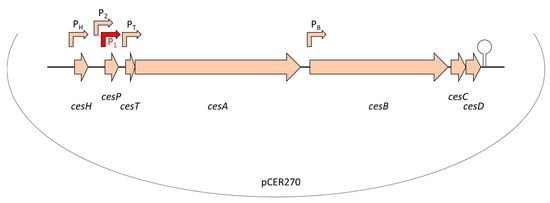
Figure 2.
Genetic organization of the ces locus encoding the genetic determinants essential for non-ribosomal assembly of the cereulide toxin, located on the pX01-like mega-plasmid pCER270. For detailed description of the ces locus see text and [154,155,161,162]. Promoters are indicated by arrows. The main promoter P1 (indicated in red) drives the polycistronic transcription of the ces operon. A hairpin indicates the terminator. Abbreviation: cesH, a hydrolase/esterase; cesP, a phosphopantetheinyl transferase; cesT, a type II thioesterase; cesA and cesB, structural cereulide synthetase genes; cesC and cesD, ABC transporter.
3.2. Regulation of Cereulide Biosynthesis
Since production of secondary metabolites via NRPSs is metabolically very costly, these multi-enzyme machineries are usually tightly regulated [37]. Six out of the seven CDSs in the ces locus are transcribed as polycistron from the main promotor P1, located upstream of cesP (see Figure 2). This ensures an orchestrated transcription of the respective genes, which show a tightly regulated transcription peak in late exponential phase [163,164]. Additional promotors have been found in the ces locus, but their exact role in ces transcription and regulation is still elusive [162]. The putative hydrolase cesH, located in the 5′ region of the ces locus, is transcribed by its own promotor. Using a mutagenesis approach, Lücking et al. showed that CesH, which structurally belongs to the 6_AlphaBeta_hydrolase subgroup of the alpha/beta-hydrolase fold superfamily, inhibits cereulide synthesis [165]. CesH overexpression led to a cereulide-negative phenotype and transcriptional analysis of the CesH overexpression mutant resulted in strongly down-regulated cesA mRNA levels, indicating that CesH is involved in the timing of Ces-NRPS expression and cereulide assembly. Thus, it could be assumed that it functions as closing signal [165]. This hypothesis is fostered by the transcriptional kinetic of cesH showing highest cesH expression in stationary growth, whereas the other genes of the ces locus are transcribed in earlier growth phases [166]. However, since a direct action of a hydrolase as a transcriptional regulator is rather unlikely, it is tempting to speculate that CesH acts indirectly by degrading quorum sensing signaling molecules or metabolites, which may impact ces transcription in later growth phases. The findings are corroborated by a recent study. In line with the results from Lücking et al. [165], demonstrating that cesH inactivation leads to accelerated cereulide production, Tian et al. [167] found increased cereulide production in a ∆cesH mutant. Recombinant CesH was reported to possess esterase activity against para-nitrophenyl acetate but, so far, attempts to show esterase activity of CesH against cereulide have failed [167]. Thus, further studies will be necessary to decipher the exact role and mechanism of CesH in cereulide biosynthesis.
Previous work demonstrated that several key transcription factors of the chromosome play a pivotal role in the onset and steep increase of the transcription of the polycistronic cesPTABCD genes. Knockout as well as in vitro promotor binding studies [163,164] showed that the ces genes are not under control of PlcR, a pleiotropic regulator known to play a central role in enteropathogenic B. cereus [168,169] (see also Section 4.1.2). By contrast, the transcriptional regulator CodY, which acts as intracellular sensor of the energetic cell status and senses branched-chain amino acids [170], plays a key role in cereulide synthesis and tightly links toxin production to cell metabolism [37,163]. Furthermore, it has been shown that the transition state transcriptional regulator AbrB suppresses ces transcription in the early growth phase and thus couples cereulide synthesis to the developmental cell status and the Spo0A-AbrB regulatory circuit [37,164].
Although transcriptional regulators play a vital role in the control of cereulide biosynthesis, other realms of regulation must be taken into consideration. For instance, there is no strict co-linearity between ces transcription and actual cereulide toxin production [163,166]. Thus, posttranscriptional regulatory mechanisms must contribute to the complex network of directing and controlling cereulide biosynthesis. Generally, NRPSs are produced as apo-carrier proteins and 4′-phosphopanthetheinyl transferases (PPTases) must activate the NRPS modules by catalyzing the transfer of a coenzyme A-derived 4′-phosphopantetheine moiety to the peptide carrier protein (PCP) domains of the NRPS in order to convert them into their holo-form [171]. The ces gene locus encodes a PPTase, designated CesP [154], but unexpectedly a cesP knockout mutant was reported to still be able to produce cereulide [165], although functionality of CesP has been proven previously [148]. Only after disruption of an additional, chromosomally encoded PPTase (designated ppt) was a toxin-negative phenotype observed, indicating that this PPTase can function as a redundant CesP-PPTase in cereulide biosynthesis [165]. Thus, in cereulide biosynthesis there is not only chromosomal-plasmid crosstalk on a transcriptional level, but also on a posttranslational level, highlighting once more the complexity of the regulatory circuits governing cereulide production. The recently discovered novel and essential role of the ABC transporter CesCD in cereulide biosynthesis, which goes beyond its canonical export function, adds another level of complexity to the posttranslational regulatory circuits in cereulide formation [161]. Since cereulide is a highly bioactive ionophore, it could be hypothesized that it would be beneficial for the cells to orchestrate both biosynthesis and export of the toxin.
However, not only intrinsic but also extrinsic, environmental factors can have a significant impact on cereulide production capacity [172]. A comprehensive study showed that temperature is a cardinal parameter for cereulide production, which exerts control on posttranscriptional level [173]. Notably, maximum growth rate and maximum cereulide production were found to be decoupled, indicating that solely the number of bacteria or growth rates are no suitable parameters to predict the risk of cereulide production. Furthermore, the study of Kranzler et al. [173] revealed not only that the total amount of cereulide produced was highly temperature-dependent, but temperature also significantly impacted the formation of the recently identified cereulide isoforms. The production of isocereulide A, which is about 10-fold more cytotoxic than cereulide, was specifically supported at low temperatures (12 and 15 °C), whilst the production of the non-toxic isocereulide B was supported by ambient temperatures [173]. The latter must be taken into consideration in terms of food safety and predictive microbiology. Apart from temperature, other food industrial relevant extrinsic parameters, such as oxygen, pH or the food matrix, could also have a significant impact. However, further study will be necessary to fully understand the impact of specific extrinsic factors (and their interplay) on cereulide and isocereulide production in complex systems such as foods. Generally, there are indications that a neutral pH, high content of starch, carbohydrates, vitamins and trace elements support cereulide production, while low oxygen levels and high NaCl concentrations have a rather negative impact [7]. A detailed overview of the current knowledge of food industrial relevant external parameters is provided in a recent review by Rouzeau-Szynalski et al. [146]. There are also some indications that certain food additives, such as polyphosphates, could affect cereulide production in a specific manner by a hitherto unknown mechanism [152]. Furthermore, it has also been reported that specific antibiotics, which provoke the production of small colony variants (SCVs), could lead to dysregulation of cereulide synthesis resulting in derailed cereulide levels [174].
3.3. Mode of Action and within Host Translocation
Food-borne intoxications caused by cereulide are characterized by heavy episodes of vomiting shortly (15 min to 6 h) after consumption of contaminated foods, which are accompanied by nausea [10]. Based on results from a Suncus murinus animal model [6], it has been assumed that cereulide interacts with 5-HT3 serotonin receptors leading to the stimulation of the afferent vagus nerve and subsequent triggering of the vomiting center in the medulla oblongata. Usually symptoms decline after 24 h, but more severe intoxications are increasingly reported [7,14,18,23]. Due to its high ionophoric activity, cereulide can lead to rhabdomyolysis, liver damage and serious multiorgan failures. Low doses of cereulide have been reported to cause dysfunction in beta-cell lines [175] and to be detrimental towards isolated porcine pancreatic Langerhans islets [176]. Furthermore, by using whole mouse pancreatic islets and Min6 cells as model, it was shown that sub-emetic doses of cereulide have a direct impact on the insulin secretory machinery, suggesting that it may contribute to the ongoing diabetes endemic [175,177]. Recently it was reported that cereulide can co-occur with other microbial toxins, such as mycotoxins, in cereal-based foods [178]. Based on the results from in vitro co-cultivation studies of cereulide and the mycotoxin deoxynivalenol (DON), it was hypothesized that frequent consumption of cereal-based foods co-contaminated with cereulide and DON may cause synergistic cytotoxic effects and may alter the immune response in the human intestine [179]. However, since these results are only based on in vitro studies, further in vivo studies, using a suitable animal model, will be necessary to assess the actual risk associated with long-time dietary exposure to cereulide or the risks linked to co-exposure of cereulide and other microbial toxins.
As pigs are a well characterized model to study the physiological effects and toxico-kinetics of mycotoxins [180,181], which are structurally similar to cereulide, we explored their potential to gain first insights into uptake, resorption and translocation of the cereulide toxin within the body. To this end, piglets were orally challenged with different doses of cereulide [182]. The clinical symptoms mimicked those known from severe human intoxication cases, including lethargy, seizures, and convulsions [14,15,17,18,19], suggesting an involvement of the central nervous system (CNS). A part of the ingested cereulide toxin was absorbed and was distributed within the body, while the other part was rapidly excreted with the feces, indicating that screening of fecal samples by stable isotope dilution assay liquid chromatography mass spectrometry (SIDA LC-MS) may represent a suitable method for detection of cereulide intoxications (see Section 3.4). The results from the chronic trial indicated a bioaccumulation of cereulide in certain tissues and organs, such as kidney, liver, muscles and fat tissues [182]. Furthermore, it was demonstrated that cereulide can cross the blood-brain-barrier (BBB) and directly act on the brain [182], which may explain the cerebral effects reported from severe human intoxication cases. For instance, an 11 year old boy in Japan showed acute encephalopathy after consumption of cereulide-contaminated food [15]. The results from the pig intoxication model, as well as the clinical data from case reports, including the rapid onset of symptoms, indicate that the CNS is an important target for cereulide, which warrants further research to fully decipher the mode of action of the cereulide toxin.
3.4. Diagnostic Toolbox for Cereulide
Throughout the last two decades, considerable progress has been made in the development of diagnostic tools for detection and identification of emetic B. cereus as well as for the identification and quantitation of the cereulide toxin and its derivatives. Furthermore, methods have been developed for transcriptional and translational analysis of the Ces-NRPS. An overview of tools available for cereulide diagnostics and research is provided in Figure 3.
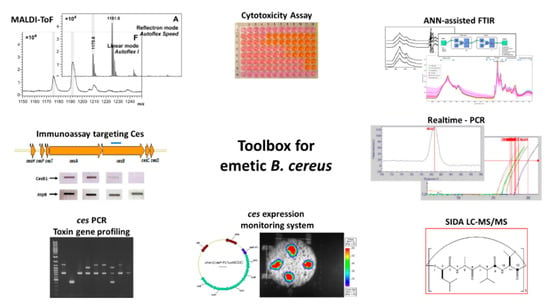
Figure 3.
Toolbox for research and diagnostics of emetic B. cereus and the cereulide toxin. Several tools have been developed, which allow the identification of emetic B. cereus by means of molecular methods, such as conventional polymerase chain reaction (PCR) and real time PCR, mass spectrometry and spectroscopy, such as Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-ToF MS) and artificial neural network assisted Fourier transform infrared spectroscopy (ANN-assisted FTIR). Furthermore, tools have been developed to monitor the ces-NRPS (Non-Ribosomal Peptide Synthetase) expression on a transcriptional and translational level, such as a lux-promotor transcription assay and an immunoassay targeting the cereulide synthetase. In addition, mass spectrometry methods for quantitation of the cereulide toxin and isocereulides and cell culture-based assays for cytotoxicity studies are available, such as stable isotope dilution assay liquid chromatography mass spectrometry (SIDA LC-MS) and Hep-2 cell culture assays. Details of the respective methods are provided in Section 3.4. Images for the composite figure are based on the following publications: [7,36,48,162,165,183,184,185].
The identification of the ces-NRPS gene cluster [155] was a breakthrough in cereulide research and diagnostics. Based on the genetic determinants responsible for non-ribosomal assembly of cereulide, PCR systems for the identification of emetic B. cereus and toxin gene profiling [48,186,187], as well as TaqMan probe-based real time PCR systems for diagnostics [185,188], have been developed. Furthermore, a lux promotor fusion bioluminescence reporter system for non-invasive real-time monitoring of cereulide synthetase promoter activity in different environments was designed [166]. The reporter system was successfully applied to classify food matrices (n = 70) into risk categories, ranging from low-risk to high-risk food [7], and to investigate the effect of food additives on cereulide production [152]. Apart from being a valuable tool in food microbiology, the lux reporter system is also a suitable tool for monitoring ces expression in vivo in the frame of mutagenesis studies [163,164]. The Ces-NRPS-specific antibody targeting the CesB1 module (see Figure 2), which became recently available [165], allows the investigation of the Ces-NRPS machinery on a translational level for gaining insights into posttranscriptional regulatory mechanisms involved in cereulide biosynthesis.
Apart from the progress made in molecular diagnostics of emetic B. cereus, the progress made in mass spectrometry and FTIR spectroscopy in recent years has also significantly improved differential diagnostics of emetic B. cereus. Recently, it was reported that emetic B. cereus and non-emetic B. cereus group strains can be differentiated by Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-ToF MS) based on certain biomarkers [189,190], and machine learning empowered FTIR spectroscopy was successfully employed to discriminate emetic B. cereus and other B. cereus group members using metabolic bacterial fingerprints [36]. In addition, MALDI-ToF MS was also found to be suitable for direct detection of cereulide in emetic B. cereus isolates [184]. For direct identification and accurate quantitation of cereulide in complex matrices such as foods [183,191] or clinical specimens [182], a SIDA LC-MS method was established [183], which provided the basis for an EN-ISO method (ISO 18465:2017) [192] for routine diagnostics. In addition, a SIDA-UPLC-MS/MS method for multiparametric quantitation of cereulide and isocereulides A-G in foods was established [193] to include the most prominent isocereulides [153] in cereulide diagnostics. Application of the latter assay in the investigation of two food-borne outbreaks linked to cereulide revealed that isocereulides were indeed present in considerable amounts in the contaminated food remnants, suggesting that isocereulides may play a yet to be explored role in the severity of food-borne intoxications.
The set of tools for research on emetic B. cereus is complemented by bioassays to investigate the in vitro cytotoxicity of the cereulide toxin and isocereulides using immortalized cell lines, such as Hep-2 or Hep-G2 cells, or, less frequently, CaCo-2 or HeLA cells [8,51,153,179,194,195]. Furthermore, boar sperms have been employed to demonstrate mitochondrial damage by cereulide [196,197].
4. The Diarrheal Enterotoxins
In this section, several features of the three main diarrheal enterotoxins Hbl, Nhe and CytK are discussed, such as their genetic organization, gene expression, toxin secretion, and mode of action. As Hbl and Nhe are both tripartite enterotoxin complexes sharing some similarities, but also significant differences, especially in their mode of action, they are summarized in Section 4.1. The β-barrel pore forming, single protein CytK is reviewed in Section 4.2.
4.1. The Tripartite Enterotoxins Hbl and Nhe
4.1.1. Organization and Evolution of the Enterotoxin Genes
The enterotoxins Hbl and Nhe consist of three protein components each, which are encoded in operons on the chromosome. The Hbl components L2, L1 and B are encoded by hblC, hblD and hblA, respectively [198]. Interestingly, two variants of the hbl operon exist among different B. cereus strains, with the more common one bearing a fourth gene, hblB, which encodes a protein described as hemolysin BL binding component precursor or Hbl B’. Figure 4A illustrates the hblCDAB operon of B. cereus strain F837/76, from which the toxin was originally purified [32,199,200]. Transcription starts 601 bp upstream of the hblC gene and the binding sequence for the global regulator PlcR is shown [201,202]. The genes hblC, D, A and B consist of 1320, 1221, 1128 and 1398 bp, respectively, with separating nucleotides of only 61 bp between hblC and D, and 36 bp between hblD and A. Between hblA and B is a space of 402 nucleotides. Sequence analyses showed 21%–70% similarity (41–82% identity) between the four Hbl components, suggesting that the hbl operon has originated by duplication from one ancestor gene [202]. The authors further suggested that, most recently, hblB has been generated by duplication of hblA and a C-terminal fusion with another ORF (open reading frame), as the C-terminal part of hblB is not similar to hblA. hblB was first identified sequencing the hblA gene of strain F837/76 [203]. Ryan et al. first showed that hblC, D and A are co-transcribed and assumed this also for hblB [198]. On the other hand, a stem loop has been identified upstream and downstream of hblB, which might act as transcriptional terminator [204]. For a long time, hblB was considered a pseudogene, as the hblCDA transcript seemed to terminate within [5,201,205] or upstream [204] of hblB. Only in 2010 did secretome analyses under different redox conditions reveal that hblB is indeed transcribed, translated and exported at detectable levels in the early secretome of the B. cereus type strain ATCC 14579 [206]. Moreover, mRNA analyses pointed to the fact that hblB may be expressed as a single, monocistronic transcript with an own promoter structure and transcriptional start side.
The three Nhe components A, B and C are encoded by nheA, nheB and nheC, respectively [207]. Figure 4B shows the nhe operon of B. cereus strain NVH 0075-95, from which the toxin components A and B were originally purified after a large food poisoning outbreak in Norway in 1995 [33,208]. Only after sequencing of the NheA and B encoding operon was the existence of NheC proven [207]. The genes nheA, nheB and nheC consist of 1161, 1209 and 1080 bp, respectively. Between nheA and nheB is a space of only 40 bp, and between nheB and nheC 109 bp. Northern blotting revealed that all three genes encoding the Nhe toxin components are transcribed in one polycistronic mRNA [209]. Nevertheless, inverted repeats between nheB and nheC and after the nheC stop codon were identified by sequencing [207], and are shown as stem loops in Figure 4B. Furthermore, the intergenic region between nheA and nheB could be amplified, but any attempt to amplify the intergenic region between nheB and nheC did not succeed [209]. Interestingly, two promoter regions with two PlcR binding sites were identified upstream of nheA [54,91,201,209]. Additionally, unusually long 5′ untranslated regions exist upstream of the hbl and nhe transcription start sites, which were not found upstream of cytK-1 or cytK-2 [210]. Though the tripartite enterotoxins are known to be encoded on the chromosome, one study provided evidence for a second operon encoding all three Nhe components (42%–56% sequence identity) on a large plasmid in B. weihenstephanensis strain KBAB4 [211]. Evidence has been found that redundant genes in the B. cereus group are often carried on plasmids, but that their transcription is differently regulated [212].
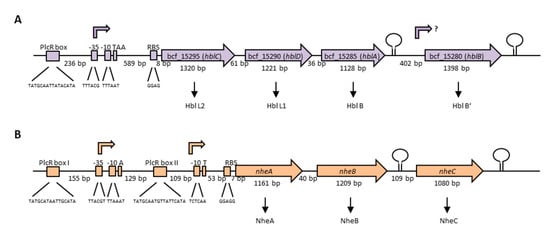
Figure 4.
Genetic organization of the operons encoding the tripartite enterotoxins of B. cereus. (A) The hblCDAB operon of strain F837/76, encoding the proteins Hbl L2, L1, B and B’ [198,199]. Upstream and downstream of hblB, a stem loop was identified [204]. The promoter region, as well as the transcriptional start TAA is shown [201]. 236 bp upstream of the promoter, a PlcR-box is located [202]. (B) The nhe operon of strain NVH 0075-95, encoding NheA, B and C [91,210]. Two PlcR-boxes as well as two possible transcriptional start sites exist [201,209,210]. Upstream and downstream of nheC, a stem loop was identified [207,210].
Figure 4.
Genetic organization of the operons encoding the tripartite enterotoxins of B. cereus. (A) The hblCDAB operon of strain F837/76, encoding the proteins Hbl L2, L1, B and B’ [198,199]. Upstream and downstream of hblB, a stem loop was identified [204]. The promoter region, as well as the transcriptional start TAA is shown [201]. 236 bp upstream of the promoter, a PlcR-box is located [202]. (B) The nhe operon of strain NVH 0075-95, encoding NheA, B and C [91,210]. Two PlcR-boxes as well as two possible transcriptional start sites exist [201,209,210]. Upstream and downstream of nheC, a stem loop was identified [207,210].
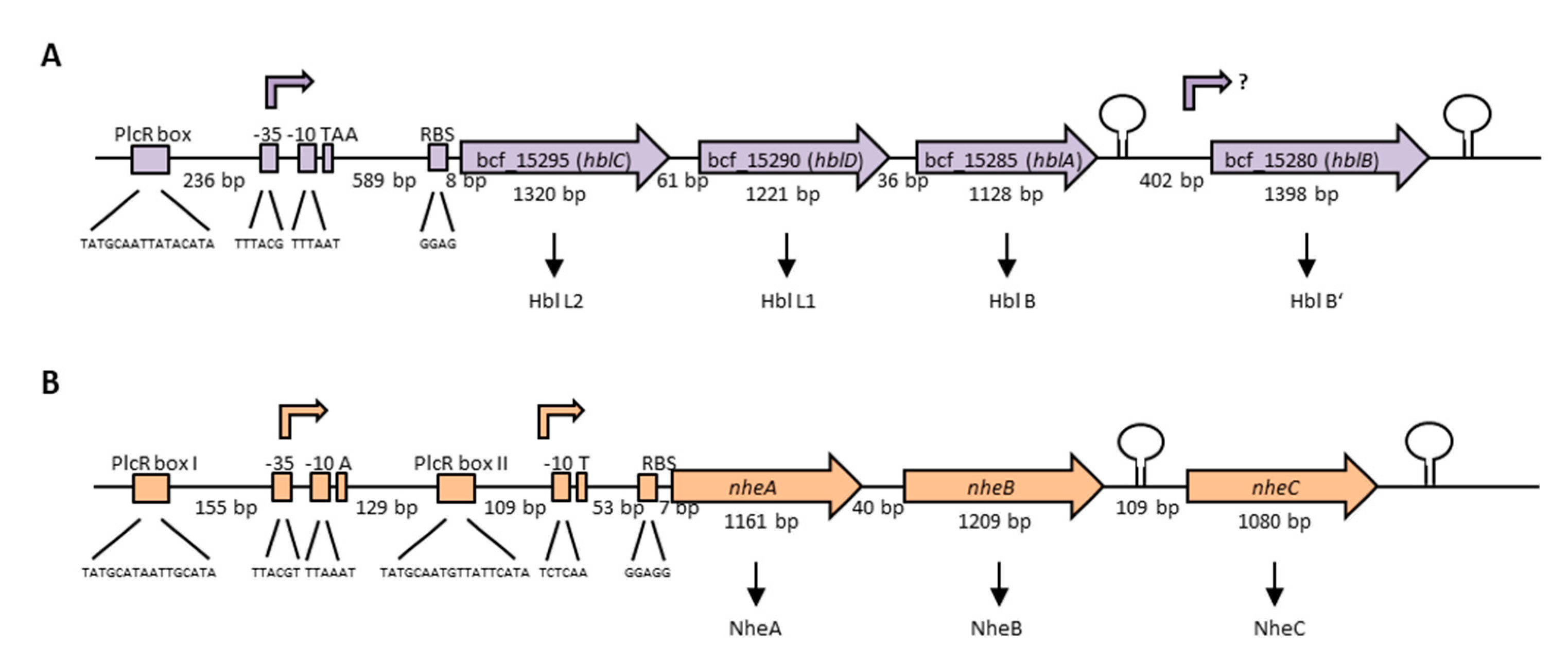
In any case, total genome sequencing of selected enteropathogenic and apathogenic strains revealed the presence of two nhe operons in moderately toxic strain 14294-3 (M6) and low toxic MHI 226, isolated from ice cream and a milk product, respectively. Moreover, two hbl operons were detected in 14294-3 (M6), moderately toxic strain 6/27/S isolated from human feces, high toxic strain RIVM BC 126 from human feces, and low toxic strain RIVM BC 934 isolated from salad [54,91]. Duplication of the enterotoxin operons seems thus not to correlate with toxic activity. It has been shown in earlier studies that different strains can harbor both variants of the hbl operon (see above), and thus, express homologous sets of Hbl proteins. These are the more common Hbl L2, L1, B (and B’) from the hblCDAB operon and the rarer variants Hbl L2a, L1a and Ba from the hblCDA operon, each set of proteins with distinct properties. While the hblCDAB operon is highly conserved among different strains (sequence identities of 97%–99%), the hblCDA operon shows 75%–82% identity towards hblCDAB [91,211,213,214]. Our own studies also suggested high conservation of the nhe operon among different B. cereus strains (93%–99% similarity); however, these were all enteropathogenic, ces-strains from phylogenetic clades I and II [91]. An earlier study showed a novel Nhe variant in three B. cytotoxicus strains with only 80% protein sequence identity compared to known Nhe proteins [215]. In a study where 81 B. cereus s. l. strains were compared, it was shown that the upstream regions of nheABC were more conserved than the downstream regions [216]. Moreover, NheA protein sequences were sorted into four groups, according to their identities ranging from 100% to 78%. NheB protein sequences were most conserved, ranging from 100% to 87% identity in three groups. The least conserved component was NheC with 100% to 73% identity assigned to three groups of strains. As observed before, B. cytotoxicus strain NVH391-98 showed least conservation [216]. Earlier observations suggested that variations appear especially in sequence and length of the intergenic regions between nheB and nheC [5].
Sequence identities have not only been found between the three components of each enterotoxin, but also between the two toxins, with Hbl L2 and NheA sharing 23% sequence identity, Hbl L1 and NheB 40%, and Hbl B and NheC 25% [217]. Thus, it is speculated that the nhe and hbl genes arose from duplication of a single ancestor gene and that the latest duplication was the generation of nheB and nheC [5]. Böhm et al. investigated the evolution of chromosomally encoded enterotoxins. Phylogenetic analyses of 142 sequenced B. cereus s. l. strains revealed evidence for horizontal gene transfer, duplication, and frequent deletion of hbl. The duplicated hbl variant hbla was found in 22% of all genomes. Earlier studies found that the hblCDAB operon is part of an approximately 17.7-kb, 11 gene fragment, which has probably been acquired by transposal insertion [202,218]. For this, further evidence could not be found [54]. In contrast to hbl, duplication or deletion of nhe was hardly observed (0.02%). Stable horizontal transfer of the operon also seems to be rare; it is almost exclusively transmitted vertically [54]. Due to its altered structure, some studies speculated about an additional function of Nhe requiring interaction with further, so far unknown proteins [219], which is supported by the strict vertical evolution, the missing horizontal gene transfer, and the rare and instable duplication of the nhe genes. Loss or horizontal transfer of nhe has even been associated with a negative impact on the survival of the bacteria. Furthermore, horizontal transfer of cytK and plcR was observed, as well as frequent deletion of cytK. Altogether, the authors concluded that the evolution of the enterotoxins inside the B. cereus group is extremely variable, for so far unknown reasons [54]. Widespread lateral gene transfer within the B. cereus group and the great importance of horizontal gene transfer in the evolution of the enterotoxins have been suggested earlier, when 47 food-borne isolates were compared using MLST [220]. The authors also found a broad distribution of nhe and hbl, while cytK was limited to some phylogenetic clusters, and probably lost in distinct lineages. It was further concluded that gene loss is not linked to a particular species, and that the hbl, nhe, cytK (and entFM) genes were already present in a common ancestor of lineage I. Moreover, three toxin gene patterns showed a widespread coinheritance: hbl and nhe; hbl, nhe and entFM; and nhe and entFM [220]. Earlier, several studies already showed that horizontal gene transfer within B. cereus s. l. is generally possible, involving plasmids or further transposable elements [218,221,222,223,224]. Evidence has been found that massive gene exchange between plasmids and the chromosome, not restricted to distinct regions, occurred during evolution of the B. cereus group [212]. However, no ces genes have been found in the chromosome so far, and plasmid-mediated horizontal gene transfer of the emetic toxin, anthrax and insecticidal toxins is already known [54]. Rasigade et al. investigated 10 complete B. cereus s. l. genomes in search of genes under positive evolutionary selection and concluded that “adaptation to animal hosts, whether as pathogens, saprophytes or symbionts, is the major driving force in the evolution of the B. cereus group” [225].
4.1.2. Enterotoxin Gene Expression and Toxin Secretion
Among the multitude of studies determining and identifying enterotoxin genes and pattern/profiles, it was repeatedly attempted to link them to cytotoxicity. By now, it is commonly accepted that the presence of enterotoxin genes hardly reveals evidence on the toxic potential of a certain isolate, especially as there are strains with identical enterotoxin gene profiles and even nearly identical toxin gene sequences producing highly variable amounts of toxins [91,226,227]. If not the enterotoxin genes, what lies beneath this extreme variability of toxin production? The answer is hidden in the complex, interwoven and not yet fully understood processes of enterotoxin gene transcription, posttranscriptional and posttranslational modification, and protein secretion.
The expression of the enterotoxin genes is highly complex and probably strain-specifically affected by environmental factors such as temperature, pH, oxygen tension, and nutrient (carbohydrate, nitrogen) availability, as well as by intrinsic factors such as the growth or the general energetic status of the cell [163,169,228,229,230,231,232,233,234]. Next to the PlcR-boxes (see Figure 4), binding sites for further transcriptional regulatory proteins such as CodY, ResD, Fnr, CcpA and SinR have been identified in the promoter regions of the hbl and nhe operons, which presumably control enterotoxin expression in a concerted action [169,210,230,233,235,236]. Many of the virulence factors secreted by B. cereus including Hbl and Nhe are under the control of the 34 kDa pleiotropic transcriptional regulator PlcR, which activates their gene transcription at the onset of the stationary growth phase [169,201,202,237,238]. Nutrient depletion leads to repression of plcR transcription itself via the sporulation regulator Spo0A-P [239]. Furthermore, the expression of plcR is positively autoregulated, but is also influenced by the YvfTU two-component system, whose encoding genes lie in proximity to plcR [238,240]. PlcR is part of a quorum-sensing system requiring also the 48 amino acid signaling peptide PapR, which is encoded 70 bp downstream of plcR and exported, processed and re-imported as heptapeptide PapR7 into the cell via the oligopeptide permease OppABCDF [168,241,242,243]. Evidence has been found that the secreted neutral protease B (NprB) is required for extracellular PapR processing [244]. At high cell densities, PapR is accumulated inside the cell and functions as autoinducer for PlcR. Especially the C-terminal regions of the two partners seem to be important for their interaction [244]. The PlcR-PapR complex can then bind the PlcR box, a specific target site in the promoter region with a conserved, palindromic sequence (TATGNANNNNTNCATA), which activates gene transcription [169,201,239,242]. Crystallization revealed an asymmetric, dimeric structure of PlcR complexed with PapR, including an N-terminal helix-turn-helix DNA binding domain and a C-terminal 11 helix domain interacting with the autoinducer. It was also suggested that this interaction causes further oligomerization of the PlcR dimers establishing a right-handed spiral interacting with the DNA [168]. Crystal structures of the apo-form of PlcR and the ternary complex with PapR and the PlcR box showed major conformational changes followed by binding of the helix-turn-helix domains in the major groove of the two half sites of the PlcR-box, revealing the mechanism of PapR-PlcR transcriptional activation [245]. Nonetheless, the PlcR-PapR system is also subject to strain-specific variations. Slamti et al. found different types of mutations in the plcR gene responsible for hemolysis- and lecithinase-negative phenotypes [246]. In B. anthracis, the PlcR regulon is silenced due to a nonsense mutation in plcR, which can be restored by the expression of a functional PlcR-PapR fusion protein [247,248]. PlcR activation due to PapR has also been shown to be strain-specific, involving especially the first amino acids of the peptide. Four classes of PlcR-PapR pairs were distinguished leading to four distinct pherotypes (based on pheromone-receptor association) among the B. cereus group [249]. Variability in enterotoxin transcription is further induced by the presence of two transcriptional start sites with two PlcR boxes upstream of the nhe operon [201,209]. Interestingly, newer studies have found a second quorum-sensing-system, consisting of PlcR-paralog PlcRa and the signaling peptide PapRa7, which is involved in oxidative stress response and cysteine metabolism. PapR variants were also able to activate PlcRa, which showed a connection between the PlcR and PlcRa regulons for the first time [250,251]. Recent studies focus on the inactivation of the PlcR-PapR quorum sensing system and, thus, the inhibition of virulence factor production due to synthetic PapR7 derivatives. Furthermore, it was found that especially proline and glutamic acid residues of PapR play an important role in PlcR regulon activation [252,253].
Next to PlcR, the pleiotropic regulator CodY is strongly involved in enterotoxin gene expression in B. cereus [163,254]. Generally, this conserved transcriptional repressor senses nutrient availability and the energy status of the cell in low GC Gram-positive bacteria by binding GTP or branched-chain amino acids. Under nutrient starvation, genes of the CodY regulon involved in motility, chemotaxis, catabolism, and virulence are derepressed in complex ways, directly or indirectly [170,255,256,257,258,259,260,261,262,263,264]. First studies with codY deletion mutants in B. cereus pointed to strong effects of the regulator on the PlcR-PapR quorum sensing system and assigned CodY a key role in pathogenicity, toxin production and adaptation to nutrient starvation [163,254]. On the one hand, CodY influences enterotoxin gene expression directly via binding to specific target sites in their promoter regions. Upstream of the nhe and hbl operons, long 5′ untranslated regions were detected, in which one (hbl) and even two (nhe) CodY binding site were identified [210]. Furthermore, the length of these regions also influences promoter activities. While PlcR binding sites are conserved inside the B. cereus group, higher variability has been found among the CodY recognition sites. This leads to diverging affinity of the regulator to its target promoter regions and thus can be one explanation for the high strain-specific variability in enterotoxin production [210]. On the other hand, CodY exerts influence on PlcR by regulating the re-import of the signaling peptide PapR. It has been shown that CodY controls the production of the oligopeptide permease OppABCDF as well as further Opp-like proteins [265,266].
In the promoter region of the hbl operon, three putative ResD and two putative Fnr binding sites are located, while two putative ResD and one putative Fnr recognition sites have been identified upstream of the nhe genes [210,229]. The two-component system ResDE was shown to control fermentative growth and enterotoxin expression under low oxidoreduction potential anaerobic conditions [229]. ResD is the response regulator and ResE the corresponding sensor kinase. ResD directly binds to the promoter regions of hbl and nhe, as well as resDE, fnr, and plcR [235]. It has also been shown that phosphorylation increases ResD interaction with resDE and fnr promoters, and that ResD and Fnr bind their target DNA in a concerted action. It was further suggested that ResD acts as anti-activator, and ResD-P as an activator of Fnr [235]. Fnr is a redox regulator necessary for fermentative growth under low oxidoreduction potential, as shown in an fnr deletion strain of B. cereus. Furthermore, expression of the hbl and nhe operons was significantly decreased in the mutant strain [234]. Interestingly, Hbl production was more strongly affected by the deletion of fnr than Nhe production [267]. It has been shown that monomeric apoFnr binds to the promoter regions of hbl and nhe, as well as fnr, resDE, and plcR, and thus, activates their expression [236]. Furthermore, Fnr is not only important for carbon source regulation in B. cereus, but also for the regulation of enterotoxin gene expression in response to carbohydrates [267]. It has also been proven that Fnr forms a ternary complex with ResD and PlcR. An Fnr monomer binds one [4Fe-4S]2+ cluster, which is, though, not required for DNA binding or formation of the complex [268]. Another intriguing fact is that Fnr positively regulates Escherichia coli ClyA (see Section 4.1.3) [269]. It has been concluded that B. cereus might induce enterotoxin gene expression as part of a compensatory metabolic pathway aiming to maintain the intracellular redox state in proximity to the intestinal epithelium [270].
The hbl and nhe operons are also controlled by CcpA-mediated catabolite repression. CcpA is a transcriptional repressor binding to promoter regions at specific, palindromic catabolite responsive elements (cre-sites), thus controlling glucose catabolism while repressing gluconeogenesis and alternative metabolic pathways [271]. Potential cre sites have been found upstream of the hbl and nhe operons [5,210,233]. During anaerobiosis, in the stationary growth phase and under glucose surplus, the expression of the operons is repressed [228,233]. Reversely, using sucrose or fructose resulted in enhanced Hbl and Nhe production compared to glucose as carbon source [272,273]. Similar to Fnr, CcpA seems to affect hbl and nhe expression differently, as deletion of ccpA resulted in higher expression of nhe compared to hbl [233].
Additionally, the phase-transition regulator SinR is involved in enterotoxin gene expression. SinR and its antagonists SinI, SlrA, and SlrR interactively regulate biofilm formation in Bacillus subtilis [274,275]. Deletion mutants revealed that Spo0A, AbrB and SinI/SinR control biofilm formation and swimming motility in B. thuringiensis [230]. Furthermore, hbl transcription was shown to be controlled by SinI/SinR together with PlcR. Interestingly, only a small subpopulation of cells in biofilms expressed hbl, which corresponded to sinI expression [230]. Furthermore, two SinR recognition sites were found upstream of the hbl operon, while one SinR binding site was identified upstream of the nhe operon, in close proximity to an Fnr binding site as well as a cre element [210]. An essential role of the spo0A-sinI-sinR regulatory circuit has also been shown for biofilm formation, cell differentiation, nematocidal activity, and bacteria-host interactions of B. cereus AR156 [276].
Beyond the six verified transcriptional regulators PlcR, CodY, ResD, Fnr, CcpA and SinR, further regulatory mechanisms of enterotoxin expression have been suggested. On the one hand, expression of hblB might be independent from the hblCDA operon due to its putative own promoter [204,206]. Microarray and qRT-PCR analyses already revealed two-fold upregulation of hblB in hyperflagellated swarm cells [277]. Furthermore, expression of hblB was found to be 126-fold down-regulated in the presence of mucin, while nheB and hblA were 100-fold and 110-fold up-regulated, respectively [278]. This observation confirmed a separate, so far unknown regulatory mechanism. Moreover, Clair et al. showed that Hbl and Nhe secretion is influenced by the OhrRA system, which is involved in response to redox changes. It was suggested that OhrA affects toxin secretion mainly post-transcriptionally and that OhrR might even be a transcriptional repressor for hblCDAB and nheAB [279]. A deletion mutant of transcriptional regulator RpoN (sigma 54) was impaired in NheA production [280]. Especially the unusually long 5′ non translated regions of the hbl and nhe operons might harbor additional regulatory mechanisms. Intergenic regions encoding small regulatory peptides or proteins are increasingly found in prokaryotes [281]. Upstream of the hbl operon, a potential ORF encoding a so far unknown protein has been identified [210]. Furthermore, these unusually long sequences might host temperature-sensitive RNA thermometers or metabolite-responsive riboswitches [210] which, however, have not been identified upstream of the enterotoxin genes in B. cereus so far.
It has also been shown that several host factors influence B. cereus enterotoxin expression. Besides temperature, oxygen tension, nutrient availability, growth phase etc. (see above), the host epithelium is of particular interest. Very recently, we identified massive transcriptional changes in B. cereus after contact with mucin, including genes encoding enterotoxins (Hbl, Nhe) and further putative virulence factors. The number of secreted enterotoxins was also increased in various B. cereus strains, depending on mucin concentration [278]. Previously, we showed that the secretome of CaCo-2 cells influences entero-toxin gene expression and toxin production [92]. Growing for only two hours in cell culture medium pre-incubated with CaCo-2 cells mimicking intestinal conditions, enterotoxin gene expression of different B. cereus strains was activated and total protein secretion was enhanced. Similarly, germination of spores from eight out of 11 enterotoxic B. cereus strains was triggered by differentiated CaCo-2 cells in an earlier study. The germinant, which still needs to be identified, is secreted by the cells, stable towards heat and proteolysis, and most likely bound or degraded by the spores [282]. Our own studies proved that the factor triggering enterotoxin production under simulated intestinal conditions also originates from the CaCo-2 cells, is used up by the bacteria, and affects high as well as low toxic B. cereus strains [92]. Furthermore, the molecule is small, <3 kDa, heat stable, is secreted by the cells long before contact with the bacteria, is not restricted to CaCo-2 cells, but is rather secreted by various cell lines from different organisms and compartments, independently from cell differentiation (unpublished data).
As mentioned above, B. cereus increases total protein and thus enterotoxin secretion when sensing the host environment [92,278]. The amino acid sequences of all Nhe and Hbl components contain N-terminal signal peptides for secretion, indicating toxin secretion via the Sec translocation pathway. Modification of this signal sequence in Hbl B led to loss of secretion and thus, to intracellular accumulation of the protein. It was additionally shown that sodium azide, an inhibitor of SecA, reduced secretion of the enterotoxins and enhanced their intracellular accumulation [283]. The Sec translocation machinery consists of SecYEG (pore), SecDF and the ATPase SecA. The number of enterotoxins in the secretome was clearly reduced in a ΔsecDF mutant. Further enzymes such as chaperones or peptidases are also involved in the secretion process [284,285]. On the other hand, evidence has been found for an involvement of flagella in the export of several virulence factors. The flagellar export apparatus, which resembles the type III secretion system in Gram-negative bacteria, can also be used for secretion of non-flagellar proteins [286]. Motility and virulence are connected in B. cereus via flagella, as flagellin expression and motility were decreased in a plcR mutant [237,287]. Deletion of flhA and lack of flagella led to defective secretion and to intracellular degradation of Hbl, respectively [288,289,290]. Furthermore, hblC transcription was impaired in the flhA mutant [291]. The correlation between swarming and hemolysin BL secretion in 42 B. cereus group isolates confirmed this connection [289]. Similarly, an flhF (encoding a signal recognition particle (SRP)-like GTPase) deletion resulted in decreased secretion of flagellin, Hbl and further virulence factors, as well as in an increase of NheB and other virulence factors in the secretome [290,292]. This was confirmed by the differential expression of 118 genes during swarming, including flagellar genes and the hbl operon [277]. Very recently, it was shown that FlhF does not interfere with the expression of hblC, but that it binds the Hbl L2 protein and recruits it to the plasma membrane for secretion [293]. Thus, motility and flagella are intensely related to pathogenicity, and especially to Hbl secretion [290,292,293,294,295,296]. As the sizes of the Hbl components found in the secretome match those calculated for the proteins lacking the N-terminal signal peptides for secretion, it has been suggested that signal peptidases collaborate with the flagellar export system for Hbl secretion [296]. Secretion of Nhe seems to be rather independent from the flagellar export system and, thus, is mediated exclusively by the Sec translocation pathway.
Multiple attempts have been made to predict the toxic potential of newly isolated B. cereus strains [27,297]. This is a difficult venture, as factors such as toxin gene expression, secretion and protein stability are highly diverse among members of the PlcR regulon within one single strain [298]. Additionally, the different regulatory mechanisms from enterotoxin gene transcription to toxin secretion summarized in this section probably underlie extreme strain-specific variations.
4.1.3. Structure and Mode of Action of the Enterotoxin Complexes
When hemolysin BL was originally purified from B. cereus strain F837/76 isolated from a postoperative wound [299], a molecular weight of 45, 36, and 35 kDa was postulated for the three components L2, L1 and B, respectively. First, only a binding and a lytic component were identified, which was later corrected to three components [204,300,301]. Hbl owes its name to its ability to lyse sheep erythrocytes [32,200]. As two Hbl encoding operons exist (see Section 4.1.1), distinct homologues of each protein varying in size can occur between B. cereus isolates or in one single isolate [54,213,214]. In early studies, it was shown that Hbl is involved in fluid accumulation in rabbit ileal loops, is active in vascular permeability tests, and has dermonecrotic, hemolytic and cytotoxic properties [32,200,213,302,303,304,305,306]. Interestingly, functional differences were found between Hbl and Hbla (see Section 4.1.1), as Hbla did not lead to the Hbl-typical ring-shaped hemolysis zone phenomenon on blood agar [213]. Though Hbl was the earlier enterotoxin to be discovered in B. cereus, determination of its toxic activity and mode of action has been difficult for a long time, as nearly all natural B. cereus isolates additionally bear and secrete Nhe (see Section 2.1). Furthermore, it was largely believed that Nhe or at least NheA might have an essential function for B. cereus [5,54], and several attempts to generate complete nhe deletion strains failed [217,307]. Further difficulties were faced creating recombinant Hbl components, and it was even suggested that they might be toxic for E. coli [198,203,304]. These issues were resolved years later, when functional recombinant Hbl proteins could be overexpressed in B. anthracis [204] and E. coli [308], and the nhe operon was successfully deleted in strain F837/76 [308].
NheA and B were first isolated from hbl-negative strain NVH 0075-95, which caused a large food poisoning outbreak in Norway [33,208]. Initially, NheC could not be identified, possibly due to its low expression compared to NheA and B, which has been proved in later studies. Basically, sequencing of the nhe operon led to the identification of nheC, and the corresponding protein was shown to be part of the Nhe toxin [207,209]. The molecular weight of the three components was determined as 41 kDa (NheA), 39.8 kDa (NheB) and 36.5 kDa (NheC) [207]. However, also for Nhe strain-specific variations exist [5]. All three recombinant Nhe protein components were successfully overexpressed in B. anthracis [204] and, just recently, in a eukaryotic cell-free protein expression system [309]. The Nhe and Hbl proteins share sequence identities, both between the three components of each complex and between the two enterotoxin complexes. Hbl L2 and NheA share 23% sequence identity, Hbl L1 and NheB 40%, and Hbl B and NheC 25%. Highest identity was found between NheB and NheC (44%) [217]. Similarities were also found when predicted transmembrane helices were compared. Of these, NheA and Hbl L2 have none, NheB and Hbl L1 have two, and NheC and Hbl B have one [5,207,310]. When all six enterotoxin components were recombinantly produced in B. anthracis, it was proven that they are not arbitrarily interchangeable among each other. Thus, the Hbl pore cannot be complemented by any Nhe component and vice versa [204].
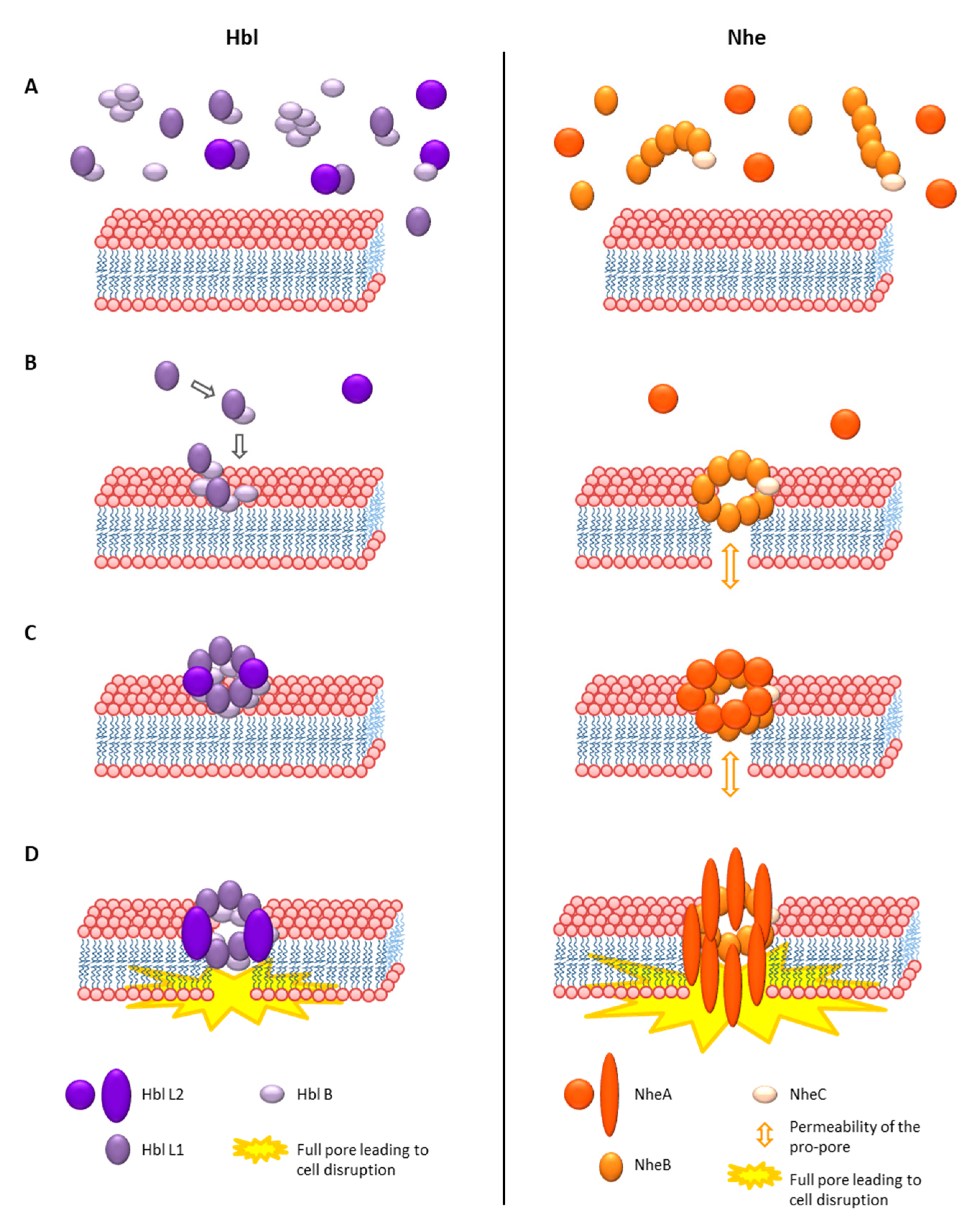
As Hbl and Nhe showed no sequence homologies to any other known toxins, they could not be assigned to a group of toxins for some time. However, the crystal structure of Hbl B, which was determined in 2008, revealed great structural similarities to Cytolysin A (ClyA) from Gram-negative bacteria [311]. Thus, Hbl and Nhe were assigned to the ClyA superfamily of α-helical pore forming toxins, which was confirmed later by the crystal structure of NheA [217,219,311,312]. The 34 kDa, hemolytic and pore-forming protein ClyA or SheA (silent hemolysin A) or HlyE (hemolysin E) is one of the best characterized α-pore-forming toxins [313]. Gene expression of clyA is repressed by the nucleoid-associated protein H-NS, and positively influenced by the regulator SlyA, as well as under anaerobic conditions by the above mentioned Fnr [314]. Cytotoxicity evolves from the formation of 3 nm cation selective pores after cholesterol binding on the cell surface [315,316]. The monomer consists of a tail domain composed of four 80–90 Å α-helices (α-A-C and α-F) and the fifth, 35 Å α-G helix, as well as a head domain composed of two antiparallel β-sheets, the characteristic β-tongue [317]. In contact with lipids, cell membranes or detergents, the protein oligomerizes and forms ring-shaped structures acting as transmembrane pores [317,318]. The overall, 400 kDa pore complex consisting of 12 monomers is formed via huge conformational changes, reorganization of the hydrophobic core and alterations of the β-sheets and the loops in the α-helices [313,317,318,319]. The mechanism of pore formation is significantly different from other known toxins. First, linear oligomers of different sizes are built, then they assemble as pairs, which are able to directly close the pore complex ring, which makes pore formation extremely fast and efficient [313,319,320]. A current study shows that ClyA can permeabilize membranes even before complete pore formation, letting particles smaller than 400 Da pass through [321]. The full pore resembles a hollow cylinder of 130 Å height, an outer diameter of 105 Å and the smallest inner diameter of 35 Å. The inside is negatively charged underlying the cation selectivity of the pore [313,315]. The hydrophobic β-tongue is assumed to be inserted into the membrane first [319].
The crystal structure of Hbl B shows some remarkable similarities to ClyA, such as the large bundle of five α-helices and the head domain with hydrophobic β-sheets. Its size is approximately 90 Å × 40 Å × 30 Å [311]. Based on these similarities, the authors suggested that Hbl B alone might be able to oligomerize to a heptamer or octamer and form a pore. In that case, Hbl L1 and L2 were either responsible for conformational changes of Hbl B or for the stabilization of the head domain. It was also speculated that these toxin components might infiltrate the cell, similarly to anthrax toxin [311]. As mentioned, Hbl L2 itself does not possess hydrophobic sequences in its head domain. On the other hand, those of Hbl L1 are even longer than the hydrophobic regions of Hbl B. Thus, the theory came up that Hbl L1 might be able to form two complete transmembrane helices [110,219]. Due to the sequence identity of NheB and NheC towards Hbl B, homology models based on the Hbl B crystal structure were established. Their structures also contain a bundle of four α-helices wrapping around each other in left-handed supercoils, a shorter fifth carboxy-terminal helix, as well as a β-hairpin correlating with the hydrophobic regions of the proteins [217]. Resolving the crystal structure of NheA showed a shortened N-terminal α-helix compared to ClyA, which might point to a significant difference in pore formation. Despite a generally similar structure to Hbl B or ClyA, the head domain of NheA is significantly enlarged [219]. Furthermore, the typical hydrophobic β-hairpin is exchanged for an amphipathic hairpin connected to the main protein in a manner known from S. aureus β-pore forming toxins. Thus, the authors concluded that this β-tongue is not able to form a ClyA-like hydrophobic transmembrane helix, leading to the thesis that NheA, after binding to the NheB-C pro-pore, forms a transmembrane β-pore, analogously to S. aureus α-hemolysin [219,322]. Despite all homologies, this would be a fundamental difference in the mechanism of pore formation of Hbl and Nhe.
Hbl pore formation was first shown by osmotic protection assays [300]. The authors also suggested that all three Hbl components can bind individually to erythrocytes and form a “membrane attack complex”, which finally leads to cell lysis. Early experiments with rabbit ileal loops also suggested equimolar amounts of Hbl L2, L1 and B for toxic activity [304], or lower amounts of Hbl L2 or L1 compared to B for maximal hemolytic activity [305]. In these early studies, the discontinuous ring-shaped hemolysis zone phenomenon was also discovered, which is characteristic for Hbl [32,300,304]. When Hbl diffuses through blood agar from a particular point, hemolysis is observed at a certain distance from that point. This is presumably caused by the variable diffusion velocity of the three single Hbl components leading to a continuous concentration gradient. Hemolytic activity occurs only at the point of optimal concentration ratio [32,300,304]. In early studies, surplus of Hbl B or L1 seemed to hinder this activity [300,323]. On the contrary, current studies show that the hemolytic activity is determined by the amount and the diffusion velocity of Hbl B. Excess of B enhances the outer hemolytic ring, and excess of L1 inhibits it, while the amount of L2 seems not to influence it [301].
What became obvious is that all three components are necessary for biological (hemolytic or cytotoxic) activity, and that a defined concentration ratio is required. More recent studies oppose the theories of a membrane attack complex or the oligomeric Hbl B pore (see above). Using Hbl proteins recombinantly produced in B. anthracis and E. coli, it was shown that only Hbl B can bind to the surface of target cells. Thus, the current model is that the three components bind to the target cell surface sequentially in the required binding order for pore formation B-L1-L2 [204,301]. Hbl B alone shows neither cytotoxic activity nor pore formation on target cells [204,301] or artificial lipid bilayers [324]. On the other hand, it has been determined that the Hbl components form complexes in solution prior to cell binding, and that Hbl B, recombinant, as well as native in B. cereus culture supernatants, is mainly present in those complexes. Recombinant Hbl B binds to L1 with a KD value of 4.7 × 10−7 M, and Hbl L2 to L1 with a KD value of 1.5 × 10−7 M. Binding of Hbl L2 to B was observed only in a certain constellation and is comparably weak, with a KD value of 3.4 × 10−6 M [308]. Furthermore, Hbl B alone was able to form up to 600 kDa multimeric complexes. Those complexes, which still showed pore-forming activity, also comprised Hbl L1 and L2 [324]. Another interesting fact is that heat treatment led to a decrease of pore-forming and cytotoxic activity of Hbl at approximately 60 °C, and a subsequent increase at higher temperatures. The latter is caused by releasing Hbl B monomers from the tight complexation observed earlier, thus enhancing Hbl B binding to the target cell surface [324]. These initially contradictory findings, of only Hbl B binding to the cell surface, and of its tight complexation, can nevertheless be equally appropriate. Similar to the above described ClyA mechanism, homo-(Hbl B) as well as hetero-oligomers (Hbl B-L1 and L1-L2) would be present in solution, letting the final assembly of the pore at the cell surface take place immediately. Rapid Hbl pore formation has been shown [227,301]. However, not only do the binding order and pre-formed complexes play a role in Hbl pore formation, but also to a large extent the concentration ratio of the three components. Most rapid pore formation was detected with ratios L2:L1:B = 1:1:10 and 10:1:10, meaning that surplus of Hbl B enhances, and surplus of Hbl L1 hinders the velocity of pore formation, while the amount of Hbl L2 seems not to be crucial [301]. It has further been proven that binding of Hbl B to the cell surface is the most essential step for pore formation and cytotoxic activity. Most interestingly, this binding step is enhanced in the presence of Hbl L1 [301], similarly to the presence of certain Hbl B-specific monoclonal antibodies, which has been described as antibody-dependent enhancement [308,325]. Again, these findings attribute a major role in Hbl pore formation to the pre-formed B-L1 complexes, which seem to be as necessary as sufficient amounts of free Hbl B. Recently, new insights into the properties of the Hbl pores were gained. Experiments on planar lipid bilayers demonstrated that the Hbl pores have a rather small single channel conductance of around 200 pS and a probable channel diameter of at least 1 nm [324]. This correlates with earlier observations from osmotic protection experiments using carbohydrates, in which the channel diameter was estimated at 1.2 nm [300]. Moreover, the Hbl pores seem to be highly instable and of a limited lifetime, as well as slightly cation selective [324].
Detailed investigations of the mode of action of Nhe have also been conducted. Generally, these made faster progress compared to Hbl, as no second enterotoxin complex disturbed the tests, and natural [326,327,328] as well as constructed [217] mutants of the single Nhe components existed. When Nhe was first discovered in the 1990s, no hemolysis on sheep blood agar could be detected, thus it was mistakenly described as “non-hemolytic”. However, Nhe shows hemolytic activity against erythrocytes depending on the species used. It is furthermore cytotoxic towards different epithelial cells, as shown by loss of ATP and LDH [217]. This study initially described Nhe as a pore forming toxin producing large conductance channels, which leads to osmotic swelling and, finally, lysis of the epithelial cells. All three Nhe components are necessary for maximum biological activity in vitro, which also requires a specific concentration ratio (NheA:B:C = 10:10:1) [209]. In a more recent study, varying ratios were detected in 12 B. cereus isolates, of which some showed a slightly higher (3:1) or a slightly lower (1:2) NheA:B ratio, which did, however, not influence the toxic activity [329]. A specific binding order of the three components is also necessary for pore formation [209]. In the study of Lindbäck et al., only NheB was able to directly bind to the surface of the target cell, and binding was abolished by excess of NheC. On the other hand, it has also been suggested that NheB as well as NheC can bind independently to the target cell surface and that NheC presence is mandatory in the first, and NheA in the final step of pore formation [328]. However, NheB and C form highly affine and stable complexes in solution (KD value of 4.8 × 10−10 M [330]), which impedes the detection of NheC in the supernatant of B. cereus cultures via specific antibodies. This might be the reason why NheC has initially not been detected as part of the Nhe toxin complex [33,207,306], and why it is generally not found in the B. cereus culture secretome or only in very low concentrations [53,226,248]. The approximately 620 kDa NheB-C complexes consist of at least one NheC molecule and up to 15 NheB molecules [330]. Furthermore, the Nhe components seem to be additionally processed in the extracellular space, after separation from the signal peptide for secretion. The shorter forms lack up to 12 additional N-terminal amino acids while remaining their cytotoxic activity [33,306,331]. A defined ratio of NheB and C in the complexes (NheB:C = 10:1), as well as a sufficient amount of free NheB, is essential for effective cell binding and the cytotoxic activity of Nhe [327]. Moreover, in an appropriate concentration ratio, the NheB-C complexes themselves are capable of forming stable transmembrane channels with a diameter of approximately 2 nm, and thus increase membrane permeability of the target cells even before formation of the whole pore [330]. To complete the pore, NheA, which is not able to interact with NheB or C in solution, binds to cell-bound NheB. For this, an NheB:C ratio of 10:1 is also mandatory. Artificial addition of NheC resulting in a ratio of NheB:C = 1:1 led to reduced cell binding of NheB [327] as well as to reduced amounts of NheA at the cell surface [329]. Evidence has been found that the N-terminus of NheA is important for attaching to cell-bound NheB-C and that NheB is the main interaction partner for NheA [329]. Several studies proved that the pore forming procedure involves massive conformational changes of all protein components and that especially NheA is highly flexible [326,329,332], similarly to ClyA (see above). Osmotic protection and lipid bilayer experiments have also pointed to a comparable size of the ClyA and the Nhe full pores [217]. Another similarity is the permeabilization of the target cell membrane even before formation of the full pore [321,330]. A recent study showed that NheABC (ratio 10:10:1) forms large, stable pores with a conductance of approximately 18 nS on planar lipid bilayers. Increased amounts of NheC led to smaller and instable pores [309]. What all three (ClyA, Nhe and Hbl) pores seem to have in common is their selectiveness for cations [217,315,324]. Distinct features of the ClyA, Hbl and Nhe pores are comparatively summarized in Table 1. Figure 5 illustrates similarities and differences in the mechanism of pore formation of Hbl and Nhe.

Table 1.
Distinct characteristics of the pores formed by ClyA, Hbl and Nhe.
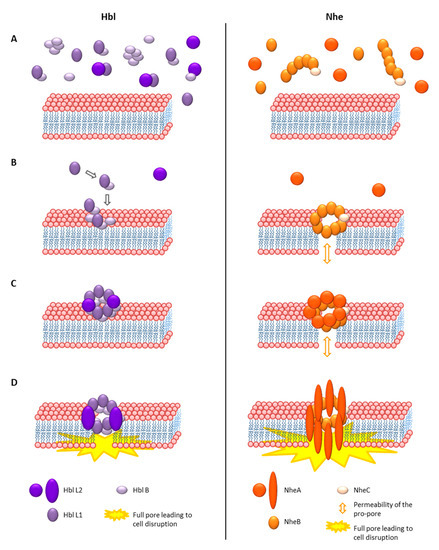
Figure 5.
Comparative depiction of the pore forming mechanism of the three-component enterotoxin complexes Hbl and Nhe. (A) Step 1: Complex formation in solution as well as presence of free Hbl and Nhe components [301,308,324,327,329,330]. (B) Step 2: Membrane attachment as Hbl B-L1 complexes [204,301], or as NheB-C complexes resulting in small, permeable “pro-pores” [327,330]. (C) Step 3: Attachment of Hbl L2 or NheA [301,324,329]. (D) Step 4: Conformational changes and completion of the full pore [301,324,329].
To sum up, both enterotoxin complexes need all three components for full biological activity [204,209,301]. In a first step, the components of both toxins form complexes in solution. Hbl B-L1, Hbl L2-L1, and Hbl L2-B form complexes with decreasing affinities [308]. Moreover, Hbl B itself oligomerizes [324]. On the other hand, NheB and C binding is highly affine, while NheA is not able to interact with the other components in solution [329,330]. In both cases, sufficient amounts of free Hbl B and NheB, the binding components, are also required [301,324,327], but complex formation seems to be a great advantage for the efficiency of the formation of both pores. In a second step, the NheB-C complexes attach to the cell membrane and form small, permeable pro-pores [327,330]. In case of Hbl, only Hbl B binds to the cell surface, but binding is enhanced in the presence of L1 [301]. Free Hbl L1 can subsequently bind to B [204,301]. Excess of Hbl L1 as well as NheC can impair the pore forming process. The optimal concentration ratio for maximum pore formation is Hbl L2:L1:B = 1:1:10, and NheA:B:C = 10:10:1 [209,301,327]. The third step consists of attachment of Hbl L2 or NheA. Especially for Hbl L2, only small amounts are needed compared to the other components [301,324,329]. Massive conformational changes, which are proven only for Nhe so far [329], lead to the formation of the full pore and permeabilization of the target cell membrane in the fourth step (see also Figure 5).
Though both are slightly cation selective, the size of the full Hbl and Nhe pores differs. Moreover, the Nhe pores seem to be more stable and persistent on planar lipid bilayers than the Hbl pores (see Table 1). On the other hand, Hbl pore formation seems to be more rapid and efficient [227,301]. In one of our own studies, Hbl-positive strains caused significantly faster influx of propidium iodide into target cells compared to solely Nhe producing isolates. Nevertheless, the latter were responsible for significantly increased cytotoxicity after 24 h [227]. Thus, it can be concluded that Hbl pores are formed more quickly, but that Nhe pores, not least due to their size, cause more damage to the target cell membrane. Once again, this indicates a different mode of action of the two enterotoxin complexes. It has been speculated that Nhe is the more dominant enterotoxin, as it is more frequently present, cytotoxicity correlates well with the amount of NheB, and toxic activity was eliminated by deletion of nhe genes [5,53,217]. On the other hand, disruption of the hbl genes also resulted in decreased cytotoxicity in a different strain, and residual cytotoxic activity was observed after removal of Nhe from culture supernatants [205,227]. In conclusion, both enterotoxin complexes seem to be (strain-specifically) important for cytotoxicity and thus the course of the diarrheal disease. If unleashed simultaneously, the Hbl pores might be responsible for a rapid, initial damage of the target cell membranes, while the Nhe pores cause delayed but stronger harm.
Despite all the progress made in exploring the mode of action of the tripartite enterotoxins, the exact regions and amino acids responsible for the protein-cell and protein-protein-interactions still remain to be identified. Nevertheless, by employing mutated NheC proteins, it was shown that its hydrophobic β-tongue is essential for cell binding, but not for the interaction with NheB in solution [328]. Based on studies with Nhe-specific, neutralizing monoclonal antibodies (mAbs), first hints were found that the C-terminal region of NheC is necessary for interaction with NheB [327,330]. Similarly, it was found that amino acids 122–150 of NheB (binding site of mAb 2B11) are the counterpart essential for the interaction with NheC, and that amino acids 321–341 (epitope of mAb 1E11) are needed for association between NheA and cell-bound NheB [326]. The authors concluded that the C-terminus is an important functional region of NheB. Furthermore, amino acids 85–100 of NheA (epitope of mAb 2G11) are relevant for attachment of NheA to the NheB-C complex on the cell surface, and amino acids 130–160 (binding site of mAb 1G4) play an important part in the change of conformation of NheA for transition of the NheABC-complex to the full-pore [329].
A great heterogeneity of enterotoxin production exists among enteropathogenic B. cereus isolates. In the supernatants of 100 strains, Moravek et al. found concentrations of 0.03–14.2 µg/mL NheB and 0.02–5.6 µg/mL Hbl L2, while the median NheB level was significantly higher for food poisoning than for food or environmental isolates [53]. A strong correlation between the amount of NheB and cytotoxic activity has been determined [53,227]. Further correlation was found for the amounts of Hbl B and L1 [227]. Defining the effective dose of the enterotoxins proved to be rather difficult. In cytotoxicity tests on Vero cells, Lund and Granum found a 50% inhibitory concentration of 20–30 ng for each Nhe component of strain NVH 0075-95 and 25–30 ng for each Hbl component of strain F837/76 [306]. In supernatants of high toxic strains, NheB amounts of approximately 10–14 µg/mL are found [53,326]. When these supernatants are tested in cellular assays, reciprocal cytotoxicity titers of usually more than 1000 are obtained [226], leading to the assumption that Nhe is toxic even at pmol concentrations. Similarly, propidium iodide influx into Vero cells as a measure of pore formation was maximal at a Hbl concentration of 68.3 pmol/mL (Hbl L2:L1:B = 1:1:1) and still detectable after four hours of measurement at a concentration of 4.7 pmol/mL [301].
Since their discovery, it was believed that the above described three-protein-component enterotoxins are a unique feature of the B. cereus group [5]. Just recently, extensive in silico search revealed Hbl and Nhe orthologues, and tripartite toxins of the ClyA family, in Gram-negative bacteria such as Serratia marcescens, Erwinia mallotivora and Aeromonas hydrophila. Furthermore, the tripartite α-pore forming toxin AhlABC of A. hydrophila was characterized in detail [336]. Briefly, the AhlC component forms a tetramer and subsequently disaggregates into monomers for target membrane binding, which leads to recruitment of AhlB. Major conformational changes initiate assembly of AhlB into a hydrophobic pore. With the subsequent binding of AhlA, the active, hydrophilic-lined pore is formed [336]. After that discovery, however, a further unique characteristic of the B. cereus group would be a toxin consisting not only of three, but of four individual, active protein components. As described in Section 4.1.1, the more common hblCDAB operon includes a fourth gene encoding Hbl B’, which shows some sequence similarities, but also differences compared to Hbl B. It has been suggested that hblB is transcribed independently from hblCDA [206]. Consensually, total transcriptome analyses revealed that hblB was down-regulated in reference strain F837/76 in the presence of mucin, while hblA was up-regulated [278]. Moreover, the protein was found in the secretome of B. cereus type strain ATCC 14579 [206]. Our own, recent data bear evidence that the Hbl B’ protein indeed plays a role in the complex formation and pore-forming activity of Hbl and it should thus be considered as the fourth component of the rather quadripartite Hbl enterotoxin complex. Furthermore, we detected it in culture supernatants of several enteropathogenic B. cereus strains, which increases its relevance (data unpublished).
4.1.4. Susceptibility and Cellular Response towards the Tripartite Enterotoxins
Several studies proved that the B. cereus enterotoxins affect a variety of different target tissues and cell lines, such as rabbit retinal tissue and ileum [303], sheep blood [300], as well as Vero (African green monkey kidney), CHO (Chinese hamster ovary), HUVEC (human umbilical vein/vascular primary endothelial cells), Hep-2 (human cervix), CaCo-2 (human colon), Hep-G2 (human liver), A549 (human lung), RPMI 8226 (human B lymphocyte), A204 (human muscle), Jurkat (human T lymphocyte), U937 (human monocyte), HT-29 (human colon), IPEC-J2 (swine colon), BMDM (primary bone marrow derived macrophages), EC (mouse lung primary endothelial), RAW 264.7 (murine macrophages), B16-BL6 (murine skin), B16-F10 (murine skin), and HT-1080 (human connective tissue) cells [204,227,306,335,337,338]. At this point, the question frequently arose whether specific target structures and receptors for Hbl and Nhe exist. Generally, pore forming toxins get to their target cell membrane in their soluble form by binding to proteinaceous receptors or by specifically interacting with different lipids [339]. The Cry toxins from B. thuringiensis, for instance, interact with insect-specific receptors such as cadherin-like proteins, aminopeptidase N, alkaline phosphatase or ATP binding transporters [340,341]. For S. aureus hemolysins, specific target proteins such as disintegrin are known, and C. perfringens enterotoxin (CPE) attaches to claudin [339]. The protective antigen (PA) from B. anthracis binds CMG2 (capillary morphogenesis gene 2 or ANTXR2) and TEM8 (tumor endothelial marker 8 or ANTXR1) receptors at the cell surface. Both are integrin-like proteins associated with proteins of the extracellular matrix, such as collagen and fibronectin [342]. On the other hand, Cyt toxins from B. thuringiensis recognize membrane lipids such as phosphatidylcholine, phosphatidylethanolamine or sphingomyelin [107,343]. Actinoporins for instance interact with sphingomyelin, perfringolysin O and listeriolysin O with cholesterol. Cholesterol is also listed as target structure for ClyA, Hbl and Nhe [339], however, this has only been experimentally proven for ClyA (see Table 1 and [316,321,334]). On the one hand, the broad range of target cells (see above), as well as the ability of NheB-C [330], NheABC [309] and Hbl [324] to form pores in artificial lipid bilayers, oppose the existence of specific (protein) receptors. On the other hand, Hbl and Nhe contribute differentially to total cytotoxicity of B. cereus supernatants. In a study from 2014, total cytotoxic activity of strain F4430/73 on HUVEC was dominated by Nhe (90%), while on A549 cells 75% of the cytotoxic effects could be attributed to Hbl. Further on, U937 cells proved to be nearly resistant towards Nhe [227]. These variations point to specific enterotoxin target structures. Specific receptors also best fit the proposed models for Hbl and Nhe pore formation and mode of action (Section 4.1.3). Moreover, it is believed that Hbl and Nhe address different receptor types, which would also account for the non-demonstrable interaction between both toxin complexes despite sequence homology [204,227]. A big step towards resolving this long-discussed issue has recently been made. Using a whole-genome CRISPRCas9 knockout screen, LPS-induced TNF-α factor (LITAF) was identified as the main receptor for Hbl. Moreover, its related protein CDIP1 was found to be an alternative Hbl receptor in the absence of LITAF. LITAF/CDIP1 double knockout cells were completely resistant towards Hbl B binding as well as Hbl toxicity, and LITAF knockout mice were resistant towards Hbl toxicity [335]. Nevertheless, the specific Nhe receptor, which is more than likely to exist, is still unidentified.
Further open questions regard the specific cellular response towards the B. cereus enterotoxins. Generally, a great variety of cellular reactions to pore-forming toxins exist. First, pore formation decreases the intracellular potassium concentration, which activates different signal pathways including the inflammasome complex (e.g., aerolysin, Vibrio cholerae cytolysin, Clostridium β-toxin or S. aureus toxins), MAPK (mitogen-activated protein kinase) signal pathways or autophagy (e.g., S. marcescens ShlA or S. aureus α-hemolysin). Pore formation in the cell membrane can also induce calcium influx, which again activates different signal cascades [339,344]. It has further been reported that pore forming toxins alter gene expression in the target cells [345]. When cells are exposed to small toxin concentrations, or over a short period of time, mechanisms for membrane repair become effective, which mostly depend on calcium. Pores can be taken up via endocytosis and further be degraded or emitted. Alternatively, membrane vesicles including the toxins can be separated [339,345]. Liu et al. showed that Nhe is able to induce apoptosis in Vero cells. As mentioned above, the formation of the Nhe pore leads to calcium influx into the cells, oxidative stress, and thus to an activation of ASK1 (apoptosis signal-regulating kinase 1) and p38 MAPK. The latter is additionally activated by Fas (tumor necrosis factor superfamily receptor). The kinases subsequently activate caspase-8 and caspase-3 for induction of apoptosis [346]. Recently, it has been shown that Hbl as well as Nhe is able to induce inflammasome-mediated mortality of macrophages [337,338]. Formation of the lytic pore at the macrophage cell membrane leads to potassium efflux, subsequent activation of the NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome, secretion of interleukin-1β and interleukin-18, and pyroptosis, an inflammatory form of cell death. The authors concluded that Hbl and Nhe operate synergistically to induce inflammation and that the detection of inflammasome-activating toxins in the cytosol is essential for recognizing a B. cereus infection [337,338]. Hence, the involvement of B. cereus and especially its enterotoxins in gastrointestinal disease and inflammation is ensured.
Beyond that, B. cereus is responsible for a variety of severe extra-gastrointestinal infections. Often neonates, elderly or immunosuppressed patients are affected [347,348], but infections of immunocompetent persons are also known [349]. These include local, especially post-operative skin and wound infections [347,349,350], septicemia [350,351,352,353,354,355,356,357,358,359,360], meningitis [361,362,363,364,365,366,367,368,369,370,371,372], pneumonia (often by B. cereus strains exhibiting B. anthracis toxin genes) [373,374,375,376,377,378] and endocarditis [379,380,381,382,383,384,385,386,387,388]. Several studies focus on B. cereus-associated endophthalmitis, which occurs mainly following post-traumatic injuries and often leads to loss of vision or even of the eye in less than 48 h. Severe inflammation is caused by the concerted action of rapid bacterial replication, migration and toxin production [389]. Early studies found evidence for an involvement of Hbl, phosphatidylcholine-preferring phospholipase C and collagenase in B. cereus endophthalmitis [302,303]. By now, it is believed that the combination of multiple virulence factors and toxins leads to intraocular virulence [389]. Using deletion mutants, it was shown that the quorum sensing regulator PlcR (see also Section 4.1.2) contributes significantly to the disease, on the one hand by controlling toxin and virulence factor expression, but also by controlling expression of cell wall components and bacterial motility [287,390,391]. Motility and swarming behavior, as well as cell wall components such as flagella and pili or the S-layer are also a trigger for intraocular inflammation [392,393,394,395]. The disease results from the interplay of various bacterial and host factors [389,396,397], which makes clinical treatment difficult. Applying sublethal doses of carvacrol, for instance, enhanced nhe and hbl gene expression and toxin production, as well as stress-induced virulence against Caenorhabditis elegans and mice [398]. Further recent studies focus on genotyping, whole genome sequencing and, thus, on the identification of other putative B. cereus virulence factors involved in endophthalmitis [399,400,401]. The most important, which may also play a role in gastrointestinal diseases, are summarized in Section 5.
4.2. Cytotoxin K
In contrast to the above described tripartite toxin complexes Hbl and Nhe, the third diarrheal enterotoxin, cytotoxin K, is a single protein, β-barrel pore-forming toxin. CytK was first isolated from thermotolerant B. cereus strain NVH 391/98, which was responsible for a severe food poisoning case in a nursing home in France in 1998. Six people suffered from bloody diarrhea, and three people died. At this time, none of the known B. cereus enterotoxins could be identified, and CytK proved to be toxic towards Vero cells [31]. Nevertheless, Nhe was identified later in this strain [211,215], which might also have been involved in the outbreak. Concurrently, CytK was also isolated from supernatant of a B. cereus strain associated with endophthalmitis and named hemolysin IV (HlyIV) [302].
The CytK toxin is encoded by the single gene cytK. First PCR analyses suggested that cytK is not particularly prevalent among different B. cereus isolates [31,402]. More detailed investigations identified two different variants of the cytK gene with high sequence homology, which were—according to their discovery—named cytK-1 and cytK-2, respectively [403,404]. The corresponding toxins CytK-1 and CytK-2 share 89% amino acid sequence identity. While cytK-2 is found in a variety of B. cereus strains, the cytK-1 variant occurs only exceptionally. Among the B. cereus group, cytK-2 was most commonly found in the mesophilic phylogenetic groups III and IV [145,404]. In a study from 2015, the occurrence of cytK-2 was lower in clinical and food poisoning isolates compared to environmental strains, which allowed the authors to conclude that CytK-2 is most likely not a relevant virulence factor for the diarrheal syndrome [405]. In our own studies, we also concluded that cytK-(2) is not a suitable marker for enteropathogenic B. cereus or their cytotoxic activity [91,297]. Basically, cytK-1-positive strains bear an unusual variant of the nhe operon [215]. For a long time, only five of these strains were known [406], which were eventually defined as a new species, B. cytotoxicus. Their 16S rRNA gene sequences show a close phylogenetic relationship to other members of the B. cereus group, but within this group the investigated B. cytotoxicus strains form their own cluster [95,404]. In more recent studies, B. cytotoxicus was identified with higher prevalence. Of 151 tested potato products, 35% were positive, with highest prevalence in dried potato powder [407]. Nine out of nine mashed potato powders were positive for B. cytotoxicus, which, however, showed highly variable cytotoxic activity [408]. The species was also identified in millet flour and potato-containing products, especially in potato flakes [409]. When the genome sequences of 14 B. cytotoxicus strains were compared, they could clearly be distinguished from other members of the B. cereus group, and a hydroxy-phenylalanine operon for utilization of tyrosine was identified as a unique feature for this species. The authors concluded that this putative evolutionary advantage might have been the reason for species differentiation [410]. Recently, Cavello et al. first reported the isolation of B. cytotoxicus from the natural environment, a geothermal area, as well as its keratinolytic activity [411]. Interestingly, cytK-2 is not present in B. anthracis [125], but has been found in B. mycoides [412], very frequently in B. thuringiensis [125,210], and also outside the B. cereus group in Paenibacillus cookie [413].
Like the hbl and nhe operons, the expression of the genes cytK-1 and cytK-2 is regulated by PlcR (compare Section 4.1.2) via binding to specific PlcR boxes in their promoter regions [31,169,246,414,415]. On the other hand, the unusually long 5′ untranslated regions, which were found upstream of hbl and nhe (compare Section 4.1.1 and 4.1.2), are not present. With approximately 100 bp, the promoter regions are comparably short. Analyzing the sequences of a variety of B. cereus group strains, Böhm et al. found high similarity in the cytK-1 promoter region, and a highly variable region as well as a strongly conserved promoter for cytK-2 [210]. Figure 6A gives an overview of the genetic organization of cytK-1 and cytK-2. These in silico analyses further revealed putative binding sites for Fnr and SinR (compare Section 4.1.2) upstream of cytK-1, which are, however, not experimentally verified to date. It has also been shown that both cytK genes are expressed independently of CcpA-mediated catabolite control [233] and that CodY does not interact with their promoter regions [210]. Furthermore, evidence has been found that the cytK genes, similarly to hbl, are inherited via horizontal transfer as well as frequent deletion [210]. In contrast to the highly conserved cytK-1 promoter region, great variability in CytK-1 production and cytotoxicity has been observed between the highly toxic type-strain NVH 391/98 and non-toxic NVH 883/00 [215,414], which might be explained by one mismatch in the PlcR binding site of strain NVH 391/98 or by further, as yet unidentified regulatory pathways [215,406]. Another study revealed that only a small subpopulation (1–2%) of B. cereus ATCC 14579 expressed cytK and produced the toxin [416], pointing again to a yet unknown regulatory mechanism.
Like the Hbl and Nhe proteins, both CytK toxins harbor Sec-type signal peptides, which proposes secretion through the Sec translocation pathway. This was proven by treatment with sodium azide, an inhibitor of SecA, and the subsequent failure of secretion and intracellular accumulation of CytK [283]. On the other hand, no evidence was found for an involvement of the flagellar export system in CytK secretion [288].
The CytK-1 protein initially isolated from strain NVH 391/98 had a size of 34 kDa, and was shown to be hemolytic, dermonecrotic, able to form pores in lipid layers, and highly toxic towards human intestinal epithelial cells [403,417]. The pores were slightly anion selective with a predicted minimum pore diameter of approximately seven Å. Furthermore, the spontaneous formation of SDS-resistant oligomers was reported [417]. CytK-2 also proved to be hemolytic, cytotoxic and pore-forming in lipid layers, but with an approximately 80% reduced cytotoxic activity compared to CytK-1. Additionally, the pores showed lower conductance. The authors further assigned the differences between CytK-1 and CytK-2 to certain regions of the proteins [403]. Nonetheless, considerable amino acid sequence homologies were found to S. aureus leukocidin, S. aureus α- and γ-hemolysin [418,419], C. perfringens β-toxin [31,420,421], and B. cereus hemolysin II [406,422]. Thus, CytK is classified as member of the family of β-barrel pore-forming toxins, and, although not yet experimentally verified, its mechanism of oligomerization and pore formation can be predicted from the closely related and well investigated S. aureus α-hemolysin and B. cereus hemolysin II. Figure 6B shows a model of the predicted mode of action in the style of Hbl and Nhe. Initially, the toxin is secreted as soluble monomer. Those monomers bind to the target cell membrane presumably due to their interaction with liposomes. The subsequent formation of SDS-, but not heat-resistant, oligomers is characteristic for β-barrel pore-forming toxins. Most likely, heptamers are formed and conformational changes occur to complete the mushroom-shaped, anion selective, β-barrel transmembrane pore with hydrophobic parts facing the lipids and hydrophilic parts facing the inside of the channel [322,419,423].
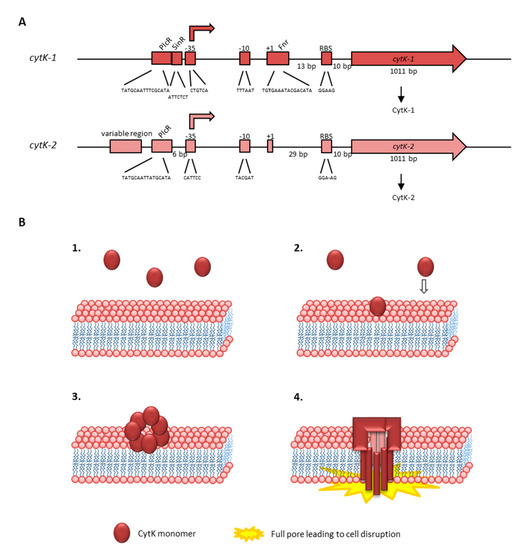
Figure 6.
Properties of the CytK enterotoxin from B. cereus. (A) The cytK-1 and cytK-2 genetic regions according to Böhm et al. [210]. The promoter regions including (putative) transcriptional regulator binding sites are shown. (B) Putative mode of action of CytK during pore formation according to related β-barrel pore-forming toxins [419,423,424]. Step 1: Soluble monomers in solution. Step 2: Target membrane binding due to putative interaction with liposomes. Step 3: Oligomerization into heptamers. Step 4: Conformational changes and pore formation.
Figure 6.
Properties of the CytK enterotoxin from B. cereus. (A) The cytK-1 and cytK-2 genetic regions according to Böhm et al. [210]. The promoter regions including (putative) transcriptional regulator binding sites are shown. (B) Putative mode of action of CytK during pore formation according to related β-barrel pore-forming toxins [419,423,424]. Step 1: Soluble monomers in solution. Step 2: Target membrane binding due to putative interaction with liposomes. Step 3: Oligomerization into heptamers. Step 4: Conformational changes and pore formation.
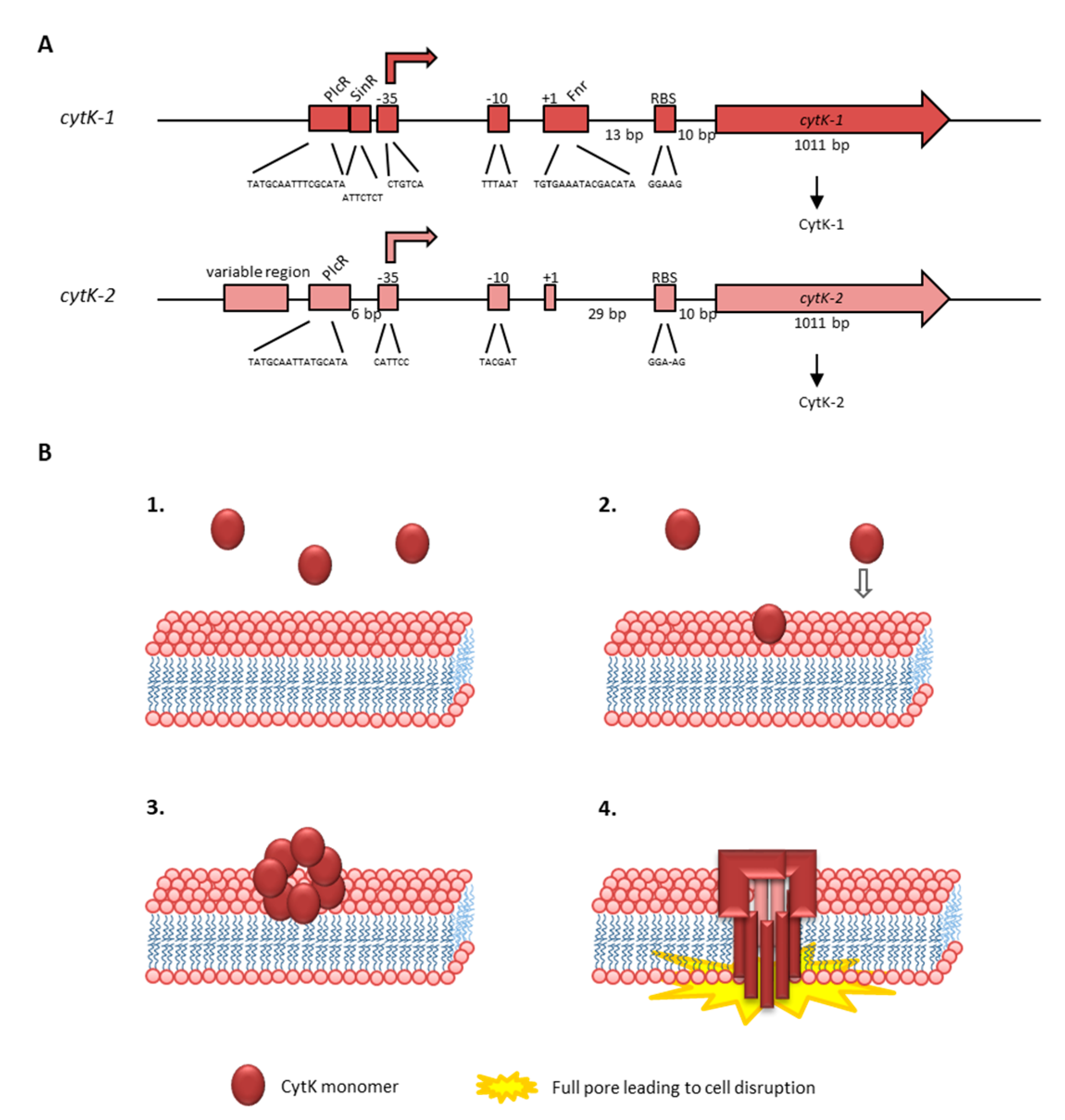
4.3. Methods for Detection of the Enterotoxins
In routine food or clinical diagnostics, presumptive B. cereus s. l. strains are usually detected on selective culture media according to international standards (ISO 7932:2005-03), highlighting the importance of toxin detection rather than species differentiation [93]. Various PCR, real-time PCR and multiplex PCR systems have been established and used for the detection of the hbl, nhe, cytK and ces genes ([48,52,70,114,185,186,187,404,412,425,426,427,428] and many others as summarized in Section 2). With progress in technology, it is also imaginable that these PCR-based techniques will be replaced by other methods such as whole genome sequencing, DNA microarray-based analysis, or fingerprinting techniques [41,54,93,120,143,210,429,430,431,432,433,434,435]. However, the detection of enterotoxin genes or patterns is not a reliable tool to predict the toxic potential of an isolate. This is not only defined by genetic prerequisites, but rather by the number of secreted enterotoxins, which can be highly variable even among strains bearing identical toxin genes [53,91,226,227].
Tools for the detection of the secreted enterotoxins in B. cereus culture supernatants are mainly based on monoclonal antibodies against the single toxin components. Three test kits were commercially available, which are Duopath® Cereus Enterotoxins (Merck) detecting NheB and Hbl L2, Oxoid™ BCET-RPLA Toxin Detection Kit specific for Hbl L2, and 3M™ Tecra™ Bacillus Diarrheal Enterotoxin (BDE) Visual Immunoassay (VIA) detecting NheA and NheB [428,436,437,438,439,440,441,442,443,444]. The latter seems to be discontinued. However, of these toxin components, only the amount of secreted NheB strongly correlates with cytotoxicity [53,227]. Using further, antibody-based systems such as sandwich enzyme immunoassays or Western and colony immunoblots, all single Hbl and Nhe components can be reliably detected and quantified [53,226,308,427,445,446,447]. However, it must be noted that these assays do not specifically detect biologically active toxins. At the moment, there is no commercially available detection kit for CytK, but the toxin has previously been detected via specific antisera [215]. As an alternative method, a MALDI-ToF MS system was established for the detection of secreted CytK-1 and Nhe [448], as well as for the differentiation of emetic and non-emetic strains [189,190].
To assess the toxic activity of the B. cereus enterotoxins, in vivo animal experiments were initially performed, such as the rabbit ileal loop, the guinea pig skin reaction, and the vascular permeability test [35,449,450], as well as monkey feeding experiments [451]. At present, these animal models are widely replaced by cytotoxicity assays carried out in cell culture [33,43,71,134,141,227,417,439,445,452,453,454,455,456,457]. In WST-1 bioassays, for instance, 50% lethal doses are determined and shown as reciprocal titers, which can easily be compared between different isolates [91,227]. Measuring the influx of fluorescent markers such as propidium iodide into the cells allows conclusions on the velocity of pore formation by the enterotoxins [217,227]. From these in vitro tests, however, only limited conclusions can be drawn regarding the actual toxicity in vivo. This means that it is hard to predict if a certain isolate is capable of causing an infection once consumed, especially as only enterotoxins produced by viable B. cereus in the intestine contribute to the diarrheal disease. Thus, many additional factors regarding the host have to be taken into account, which we recently investigated and summarized [27,92,278,297].
5. Further Virulence Factors and Toxins
Next to the three enterotoxins, the involvement of further secreted virulence factors in the diarrheal disease is extensively discussed in the literature. The 45 kDa EntFM was first described as enterotoxin in 1991 [455]. Its corresponding genes exist in B. cereus as well as in B. thuringiensis [458]. In a wide study with 616 isolates, entFM was found in all tested strains similarly to nheABC [122]. EntFM was described as cell wall peptidase involved in adhesion, motility, and biofilm formation, as well as virulence and cytotoxicity of B. cereus [459,460]. The prevalence of entFM was assessed in several studies characterizing B. cereus group isolates from food or environmental samples (see Section 2.1).
Moreover, four different types of hemolysins are known in B. cereus. Hemolysin I or cereolysin O is a member of the family of cholesterol-dependent cytolysins, as for example listeriolysin O [406]. The hemolytic activity of this toxin was first described in 1983 [461]. The 42 kDa hemolysin II belongs, like CytK, which is also described as hemolysin IV, to the family of β-barrel pore forming toxins (see Section 4.2 and [406,422]). HlyII is able to induce apoptosis in macrophages [462]. Interestingly, the corresponding gene hlyII is not—unlike most described virulence factors [169,248]—regulated by PlcR, but negatively by the TetR-like transcriptional regulator HlyIIR [463,464]. The hlyII gene is additionally regulated by the ferric uptake regulator Fur, and deletion of fur reduced virulence of B. cereus in an insect model [465,466]. The hlyII gene has been shown to appear more often in B. thuringiensis than in B. cereus [467], and the structure and properties of its β-barrel pore have been elucidated [423,424,468,469,470]. HlyII has so far not been described as cause of the diarrheal syndrome, as it might be inactivated by trypsin in the small intestine [5]. The 24 kDa hemolysin III is also a pore forming hemolysin [471,472], but so far it has not been proven to be secreted by B. cereus. Thus, its involvement in pathogenicity remains speculative [406]. Cadot et al. suggested HlyII as well as the two metalloproteases InhA1 and NprA as potential markers for differentiation between virulent and non-virulent B. cereus strains, as hlyII appeared in their study only in virulent strains, and the expression of inhA1 and nprA was clearly increased compared to non-virulent strains [473].
The metalloproteases InhA1, InhA2 and InhA3 were identified as important virulence factors in an insect model; InhA1, furthermore, seemed to counteract macrophages [474,475,476]. B. cereus spores are able to survive inside and escape macrophages. It has been shown recently that the most important effector for this is the mature NprA protein, which is cleaved by InhA1 [477]. Additionally, the phosphatidylinositol- and phosphocholine-specific phospholipase C (PI-PLC and PC-PLC) as well as the collagenase ColA are considered as pathogenicity factors [105,110,478,479,480,481]. The bifunctional protein CalY, formerly known as camelysin, has been shown to play an important role in cell surface adhesion and virulence, as well as in biofilms [482,483,484,485]. Proteome analyses identified the exoprotein EntD as a further important virulence-associated factor for B. cereus, and an entD deletion strain showed reduced growth, motility and cytotoxicity, suggesting that the protein is strongly involved in motility and toxin production [486]. In this context it is important to note that direct involvement of these virulence factors in the diarrheal form of disease has so far not been verified. In one of our own previous studies, no cytotoxic activity of B. cereus culture supernatants could be detected after removal of NheB, Hbl L2 and Hbl B via immunoaffinity chromatography [227]. Only for sphingomyelinase (SMase) could a direct impact on in vitro cytotoxicity be shown. B. cereus ∆sph and ∆nheBC seemed to complement each other for maximum virulence [487]. Interaction of SMase with Hbl has also been seen in an earlier study [323]. Similarly, SMase causes lysis of erythrocytes synergistically with PC-PLC as cereolysin AB complex [406]. It was further described as an important virulence factor in sepsis and connected to a reduction of phagocytosis [488,489,490]. In a recent study, we showed that NheB and SMase expression, together with exoprotease activity, correlates well with a complex virulence analysis scheme and might serve as template for fast and easy risk assessment in routine diagnostics [297].
6. Conclusions
The processes leading to the diarrheal syndrome caused by enteropathogenic B. cereus, as well as the physiological procedures involved in the emetic syndrome caused by the cereulide toxins, are highly complex and—despite extensive research, which is summarized here—still not completely understood. Massive progress has been made investigating the mode of action of the three-component enterotoxin complexes Hbl and Nhe, and first insights into the mechanism of the emetic toxin cereulide have been gained. Nevertheless, there are still many open questions regarding B. cereus diarrheal and emetic toxins. For instance, we are just at the beginning of understanding the interplay of the single enterotoxin components and the basic mode of action of cereulide. Moreover, a specific receptor structure for Nhe, or the involvement of the putative fourth component of Hbl, Hbl B’, need to be explored, and information on the exact molecular targets for cereulide and isocereulides are still very limited. Besides, B. cereus secretes an entire battery of (putative) further virulence factors, which possibly contribute to pathogenicity, either by interacting directly with the enterotoxins or the epithelia or by influencing further stages of the disease, such as adhesion, immune escape, etc.
Author Contributions
Conceptualization, R.D., N.J., E.M. and P.E.G.; writing—original draft preparation, R.D., N.J. and M.E.-S.; writing—review and editing, E.M. and P.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
Parts of this project were supported by Deutsche Forschungsgemeinschaft, grant number JE 871/2-1, and by the FEI via AiF within the program for promoting the Industrial Collective Research (IGF) of the German Ministry of Economic Affairs and Energy (BMWi), based on a resolution of the German Parliament, projects AiF 16845 N, AiF 17506 N, AiF 18677 N and AiF 19659 N.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the writing of the manuscript, or in the decision to publish the results.
References
- Grutsch, A.A.; Nimmer, P.S.; Pittsley, R.H.; Kornilow, K.G.; McKillip, J.L. Molecular pathogenesis of Bacillus spp., with emphasis on the dairy industry. Fine Focus 2018, 4, 203–222. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union one health 2018 zoonoses report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Agata, N.; Ohta, M.; Mori, M.; Isobe, M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 1995, 129, 17–19. [Google Scholar] [CrossRef]
- Messelhäusser, U.; Frenzel, E.; Blöchinger, C.; Zucker, R.; Kämpf, P.; Ehling-Schulz, M. Emetic Bacillus cereus are more volatile than thought: Recent foodborne outbreaks and prevalence studies in bavaria (2007–2013). BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Shinagawa, K.; Ueno, Y.; Hu, D.; Ueda, S.; Sugii, S. Mouse lethal activity of a HEp-2 vacuolation factor, cereulide, produced by Bacillus cereus isolated from vomiting-type food poisoning. J. Vet. Med. Sci. 1996, 58, 1027–1029. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Messelhäusser, U. One pathogen but two different types of foodborne outbreaks: Bacillus cereus in catering facilities in Germany. In Case Studies in Food Safety and Quality Management: Lessons from Real-Life Situations; Horfaar, J., Ed.; Woodhead: Cambridge, UK, 2012; pp. 63–70. [Google Scholar]
- Ehling-Schulz, M.; Fricker, M.; Scherer, S. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol. Nutr. Food Res. 2004, 48, 479–487. [Google Scholar] [CrossRef]
- Granum, P.E.; Lund, T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 2006, 157, 223–228. [Google Scholar] [CrossRef]
- Rajkovic, A.; Uyttendaele, M.; Vermeulen, A.; Andjelkovic, M.; Fitz-James, I.; in`t Veld, P.; Denon, Q.; Vérhe, R.; Debevere, J. Heat resistance of Bacillus cereus emetic toxin, cereulide. Lett. Appl. Microbiol. 2008, 46, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, K.; Koeppel, M.B.; Wallner, C.-P.; Marx, T.; Wagener, J.; Ney, L. Food poisoning: An underestimated cause of Boerhaave syndrome. Infection 2019, 48, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Dierick, K.; Van Coillie, E.; Swiecicka, I.; Meyfroidt, G.; Devlieger, H.; Meulemans, A.; Hoedemaekers, G.; Fourie, L.; Heyndrickx, M.; Mahillon, J. Fatal family outbreak of Bacillus cereus—associated food poisoning. J. Clin. Microbiol. 2005, 43, 4277–4279. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Gakumazawa, M.; Inaba, A.; Shiga, K.; Takeshita, S.; Mori, M.; Kikuchi, N. Acute encephalopathy of Bacillus cereus mimicking Reye syndrome. Brain Dev. 2010, 32, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Latsios, G.; Petrogiannopoulos, C.; Hartzoulakis, G.; Kondili, L.; Bethimouti, K.; Zaharof, A. Liver abscess due to Bacillus cereus: A case report. Clin. Microbiol. Infect. 2003, 9, 1234–1237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahler, H.E.M.; Asi, A.U.P.; Kramer, J.M.; Schulte, P.; Scoging, A.C.; Är, W.A.B.; Rähenbühl, S.T.K. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 1997, 336, 1142–1148. [Google Scholar] [CrossRef]
- Naranjo, M.; Denayer, S.; Botteldoorn, N.; Delbrassinne, L.; Veys, J.; Waegenaere, J.; Sirtaine, N.; Driesen, R.B.; Sipido, K.R.; Mahillon, J.; et al. Sudden death of a young adult associated with Bacillus cereus food poisoning. J. Clin. Microbiol. 2011, 49, 4379–4381. [Google Scholar] [CrossRef]
- Posfay-Barbe, K.M.; Schrenzel, J.; Frey, J.; Studer, R.; Korff, C.; Belli, D.C.; Parvex, P.; Rimensberger, P.C.; Schäppi, M.G. Food poisoning as a cause of acute liver failure. Pediatr. Infect. Dis. J. 2008, 27, 846–847. [Google Scholar] [CrossRef]
- Saleh, M.; Al Nakib, M.; Doloy, A.; Jacqmin, S.; Ghiglione, S.; Verroust, N.; Poyart, C.; Ozier, Y. Bacillus cereus, an unusual cause of fulminant liver failure: Diagnosis may prevent liver transplantation. J. Med Microbiol. 2012, 61, 743–745. [Google Scholar] [CrossRef]
- Shiota, M.; Saitou, K.; Mizumoto, H.; Matsusaka, M.; Agata, N.; Nakayama, M.; Kage, M.; Tatsumi, S.; Okamoto, A.; Yamaguchi, S.; et al. Rapid Detoxification of cereulide in Bacillus cereus food poisoning. Pediatrics 2010, 125, e951–e955. [Google Scholar] [CrossRef]
- Takabe, F.; Oya, M. An autopsy case of food poisoning associated with Bacillus cereus. Forensic Sci. 1976, 7, 97–101. [Google Scholar] [CrossRef]
- Tschiedel, E.; Rath, P.-M.; Steinmann, J.; Becker, H.; Dietrich, R.; Paul, A.; Felderhoff-Müser, U.; Dohna-Schwake, C. Lifesaving liver transplantation for multi-organ failure caused by Bacillus cereus food poisoning. Pediatr. Transplant. 2014, 19, E11–E14. [Google Scholar] [CrossRef] [PubMed]
- Carlin, F. Origin of bacterial spores contaminating foods. Food Microbiol. 2011, 28, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.B.; Hansen, B.M.; Eilenberg, J.; Mahillon, J. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 2003, 5, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spore resistance properties. Microbiol. Spectr. 2014, 2, 201–215. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Granum, P.E.; Märtlbauer, E. The Bacillus cereus food infection as multifactorial process. Toxins 2020, 12, 701. [Google Scholar] [CrossRef]
- Clavel, T.; Carlin, F.; Lairon, D.; Nguyen-The, C.; Schmitt, P. Survival of Bacillus cereus spores and vegetative cells in acid media simulating human stomach. J. Appl. Microbiol. 2004, 97, 214–219. [Google Scholar] [CrossRef]
- Logan, N. Bacillus and relatives in foodborne illness. J. Appl. Microbiol. 2011, 112, 417–429. [Google Scholar] [CrossRef]
- European Food Safety Authority. Opinion of the Scientific Panel on biological hazards (BIOHAZ) on Bacillus cereus and other Bacillus spp in foodstuffs. EFSA J. 2005, 3, 1–48. [Google Scholar] [CrossRef]
- Lund, T.; De Buyser, M.-L.; Granum, P.E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef]
- Beecher, D.J.; Macmillan, J.D. Characterization of the components of hemolysin BL from Bacillus cereus. Infect. Immun. 1991, 59, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.; Granum, P.E. Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett. 1996, 141, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kotiranta, A.; Lounatmaa, K.; Haapasalo, M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000, 2, 189–198. [Google Scholar] [CrossRef]
- Kramer, J.M.; Gilbert, R.J. Bacillus cereus and other Bacillus species. In Foodborne Bacterial Pathogens; Doyle, M.P., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1989; pp. 21–50. [Google Scholar]
- Bağcioğlu, M.; Fricker, M.; Johler, S.; Ehling-Schulz, M. Detection and identification of Bacillus cereus, Bacillus cytotoxicus, Bacillus thuringiensis, Bacillus mycoides and Bacillus weihenstephanensis via machine learning based FTIR spectroscopy. Front. Microbiol. 2019, 10, 902. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Frenzel, E.; Gohar, M. Food–bacteria interplay: Pathometabolism of emetic Bacillus cereus. Front. Microbiol. 2015, 6, 704. [Google Scholar] [CrossRef]
- Agata, N.; Ohta, M.; Arakawa, Y.; Mori, M. The bceT gene of Bacillus cereus encodes an enterotoxic protein. Microbiology 1995, 141, 983–988. [Google Scholar] [CrossRef]
- Choma, C.; Granum, P.E. The enterotoxin T (BcET) from Bacillus cereus can probably not contribute to food poisoning. FEMS Microbiol. Lett. 2002, 217, 115–119. [Google Scholar] [CrossRef]
- Hansen, B.M.; Hoiby, P.E.; Jensen, G.B.; Hendriksen, N.B.; Høiby, P.E. The Bacillus cereus bceT enterotoxin sequence reappraised. FEMS Microbiol. Lett. 2003, 223, 21–24. [Google Scholar] [CrossRef][Green Version]
- Carter, L.; Chase, H.R.; Gieseker, C.M.; Hasbrouck, N.R.; Stine, C.B.; Khan, A.; Ewing-Peeples, L.J.; Tall, B.D.; Gopinath, G.R. Analysis of enterotoxigenic Bacillus cereus strains from dried foods using whole genome sequencing, multi-locus sequence analysis and toxin gene prevalence and distribution using endpoint PCR analysis. Int. J. Food Microbiol. 2018, 284, 31–39. [Google Scholar] [CrossRef]
- Dréan, P.; McAuley, C.M.; Moore, S.C.; Fegan, N.; Fox, E.M. Characterization of the spore-forming Bacillus cereus sensu lato group and Clostridium perfringens bacteria isolated from the Australian dairy farm environment. BMC Microbiol. 2015, 15, 38. [Google Scholar] [CrossRef]
- Hsieh, Y.M.; Sheu, S.J.; Chen, Y.L.; Tsen, H.Y. Enterotoxigenic profiles and polymerase chain reaction detection of Bacillus cereus group cells and B. cereus strains from foods and food-borne outbreaks. J. Appl. Microbiol. 1999, 87, 481–490. [Google Scholar] [CrossRef]
- Kindle, P.; Etter, D.; Stephan, R.; Johler, S. Population structure and toxin gene profiles of Bacillus cereus sensu lato isolated from flour products. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Saleh-Lakha, S.; Leon-Velarde, C.G.; Chen, S.; Lee, S.; Shannon, K.; Fabri, M.; Downing, G.; Keown, B. A study to assess the numbers and prevalence of Bacillus cereus and its toxins in pasteurized fluid milk. J. Food Prot. 2017, 80, 1085–1089. [Google Scholar] [CrossRef]
- Samapundo, S.; Heyndrickx, M.; Xhaferi, R.; Devlieghere, F. Incidence, diversity and toxin gene characteristics of Bacillus cereus group strains isolated from food products marketed in Belgium. Int. J. Food Microbiol. 2011, 150, 34–41. [Google Scholar] [CrossRef]
- Chica, J.S.; Correa, M.M.; Aceves-Diez, A.E.; Rasschaert, G.; Heyndrickx, M.; Castañeda-Sandoval, L.M. Genomic and toxigenic heterogeneity of Bacillus cereus sensu lato isolated from ready-to-eat foods and powdered milk in day care centers in Colombia. Foodborne Pathog. Dis. 2020, 17, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Guinebretière, M.-H.; Monthán, A.; Berge, O.; Fricker, M.; Svensson, B. Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol. Lett. 2006, 260, 232–240. [Google Scholar] [CrossRef]
- Fiedler, G.; Schneider, C.; Igbinosa, E.O.; Kabisch, J.; Brinks, E.; Becker, B.; Stoll, D.A.; Cho, G.-S.; Huch, M.; Franz, C.M.A.P. Antibiotics resistance and toxin profiles of Bacillus cereus-group isolates from fresh vegetables from German retail markets. BMC Microbiol. 2019, 19, 250. [Google Scholar] [CrossRef]
- Svensson, B.; Ekelund, K.; Ogura, H.; Christiansson, A. Characterisation of Bacillus cereus isolated from milk silo tanks at eight different dairy plants. Int. Dairy J. 2004, 14, 17–27. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Svensson, B.; Guinebretière, M.-H.; Lindbäck, T.; Andersson, M.; Schulz, A.; Fricker, M.; Christiansson, A.; Granum, P.E.; Märtlbauer, E.; et al. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 2005, 151, 183–197. [Google Scholar] [CrossRef]
- Guinebretière, M.-H.; Broussolle, V.; Nguyen-The, C. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 2002, 40, 3053–3056. [Google Scholar] [CrossRef]
- Moravek, M.; Dietrich, R.; Buerk, C.; Broussolle, V.; Guinebretière, M.-H.; Granum, P.E.; Nguyen-The, C.; Märtlbauer, E. Determination of the toxic potential of Bacillus cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 2006, 257, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.-E.; Huptas, C.; Krey, V.M.; Scherer, S. Massive horizontal gene transfer, strictly vertical inheritance and ancient duplications differentially shape the evolution of Bacillus cereus enterotoxin operons hbl, cytK and nhe. BMC Evol. Biol. 2015, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Alegro, L.C.; Palcich, G.; Lopes, G.V.; Ribeiro, V.B.; Landgraf, M.; Destro, M.T. Enterotoxigenic and genetic profiles of Bacillus cereus strains of food origin in Brazil. J. Food Prot. 2008, 71, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.Q.; Pires, E.S.; Vivoni, A.M. Genetic diversity, antimicrobial resistance and toxigenic profiles of Bacillus cereus isolated from food in Brazil over three decades. Int. J. Food Microbiol. 2011, 147, 12–16. [Google Scholar] [CrossRef]
- Ankolekar, C.; Rahmati, T.; Labbé, R.G. Detection of toxigenic Bacillus cereus and Bacillus thuringiensis spores in U.S. rice. Int. J. Food Microbiol. 2009, 128, 460–466. [Google Scholar] [CrossRef]
- Batchoun, R.; Al-Sha’Er, A.I.; Khabour, O.F. Molecular characterization of Bacillus cereus toxigenic strains isolated from different food matrices in Jordan. Foodborne Pathog. Dis. 2011, 8, 1153–1158. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Dietrich, R.; Märtlbauer, E.; Cao, J.; Ding, S.; Zhu, K. Characterization of Bacillus cereus isolates from local dairy farms in China. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef]
- Forghani, F.; Kim, J.-B.; Oh, D.-H. Enterotoxigenic profiling of emetic toxin- and enterotoxin-producing Bacillus cereus, isolated from food, environmental, and clinical samples by multiplex PCR. J. Food Sci. 2014, 79, M2288–M2293. [Google Scholar] [CrossRef]
- Gao, T.; Ding, Y.; Wu, Q.; Wang, J.; Zhang, J.; Yu, S.; Yu, P.; Liu, C.; Kong, L.; Feng, Z.; et al. Prevalence, virulence genes, antimicrobial susceptibility, and genetic diversity of Bacillus cereus isolated from pasteurized milk in China. Front. Microbiol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Park, K.M.; Jeong, M.; Park, K.J.; Koo, M. Prevalence, enterotoxin genes, and antibiotic resistance of Bacillus cereus isolated from raw vegetables in Korea. J. Food Prot. 2018, 81, 1590–1597. [Google Scholar] [CrossRef]
- Rana, N.; Panda, A.K.; Pathak, N.; Gupta, T.; Thakur, S.D. Bacillus cereus: Public health burden associated with ready-to-eat foods in Himachal Pradesh, India. J. Food Sci. Technol. 2020, 57, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Thaenthanee, S.; Wong, A.; Panbangred, W. Phenotypic and genotypic comparisons reveal a broad distribution and heterogeneity of hemolysin BL genes among Bacillus cereus isolates. Int. J. Food Microbiol. 2005, 105, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.-H.; Kim, K.-Y.; Chon, J.-W.; Kim, D.-H.; Kim, H.-S.; Choi, D.-S.; Choi, I.-S.; Seo, K.-H. Incidence, antibiotic susceptibility, and toxin profiles of Bacillus cereus sensu lato isolated from Korean fermented soybean products. J. Food Sci. 2015, 80, M1266–M1270. [Google Scholar] [CrossRef] [PubMed]
- Amor, M.G.-B.; Jan, S.; Baron, F.; Grosset, N.; Culot, A.; Gdoura, R.; Gautier, M.; Techer, C. Toxigenic potential and antimicrobial susceptibility of Bacillus cereus group bacteria isolated from Tunisian foodstuffs. BMC Microbiol. 2019, 19, 196. [Google Scholar] [CrossRef]
- Ouoba, L.; Thorsen, L.; Varnam, A.H. Enterotoxins and emetic toxins production by Bacillus cereus and other species of Bacillus isolated from Soumbala and Bikalga, African alkaline fermented food condiments. Int. J. Food Microbiol. 2008, 124, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Kwarteng, J.; Wuni, A.; Akabanda, F.; Debrah, K.T.; Jespersen, L. Prevalence, virulence factor genes and antibiotic resistance of Bacillus cereus sensu lato isolated from dairy farms and traditional dairy products. BMC Microbiol. 2017, 17, 65. [Google Scholar] [CrossRef]
- Drewnowska, J.M.; Stefanska, N.; Czerniecka, M.; Zambrowski, G.; Swiecicka, I. Potential Enterotoxicity of phylogenetically diverse Bacillus cereus sensu lato soil isolates from different geographical locations. Appl. Environ. Microbiol. 2020, 86, 86. [Google Scholar] [CrossRef]
- Mäntynen, V.; Lindström, K. A rapid PCR-based DNA test for enterotoxic Bacillus cereus. Appl. Environ. Microbiol. 1998, 64, 1634–1639. [Google Scholar] [CrossRef]
- Prüß, B.M.; Dietrich, R.; Nibler, B.; Märtlbauer, E.; Scherer, S. The hemolytic enterotoxin HBL is broadly distributed among species of the Bacillus cereus group. Appl. Environ. Microbiol. 1999, 65, 5436–5442. [Google Scholar] [CrossRef]
- Zahner, V.; Cabral, D.A.; Régua-Mangia, A.H.; Rabinovitch, L.; Moreau, G.; McIntosh, D. Distribution of genes encoding putative virulence factors and fragment length polymorphisms in the vrra gene among brazilian isolates of Bacillus cereus and Bacillus thuringiensis. Appl. Environ. Microbiol. 2005, 71, 8107–8114. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, L.; Xu, H.; Liu, C.; Shah, N.P.; Wei, H. Detection of viable enterotoxin-producing Bacillus cereus and analysis of toxigenicity from ready-to-eat foods and infant formula milk powder by multiplex PCR. J. Dairy Sci. 2016, 99, 1047–1055. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Pluta, A.; Garbowska, M.; Stefańska, I.; Pluta, B. Prevalence and toxicity characterization of Bacillus cereus in food products from Poland. Foods 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.M.; Silva, H.O.; Aguilar, C.E.G.; Rochetti, A.L.; Pascoe, B.; Meric, G.; Mourkas, E.; Hitchings, M.D.; Mathias, L.A.; Ruiz, V.L.D.A.; et al. Comparative genomic survey of Bacillus cereus sensu stricto isolates from the dairy production chain in Brazil. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Park, J.-H. Characteristics of enterotoxin distribution, hemolysis, lecithinase, and starch hydrolysis of Bacillus cereus isolated from infant formulas and ready-to-eat foods. J. Dairy Sci. 2015, 98, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.A.; Almeida, F.; Guimarães, A.G.; Abrahão, W.; Arantes, O.; Vilas-Bôas, G. RE-PCR variability and toxigenic profile of food poisoning, foodborne and soil-associated Bacillus cereus isolates from Brazil. Int. J. Food Microbiol. 2011, 151, 277–283. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Knutsson, R.; Scherer, S. Bacillus cereus. In Genomes of Food and Water-Borne Pathogens; Kathariou, S., Fratamico, P., Liu, Y., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 147–164. [Google Scholar]
- Thorsen, L.; Hansen, B.M.; Nielsen, K.F.; Hendriksen, N.B.; Phipps, R.K.; Budde, B.B. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl. Environ. Microbiol. 2006, 72, 5118–5121. [Google Scholar] [CrossRef]
- Chaves, J.Q.; Cavados, C.D.F.G.; Vivoni, A.M. Molecular and toxigenic characterization of Bacillus cereus and Bacillus thuringiensis strains isolated from commercial ground roasted coffee. J. Food Prot. 2012, 75, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Chon, J.; Kim, J.; Lee, S.; Hyeon, J.; Song, K.; Park, C.; Seo, K.-H. Prevalence, phenotypic traits and molecular characterization of emetic toxin-producing Bacillus cereus strains isolated from human stools in Korea. J. Appl. Microbiol. 2012, 112, 1042–1049. [Google Scholar] [CrossRef]
- López, A.C.; Minnaard, J.; Pérez, P.F.; Alippi, A.M. A case of intoxication due to a highly cytotoxic Bacillus cereus strain isolated from cooked chicken. Food Microbiol. 2015, 46, 195–199. [Google Scholar] [CrossRef]
- Rahimi, E.; Abdos, F.; Momtaz, H.; Baghbadorani, Z.T.; Jalali, M. Bacillus cereus in infant foods: Prevalence study and distribution of enterotoxigenic virulence factors in isfahan province, Iran. Sci. World J. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Zeighami, H.; Nejad-Dost, G.; Parsadanians, A.; Daneshamouz, S.; Haghi, F. Frequency of hemolysin BL and non-hemolytic enterotoxin complex genes of Bacillus cereus in raw and cooked meat samples in Zanjan, Iran. Toxicol. Rep. 2020, 7, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Chon, J.-W.; Kim, J.-H.; Lee, S.-J.; Hyeon, J.-Y.; Seo, K.-H. Toxin profile, antibiotic resistance, and phenotypic and molecular characterization of Bacillus cereus in Sunsik. Food Microbiol. 2012, 32, 217–222. [Google Scholar] [CrossRef]
- Chon, J.-W.; Yim, J.-H.; Kim, H.-S.; Kim, D.-H.; Kim, H.; Oh, D.-H.; Kim, S.-K.; Seo, K.-H. Quantitative prevalence and toxin gene profile of Bacillus cereus from ready-to-eat vegetables in South Korea. Foodborne Pathog. Dis. 2015, 12, 795–799. [Google Scholar] [CrossRef]
- Flores-Urbán, K.A.; Natividad-Bonifacio, I.; Vázquez-Quiñones, C.R.; Vázquez-Salinas, C.; Quiñones-Ramírez, E.I. Detection of toxigenic Bacillus cereus strains isolated from vegetables in Mexico city. J. Food Prot. 2014, 77, 2144–2147. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Kim, J.-M.; Cho, S.-H.; Oh, H.-S.; Choi, N.J.; Oh, D.-H. Toxin genes profiles and toxin production ability of Bacillus cereus isolated from clinical and food samples. J. Food Sci. 2011, 76, T25–T29. [Google Scholar] [CrossRef]
- Lee, N.; Sun, J.M.; Kwon, K.Y.; Kim, H.J.; Koo, M.; Chun, H.S. Genetic diversity, antimicrobial resistance, and toxigenic profiles of Bacillus cereus strains isolated from Sunsik. J. Food Prot. 2012, 75, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.A.; Jian, J.; Beno, S.M.; Wiedmann, M.; Kovac, J. Intraclade variability in toxin production and cytotoxicity of Bacillus cereus group type strains and dairy-associated isolates. Appl. Environ. Microbiol. 2018, 84, 84. [Google Scholar] [CrossRef]
- Jessberger, N.; Krey, V.M.; Rademacher, C.; Böhm, M.-E.; Mohr, A.-K.; Ehling-Schulz, M.; Scherer, S.; Märtlbauer, E. From genome to toxicity: A combinatory approach highlights the complexity of enterotoxin production in Bacillus cereus. Front. Microbiol. 2015, 6, 560. [Google Scholar] [CrossRef]
- Jessberger, N.; Rademacher, C.; Krey, V.M.; Dietrich, R.; Mohr, A.-K.; Böhm, M.-E.; Scherer, S.; Ehling-Schulz, M.; Märtlbauer, E. Simulating intestinal growth conditions enhances toxin production of enteropathogenic Bacillus cereus. Front. Microbiol. 2017, 8, 627. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Messelhäusser, U. Bacillus “next generation” diagnostics: Moving from detection toward subtyping and risk-related strain profiling. Front. Microbiol. 2013, 4, 32. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 7932:2004 Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus cereus—Colony-Count Technique at 30 °C. Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=382192004 (accessed on 29 June 2020).
- Guinebretière, M.-H.; Auger, S.; Galleron, N.; Contzen, M.; De Sarrau, B.; De Buyser, M.-L.; Lamberet, G.; Fagerlund, A.; Granum, P.E.; Lereclus, D.; et al. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int. J. Syst. Evol. Microbiol. 2013, 63, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Guinebretière, M.-H.; Thompson, F.L.; Sorokin, A.; Normand, P.; Dawyndt, P.; Ehling-Schulz, M.; Svensson, B.; Sanchis, V.; Nguyen-The, C.; Heyndrickx, M.; et al. Ecological diversification in the Bacillus cereus group. Environ. Microbiol. 2008, 10, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; Urdiain, M.; Cifuentes, A.; López-López, A.; Blanch, A.R.; Tamames, J.; Kämpfer, P.; Kolstø, A.-B.; Ramón, D.; Martínez, J.F.; et al. Description of Bacillus toyonensis sp. nov., a novel species of the Bacillus cereus group, and pairwise genome comparisons of the species of the group by means of ANI calculations. Syst. Appl. Microbiol. 2013, 36, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lai, Q.; Göker, M.; Meier-Kolthoff, J.P.; Wang, M.; Sun, Y.; Wang, L.; Shao, Z. Genomic insights into the taxonomic status of the Bacillus cereus group. Sci. Rep. 2015, 5, srep14082. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, J.-S.; Paek, W.K.; Lim, J.; Lee, H.; Kim, P.I.; Ma, J.Y.; Kim, W.; Chang, Y.-H. Bacillus manliponensis sp. nov., a new member of the Bacillus cereus group isolated from foreshore tidal flat sediment. J. Microbiol. 2011, 49, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-Y.; Paek, W.K.; Park, I.-S.; Han, J.-R.; Sin, Y.; Paek, J.; Rhee, M.-S.; Kim, H.; Song, H.S.; Chang, Y.-H. Bacillus gaemokensis sp. nov., isolated from foreshore tidal flat sediment from the Yellow Sea. J. Microbiol. 2010, 48, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, G.-H.; Hu, G.-P.; Cetin, S.; Lin, N.-Q.; Tang, J.-Y.; Tang, W.-Q.; Lin, Y.-Z. Bacillus bingmayongensis sp. nov., isolated from the pit soil of Emperor Qin’s Terra-cotta warriors in China. Antonie van Leeuwenhoek 2014, 105, 501–510. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Lai, Q.; Zeng, R.; Ye, D.; Xu, J.; Shao, Z. Proposal of nine novel species of the Bacillus cereus group. Int. J. Syst. Evol. Microbiol. 2017, 67, 2499–2508. [Google Scholar] [CrossRef]
- Miller, R.A.; Beno, S.M.; Kent, D.J.; Carroll, L.M.; Martin, N.H.; Boor, K.J.; Kovac, J. Bacillus wiedmannii sp. nov., a psychrotolerant and cytotoxic Bacillus cereus group species isolated from dairy foods and dairy environments. Int. J. Syst. Evol. Microbiol. 2016, 66, 4744–4753. [Google Scholar] [CrossRef]
- Okinaka, R.T.; Keim, P.S. The phylogeny of Bacillus cereus sensu lato. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.M.; Wiedmann, M.; Kovac, J. Proposal of a taxonomic nomenclature for the Bacillus cereus group which reconciles genomic definitions of bacterial species with clinical and industrial phenotypes. mBio 2020, 11, e00034-20. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, P.; Banerjee, G. Recent advancement on chemical arsenal of Bt toxin and its application in pest management system in agricultural field. 3 Biotech 2018, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [CrossRef]
- Celandroni, F.; Salvetti, S.; Senesi, S.; Ghelardi, E. Bacillus thuringiensis membrane-damaging toxins acting on mammalian cells. FEMS Microbiol. Lett. 2014, 361, 95–103. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kang, S.-H.; Lee, Y.-E.; Kim, S.-J.; Yoo, Y.-B.; Bak, Y.-S.; Kim, J.-B. Distribution of toxin genes and enterotoxins in Bacillus thuringiensis isolated from microbial insecticide products. J. Microbiol. Biotechnol. 2015, 25, 2043–2048. [Google Scholar] [CrossRef]
- Frentzel, H.; Kraushaar, B.; Krause, G.; Bodi, D.; Wichmann-Schauer, H.; Appel, B.; Mader, A. Phylogenetic and toxinogenic characteristics of Bacillus cereus group members isolated from spices and herbs. Food Control 2018, 83, 90–98. [Google Scholar] [CrossRef]
- Rivera, A.M.G.; Granum, P.E.; Priest, F.G. Common occurrence of enterotoxin genes and enterotoxicity in Bacillus thuringiensis. FEMS Microbiol. Lett. 2000, 190, 151–155. [Google Scholar] [CrossRef]
- Hansen, B.M.; Hendriksen, N.B. Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl. Environ. Microbiol. 2001, 67, 185–189. [Google Scholar] [CrossRef]
- Hariram, U.; Labbé, R.G. Spore prevalence and toxigenicity of Bacillus cereus and Bacillus thuringiensis isolates from U.S. retail spices. J. Food Prot. 2015, 78, 590–596. [Google Scholar] [CrossRef]
- Johler, S.; Kalbhenn, E.M.; Heini, N.; Brodmann, P.; Gautsch, S.; Bağcioğlu, M.; Contzen, M.; Stephan, R.; Ehling-Schulz, M. Enterotoxin production of Bacillus thuringiensis isolates from biopesticides, foods, and outbreaks. Front. Microbiol. 2018, 9, 1915. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bang, J.; Kim, H.; Kim, Y.; Kim, B.-S.; Beuchat, L.R.; Ryu, J. Bacillus cereus and Bacillus thuringiensis spores in Korean rice: Prevalence and toxin production as affected by production area and degree of milling. Food Microbiol. 2014, 42, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Choi, O.-K.; Kwon, S.-M.; Cho, S.-H.; Park, B.-J.; Jin, N.Y.; Yu, Y.-M.; Oh, D.-H. Prevalence and toxin characteristics of Bacillus thuringiensis isolated from organic vegetables. J. Microbiol. Biotechnol. 2017, 27, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Han, J.-K.; Park, J.-S.; Lee, J.-S.; Lee, S.-H.; Cho, J.-I.; Kim, K.-S. Various enterotoxin and other virulence factor genes widespread among Bacillus cereus and Bacillus thuringiensis strains. J. Microbiol. Biotechnol. 2015, 25, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.; Cheng, R.A.; Carroll, L.M.; Kent, D.J.; Jian, J.; Beno, S.M.; Wiedmann, M. Production of hemolysin BL by Bacillus cereus group isolates of dairy origin is associated with whole-genome phylogenetic clade. BMC Genom. 2016, 17, 581. [Google Scholar] [CrossRef]
- Kyei-Poku, G.; Gauthier, D.; Pang, A.; Van Frankenhuyzen, K. Detection of Bacillus cereus virulence factors in commercial products of Bacillus thuringiensis and expression of diarrheal enterotoxins in a target insect. Can. J. Microbiol. 2007, 53, 1283–1290. [Google Scholar] [CrossRef]
- Ngamwongsatit, P.; Buasri, W.; Pianariyanon, P.; Pulsrikarn, C.; Ohba, M.; Assavanig, A.; Panbangred, W. Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int. J. Food Microbiol. 2008, 121, 352–356. [Google Scholar] [CrossRef]
- Schwenk, V.; Riegg, J.; Lacroix, M.; Märtlbauer, E.; Jessberger, N. Enteropathogenic potential of Bacillus thuringiensis isolates from soil, animals, food and biopesticides. Foods 2020, 9, 1484. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; He, J.; Yuan, Y.; Yuan, Z. The occurrence of Bacillus cereus, B. thuringiensis and B. mycoides in Chinese pasteurized full fat milk. Int. J. Food Microbiol. 2008, 121, 195–200. [Google Scholar] [CrossRef]
- Swiecicka, I.; Van Der Auwera, G.A.; Mahillon, J. Hemolytic and nonhemolytic enterotoxin genes are broadly distributed among Bacillus thuringiensis isolated from wild mammals. Microb. Ecol. 2006, 52, 544–551. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, 4524. [Google Scholar] [CrossRef]
- Hoton, F.M.; Fornelos, N.; N’Guessan, E.; Hu, X.; Swiecicka, I.; Dierick, K.; Jääskeläinen, E.; Salkinoja-Salonen, M.; Mahillon, J. Family portrait of Bacillus cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ. Microbiol. Rep. 2009, 1, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, A. Studies on Bacillus thuringiensis strains isolated from Swedish soils: Insect toxicity and production of B. cereus-diarrhoeal-type enterotoxin. World J. Microbiol. Biotechnol. 1994, 10, 406–409. [Google Scholar] [CrossRef]
- Damgaard, P.H. Diarrhoeal enterotoxin production by strains of Bacillus thuringiensis isolated from commercial Bacillus thuringiensis-based insecticides. FEMS Immunol. Med Microbiol. 1995, 12, 245–249. [Google Scholar] [CrossRef]
- Damgaard, P.; Larsen, H.; Hansen, B.; Bresciani, J.; Jørgensen, K. Enterotoxin-producing strains of Bacillus thuringiensis isolated from food. Lett. Appl. Microbiol. 1996, 23, 146–150. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Pang, J.-C.; Kao, S.-S.; Tsen, H.-Y. Enterotoxigenicity and cytotoxicity of Bacillus thuringiensis strains and development of a process for Cry1Ac production. J. Agric. Food Chem. 2003, 51, 100–105. [Google Scholar] [CrossRef]
- McIntyre, L.; Bernard, K.; Beniac, D.; Isaac-Renton, J.L.; Naseby, D.C. Identification of Bacillus cereus group species associated with food poisoning outbreaks in British Columbia, Canada. Appl. Environ. Microbiol. 2008, 74, 7451–7453. [Google Scholar] [CrossRef]
- Melnick, R.L.; Testen, A.; Poleatewich, A.; Backman, P.; Bailey, B. Detection and expression of enterotoxin genes in endophytic strains of Bacillus cereus. Lett. Appl. Microbiol. 2012, 54, 468–474. [Google Scholar] [CrossRef]
- Beattie, S.H.; Williams, A. Detection of toxigenic strains of Bacillus cereus and other Bacillus spp. with an improved cytotoxicity assay. Lett. Appl. Microbiol. 1999, 28, 221–225. [Google Scholar] [CrossRef]
- Abdulmawjood, A.; Herrmann, J.; Riede, S.; Jimenez, G.; Becker, A.; Breves, G. Evaluation of enterotoxin gene expression and enterotoxin production capacity of the probiotic strain Bacillus toyonensis BCT-7112T. PLoS ONE 2019, 14, e0214536. [Google Scholar] [CrossRef]
- Rejasse, A.; Gilois, N.; Barbosa, I.; Huillet, E.; Bevilacqua, C.; Tran, S.; Ramarao, N.; Stenfors Arnesen, L.P.; Sanchis-Borja, V. Temperature-dependent production of various plcr-controlled virulence factors in Bacillus weihenstephanensis strain KBAB4. Appl. Environ. Microbiol. 2012, 78, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, L.P.; Mayr, R.; Scherer, S.; Granum, P.E. Pathogenic potential of fifty Bacillus weihenstephanensis strains. FEMS Microbiol. Lett. 2002, 215, 47–51. [Google Scholar] [CrossRef] [PubMed]
- McKillip, J.L. Prevalence and expression of enterotoxins in Bacillus cereus and other Bacillus spp., a literature review. Antonie van Leeuwenhoek 2000, 77, 393–399. [Google Scholar] [CrossRef]
- Özdemir, F.; Arslan, S. Molecular characterization and toxin profiles of Bacillus spp. isolated from retail fish and ground beef. J. Food Sci. 2019, 84, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Phelps, R.J.; McKillip, J.L. Enterotoxin production in natural isolates of bacillaceae outside the Bacillus cereus group. Appl. Environ. Microbiol. 2002, 68, 3147–3151. [Google Scholar] [CrossRef]
- From, C.; Pukall, R.; Schumann, P.; Hormazábal, V.; Granum, P.E. Toxin-producing ability among Bacillus spp. outside the Bacillus cereus group. Appl. Environ. Microbiol. 2005, 71, 1178–1183. [Google Scholar] [CrossRef]
- Didelot, X.; Barker, M.; Falush, D.; Priest, F.G. Evolution of pathogenicity in the Bacillus cereus group. Syst. Appl. Microbiol. 2009, 32, 81–90. [Google Scholar] [CrossRef]
- Okutani, A.; Inoue, S.; Noguchi, A.; Kaku, Y.; Morikawa, S. Whole-genome sequence-based comparison and profiling of virulence-associated genes of Bacillus cereus group isolates from diverse sources in Japan. BMC Microbiol. 2019, 19, 296. [Google Scholar] [CrossRef]
- Carroll, L.M.; Kovac, J.; Miller, R.A.; Wiedmann, M. Rapid, High-throughput identification of anthrax-causing and emetic Bacillus cereus group genome assemblies via BTyper, a computational tool for virulence-based classification of Bacillus cereus group isolates by using nucleotide sequencing data. Appl. Environ. Microbiol. 2017, 83, e01096-17. [Google Scholar] [CrossRef]
- Guinebretière, M.-H.; Velge, P.; Couvert, O.; Carlin, F.; Debuyser, M.-L.; Nguyen-The, C. Ability of Bacillus cereus group strains to cause food poisoning varies according to phylogenetic affiliation (groups I to VII) rather than species affiliation. J. Clin. Microbiol. 2010, 48, 3388–3391. [Google Scholar] [CrossRef] [PubMed]
- Rouzeau-Szynalski, K.; Stollewerk, K.; Messelhäusser, U.; Ehling-Schulz, M. Why be serious about emetic Bacillus cereus: Cereulide production and industrial challenges. Food Microbiol. 2020, 85, 103279. [Google Scholar] [CrossRef] [PubMed]
- Agata, N.; Mori, M.; Ohta, M.; Suwan, S.; Ohtani, I.; Isobe, M. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol. Lett. 1994, 121, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Magarvey, N.A.; Ehling-Schulz, M.; Walsh, C.T. Characterization of the cereulide NRPS α-hydroxy acid specifying modules: Activation of α-keto acids and chiral reduction on the assembly line. J. Am. Chem. Soc. 2006, 128, 10698–10699. [Google Scholar] [CrossRef]
- Suwan, S.; Ohtani, I.; Agata, N.; Isobe, M.; Mori, M.; Ohta, M. Structure of cereulide, a cyclic dodecadepsipeptide toxin from Bacillus cereus and studies on NMR characteristics of its alkali metal complexes including a conformational structure of the K+ complex. J. Chem. Soc. Perkin Trans. 1 1995, 1, 765–775. [Google Scholar] [CrossRef]
- Mikkola, R.; Saris, N.-E.L.; Grigoriev, P.A.; Andersson, M.A.; Salkinoja-Salonen, M. Ionophoretic properties and mitochondrial effects of cereulide: The emetic toxin of B. cereus. JBIC J. Biol. Inorg. Chem. 1999, 263, 112–117. [Google Scholar] [CrossRef]
- Teplova, V.V.; Mikkola, R.; Tonshin, A.A.; Saris, N.-E.L.; Salkinoja-Salonen, M.S. The higher toxicity of cereulide relative to valinomycin is due to its higher affinity for potassium at physiological plasma concentration. Toxicol. Appl. Pharmacol. 2006, 210, 39–46. [Google Scholar] [CrossRef]
- Frenzel, E.; Letzel, T.; Scherer, S.; Ehling-Schulz, M. Inhibition of cereulide toxin synthesis by emetic Bacillus cereus via long-chain polyphosphates. Appl. Environ. Microbiol. 2010, 77, 1475–1482. [Google Scholar] [CrossRef]
- Marxen, S.; Stark, T.D.; Frenzel, E.; Rütschle, A.; Lücking, G.; Pürstinger, G.; Pohl, E.E.; Scherer, S.; Ehling-Schulz, M.; Hofmann, T. Chemodiversity of cereulide, the emetic toxin of Bacillus cereus. Anal. Bioanal. Chem. 2015, 407, 2439–2453. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Fricker, M.; Grallert, H.; Rieck, P.; Wagner, M.; Scherer, S. Cereulide synthetase gene cluster from emetic Bacillus cereus: Structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol. 2006, 6, 20. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Vukov, N.; Schulz, A.; Shaheen, R.; Andersson, M.; Märtlbauer, E.; Scherer, S. Identification and partial characterization of the nonribosomal peptide synthetase gene responsible for cereulide production in emetic Bacillus cereus. Appl. Environ. Microbiol. 2005, 71, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; De Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum information about a biosynthetic gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Mousa, J.J.; Bruner, S.D. Structural and mechanistic diversity of multidrug transporters. Nat. Prod. Rep. 2016, 33, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.A.; Marahiel, M.A. Molecular mechanisms underlying nonribosomal peptide synthesis: Approaches to new antibiotics. Chem. Rev. 2005, 105, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, D.; Mootz, H.D.; Linne, U.; Marahiel, M.A. Regeneration of misprimed nonribosomal peptide synthetases by type II thioesterases. Proc. Natl. Acad. Sci. USA 2002, 99, 14083–14088. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Rosovitz, M.J.; Økstad, O.A.; Fouts, D.E.; Jiang, L.; Cer, R.Z.; Kolstø, A.-B.; Gill, S.R.; Ravel, J. Complete sequence analysis of novel plasmids from emetic and periodontal Bacillus cereus isolates reveals a common evolutionary history among the B. cereus-group plasmids, including Bacillus anthracis pXO1. J. Bacteriol. 2006, 189, 52–64. [Google Scholar] [CrossRef]
- Gacek-Matthews, A.; Chromiková, Z.; Sulyok, M.; Lücking, G.; Barak, I.; Ehling-Schulz, M. Beyond toxin transport: Novel role of ABC transporter for enzymatic machinery of cereulide NRPS assembly line. mBio 2020, 11, 01577-20. [Google Scholar] [CrossRef]
- Dommel, M.K.; Frenzel, E.; Strasser, B.; Blöchinger, C.; Scherer, S.; Ehling-Schulz, M. Identification of the main promoter directing cereulide biosynthesis in emetic Bacillus cereus and its application for real-time monitoring of ces gene expression in foods. Appl. Environ. Microbiol. 2009, 76, 1232–1240. [Google Scholar] [CrossRef]
- Frenzel, E.; Doll, V.; Pauthner, M.; Lücking, G.; Scherer, S.; Ehling-Schulz, M. CodY orchestrates the expression of virulence determinants in emetic Bacillus cereus by impacting key regulatory circuits. Mol. Microbiol. 2012, 85, 67–88. [Google Scholar] [CrossRef]
- Lücking, G.; Dommel, M.K.; Scherer, S.; Fouet, A.; Ehling-Schulz, M. Cereulide synthesis in emetic Bacillus cereus is controlled by the transition state regulator AbrB, but not by the virulence regulator PlcR. Microbiology 2009, 155, 922–931. [Google Scholar] [CrossRef]
- Lücking, G.; Frenzel, E.; Rütschle, A.; Marxen, S.; Stark, T.D.; Hofmann, T.; Scherer, S.; Ehling-Schulz, M. Ces locus embedded proteins control the non-ribosomal synthesis of the cereulide toxin in emetic Bacillus cereus on multiple levels. Front. Microbiol. 2015, 6, 1101. [Google Scholar] [CrossRef] [PubMed]
- Dommel, M.K.; Lücking, G.; Scherer, S.; Ehling-Schulz, M. Transcriptional kinetic analyses of cereulide synthetase genes with respect to growth, sporulation and emetic toxin production in Bacillus cereus. Food Microbiol. 2011, 28, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xiong, H.; Geng, P.; Yuan, Z.; Hu, X. CesH represses cereulide synthesis as an alpha/beta fold hydrolase in Bacillus cereus. Toxins 2019, 11, 231. [Google Scholar] [CrossRef]
- Declerck, N.; Bouillaut, L.; Chaix, D.; Rugani, N.; Slamti, L.; Hoh, F.; Lereclus, D.; Arold, S.T. Structure of PlcR: Insights into virulence regulation and evolution of quorum sensing in Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 18490–18495. [Google Scholar] [CrossRef] [PubMed]
- Gohar, M.; Faegri, K.; Perchat, S.; Ravnum, S.; Økstad, O.A.; Gominet, M.; Kolstø, A.-B.; Lereclus, D. The PlcR virulence regulon of Bacillus cereus. PLoS ONE 2008, 3, e2793. [Google Scholar] [CrossRef] [PubMed]
- Shivers, R.P.; Sonenshein, A.L. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol. Microbiol. 2004, 53, 599–611. [Google Scholar] [CrossRef]
- Walsh, C.T. Insights into the chemical logic and enzymatic machinery of NRPS assembly lines. Nat. Prod. Rep. 2015, 33, 127–135. [Google Scholar] [CrossRef]
- Häggblom, M.; Apetroaie, C.; Andersson, M.A.; Salkinoja-Salonen, M.S. Quantitative analysis of cereulide, the emetic toxin of Bacillus cereus, produced under various conditions. Appl. Environ. Microbiol. 2002, 68, 2479–2483. [Google Scholar] [CrossRef]
- Kranzler, M.; Stollewerk, K.; Rouzeau-Szynalski, K.; Blayo, L.; Sulyok, M.; Ehling-Schulz, M. Temperature exerts control of Bacillus cereus emetic toxin production on post-transcriptional levels. Front. Microbiol. 2016, 7, 1640. [Google Scholar] [CrossRef]
- Frenzel, E.; Kranzler, M.; Stark, T.D.; Hofmann, T.; Ehling-Schulz, M. The endospore-forming pathogen Bacillus cereus exploits a small colony variant-based diversification strategy in response to aminoglycoside exposure. mBio 2015, 6, e01172-15. [Google Scholar] [CrossRef]
- Vangoitsenhoven, R.; Rondas, D.; Crèvecoeur, I.; D’Hertog, W.; Baatsen, P.; Masini, M.; Andjelkovic, M.; Van Loco, J.; Matthys, C.; Mathieu, C.; et al. Foodborne cereulide causes beta-cell dysfunction and apoptosis. PLoS ONE 2014, 9, e104866. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.M.; Roivainen, M.; Andersson, M.A.; Ylipaasto, P.; Hoornstra, D.; Mikkola, R.; Salkinoja-Salonen, M.S. In vitro toxicity of cereulide on porcine pancreatic Langerhans islets. Toxicon 2008, 51, 1029–1037. [Google Scholar] [CrossRef]
- Vangoitsenhoven, R.; Maris, M.; Overbergh, L.; Van Loco, J.; Mathieu, C.; Van Der Schueren, B. Cereulide food toxin, beta cell function and diabetes: Facts and hypotheses. Diabetes Res. Clin. Pract. 2015, 109, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Abia, W.A.; Warth, B.; Ezekiel, C.N.; Sarkanj, B.; Turner, P.C.; Marko, D.; Krska, R.; Sulyok, M. Uncommon toxic microbial metabolite patterns in traditionally home-processed maize dish (fufu) consumed in rural Cameroon. Food Chem. Toxicol. 2017, 107, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Beisl, J.; Pahlke, G.; Abeln, H.; Ehling-Schulz, M.; Del Favero, G.; Varga, E.; Warth, B.; Sulyok, M.; Abia, W.A.; Ezekiel, C.N.; et al. Combinatory effects of cereulide and deoxynivalenol on in vitro cell viability and inflammation of human Caco-2 cells. Arch. Toxicol. 2020, 94, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Dilkin, P.; Direito, G.; Simas, M.M.S.; Mallmann, C.A.; Corrêa, B. Toxicokinetics and toxicological effects of single oral dose of fumonisin B1 containing Fusarium verticillioides culture material in weaned piglets. Chem. Interact. 2010, 185, 157–162. [Google Scholar] [CrossRef]
- Haschek, W.M.; Motelin, G.; Ness, D.K.; Harlin, K.S.; Hall, W.F.; Vesonder, R.F.; Peterson, R.E.; Beasley, V.R. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia 1992, 117, 83–96. [Google Scholar] [CrossRef]
- Bauer, T.; Sipos, W.; Stark, T.D.; Käser, T.; Knecht, C.; Brunthaler, R.; Saalmüller, A.; Hofmann, T.; Ehling-Schulz, M. First insights into within host translocation of the Bacillus cereus toxin cereulide using a porcine model. Front. Microbiol. 2018, 9, 2652. [Google Scholar] [CrossRef]
- Bauer, T.; Stark, T.; Hofmann, T.; Ehling-Schulz, M. Development of a stable isotope dilution analysis for the quantification of the Bacillus cereus toxin cereulide in foods. J. Agric. Food Chem. 2010, 58, 1420–1428. [Google Scholar] [CrossRef]
- Doellinger, J.; Schneider, A.; Stark, T.D.; Ehling-Schulz, M.; Lasch, P. Evaluation of MALDI-ToF mass spectrometry for rapid detection of cereulide from Bacillus cereus cultures. Front. Microbiol. 2020, 11, 2483. [Google Scholar] [CrossRef]
- Fricker, M.; Messelhäusser, U.; Busch, U.; Scherer, S.; Ehling-Schulz, M. Diagnostic real-time PCR assays for the detection of emetic Bacillus cereus strains in foods and recent food-borne outbreaks. Appl. Environ. Microbiol. 2007, 73, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Fricker, M.; Scherer, S. Identification of emetic toxin producing Bacillus cereus strains by a novel molecular assay. FEMS Microbiol. Lett. 2004, 232, 189–195. [Google Scholar] [CrossRef]
- Wehrle, E.; Didier, A.; Moravek, M.; Dietrich, R.; Märtlbauer, E. Detection of Bacillus cereus with enteropathogenic potential by multiplex real-time PCR based on SYBR green I. Mol. Cell. Probes 2010, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Dzieciol, M.; Fricker, M.; Wagner, M.; Hein, I.; Ehling-Schulz, M. A novel diagnostic real-time PCR assay for quantification and differentiation of emetic and non-emetic Bacillus cereus. Food Control. 2013, 32, 176–185. [Google Scholar] [CrossRef]
- Ulrich, S.; Gottschalk, C.; Dietrich, R.; Märtlbauer, E.; Gareis, M. Identification of cereulide producing Bacillus cereus by MALDI-TOF MS. Food Microbiol. 2019, 82, 75–81. [Google Scholar] [CrossRef]
- Fiedoruk, K.; Daniluk, T.; Fiodor, A.; Drewicka, E.; Buczynska, K.; Leszczynska, K.; Bideshi, D.K.; Swiecicka, I. MALDI-TOF MS portrait of emetic and non-emetic Bacillus cereus group members. Electrophoresis 2016, 37, 2235–2247. [Google Scholar] [CrossRef]
- Biesta-Peters, E.G.; Reij, M.W.; Blaauw, R.H.; in`t Veld, P.H.; Rajkovic, A.; Ehling-Schulz, M.; Abee, T. Quantification of the emetic toxin cereulide in food products by liquid chromatography-mass spectrometry using synthetic cereulide as a standard. Appl. Environ. Microbiol. 2010, 76, 7466–7472. [Google Scholar] [CrossRef]
- in`t Veld, P.; Van Der Laak, L.; Van Zon, M.; Biesta-Peters, E. Elaboration and validation of the method for the quantification of the emetic toxin of Bacillus cereus as described in EN-ISO 18465—Microbiology of the food chain—Quantitative determination of emetic toxin (cereulide) using LC-MS/MS. Int. J. Food Microbiol. 2019, 288, 91–96. [Google Scholar] [CrossRef]
- Marxen, S.; Stark, T.D.; Rütschle, A.; Lücking, G.; Frenzel, E.; Scherer, S.; Ehling-Schulz, M.; Hofmann, T. Multiparametric quantitation of the Bacillus cereus toxins cereulide and isocereulides A–G in foods. J. Agric. Food Chem. 2015, 63, 8307–8313. [Google Scholar] [CrossRef]
- Decleer, M.; Jovanovic, J.; Vakula, A.; Udovicki, B.; Agoua, R.-S.E.K.; Madder, A.; De Saeger, S.; Rajkovic, A. Oxygen consumption rate analysis of mitochondrial dysfunction caused by Bacillus cereus cereulide in Caco-2 and HepG2 cells. Toxins 2018, 10, 266. [Google Scholar] [CrossRef]
- García-Calvo, J.; Ibeas, S.; Antón-García, E.; Torroba, T.; Aguilar, G.G.; Antunes, W.; González-Lavado, E.; Fanarraga, M.L. Potassium-ion-selective fluorescent sensors to detect cereulide, the emetic toxin of B. cereus, in food samples and HeLa cells. ChemistryOpen 2017, 6, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.A.; Mikkola, R.; Helin, J.; Salkinoja-Salonen, M. A novel sensitive bioassay for detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl. Environ. Microbiol. 1998, 64, 1338–1343. [Google Scholar] [CrossRef]
- Hoornstra, D.; Andersson, M.A.; Mikkola, R.; Salkinoja-Salonen, M. A new method for in vitro detection of microbially produced mitochondrial toxins. Toxicol. Vitr. 2003, 17, 745–751. [Google Scholar] [CrossRef]
- Ryan, P.A.; Macmillan, J.D.; Zilinskas, B.A. Molecular cloning and characterization of the genes encoding the L1 and L2 components of hemolysin BL from Bacillus cereus. J. Bacteriol. 1997, 179, 2551–2556. [Google Scholar] [CrossRef]
- Auger, S.; Galleron, N.; Ségurens, B.; Dossat, C.; Bolotin, A.; Wincker, P.; Sorokin, A. Complete genome sequence of the highly hemolytic strain Bacillus cereus F837/76. J. Bacteriol. 2012, 194, 1630. [Google Scholar] [CrossRef]
- Beecher, D.J.; Macmillan, J.D. A novel bicomponent hemolysin from Bacillus cereus. Infect. Immun. 1990, 58, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Agaisse, H.; Gominet, M.; Økstad, O.A.; Kolstø, A.-B.; Lereclus, D. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 1999, 32, 1043–1053. [Google Scholar] [CrossRef]
- Økstad, O.A.; Gominet, M.; Purnelle, B.; Rose, M.; Lereclus, D.; Kolstø, A.-B. Sequence analysis of three Bacillus cereus loci carrying PlcR-regulated genes encoding degradative enzymes and enterotoxin. Microbiology 1999, 145, 3129–3138. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, J.H.; Beecher, D.J.; Macmillan, J.D.; Zilinskas, B.A. Molecular cloning and characterization of the hblA gene encoding the B component of hemolysin BL from Bacillus cereus. J. Bacteriol. 1993, 175, 6760–6766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sastalla, I.; Fattah, R.; Coppage, N.; Nandy, P.; Crown, D.; Pomerantsev, A.P.; Leppla, S.H. The Bacillus cereus Hbl and Nhe tripartite enterotoxin components assemble sequentially on the surface of target cells and are not interchangeable. PLoS ONE 2013, 8, e76955. [Google Scholar] [CrossRef]
- Lindbäck, T.; Økstad, O.A.; Rishovd, A.-L.; Kolstø, A.-B. Insertional inactivation of hblC encoding the L2 component of Bacillus cereus ATCC 14579 haemolysin BL strongly reduces enterotoxigenic activity, but not the haemolytic activity against human erythrocytes. Microbiology 1999, 145, 3139–3146. [Google Scholar] [CrossRef]
- Clair, G.; Roussi, S.; Armengaud, J.; Duport, C. Expanding the known repertoire of virulence factors produced by Bacillus cereus through early secretome profiling in three redox conditions. Mol. Cell. Proteom. 2010, 9, 1486–1498. [Google Scholar] [CrossRef]
- Granum, P.E.; O’Sullivan, K.; Lund, T. The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol. Lett. 1999, 177, 225–229. [Google Scholar] [CrossRef]
- Granum, P.E. An outbreak of Bacillus cereus food poisoning during the Norwegian Ski Championships for juniors. Norsk Veterinærtidskrift 1995, 107, 945–948. [Google Scholar]
- Lindbäck, T.; Fagerlund, A.; Rødland, M.S.; Granum, P.E. Characterization of the Bacillus cereus Nhe enterotoxin. Microbiology 2004, 150, 3959–3967. [Google Scholar] [CrossRef]
- Böhm, M.-E.; Krey, V.M.; Jessberger, N.; Frenzel, E.; Scherer, S. Comparative bioinformatics and experimental analysis of the intergenic regulatory regions of Bacillus cereus hbl and nhe enterotoxin operons and the impact of CodY on virulence heterogeneity. Front. Microbiol. 2016, 7, 768. [Google Scholar] [CrossRef]
- Lapidus, A.; Goltsman, E.; Auger, S.; Galleron, N.; Ségurens, B.; Dossat, C.; Land, M.L.; Broussolle, V.; Brillard, J.; Guinebretière, M.-H.; et al. Extending the Bacillus cereus group genomics to putative food-borne pathogens of different toxicity. Chem. Interact. 2008, 171, 236–249. [Google Scholar] [CrossRef]
- Zheng, J.; Guan, Z.; Cao, S.; Peng, D.; Ruan, L.; Jiang, D.; Sun, M. Plasmids are vectors for redundant chromosomal genes in the Bacillus cereus group. BMC Genom. 2015, 16, 6. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C.L. Tripartite haemolysin BL: Isolation and characterization of two distinct homologous sets of components from a single Bacillus cereus isolate. Microbiology 2000, 146, 1371–1380. [Google Scholar] [CrossRef]
- Schoeni, J.L.; Wong, A.C. Heterogeneity observed in the components of hemolysin BL, an enterotoxin produced by Bacillus cereus. Int. J. Food Microbiol. 1999, 53, 159–167. [Google Scholar] [CrossRef]
- Fagerlund, A.; Brillard, J.; Fürst, R.; Guinebretière, M.-H.; Granum, P.E. Toxin production in a rare and genetically remote cluster of strains of the Bacillus cereus group. BMC Microbiol. 2007, 7, 43. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, T.; Xu, Y.; Zhou, G.; Zou, P.; Zeng, G.; Liu, X. Genetic and genomic diversity of NheABC locus from Bacillus strains. Arch. Microbiol. 2017, 199, 775–785. [Google Scholar] [CrossRef]
- Fagerlund, A.; Lindbäck, T.; Storset, A.K.; Granum, P.E.; Hardy, S.P. Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 2008, 154, 693–704. [Google Scholar] [CrossRef]
- Han, C.S.; Xie, G.; Challacombe, J.F.; Altherr, M.R.; Bhotika, S.S.; Bruce, D.; Campbell, C.S.; Campbell, M.L.; Chen, J.; Chertkov, O.; et al. Pathogenomic sequence analysis of Bacillus cereus and Bacillus thuringiensis isolates closely related to Bacillus anthracis. J. Bacteriol. 2006, 188, 3382–3390. [Google Scholar] [CrossRef]
- Ganash, M.; Phung, D.; Sedelnikova, S.E.; Lindbäck, T.; Granum, P.; Artymiuk, P.J. Structure of the NheA component of the Nhe toxin from Bacillus cereus: Implications for function. PLoS ONE 2013, 8, e74748. [Google Scholar] [CrossRef]
- Cardazzo, B.; Negrisolo, E.; Carraro, L.; Alberghini, L.; Patarnello, T.; Giaccone, V. Multiple-locus sequence typing and analysis of toxin genes in Bacillus cereus food-borne isolates. Appl. Environ. Microbiol. 2007, 74, 850–860. [Google Scholar] [CrossRef]
- Helgason, E.; Tourasse, N.J.; Meisal, R.; Caugant, D.A.; Kolstø, A.-B. Multilocus sequence typing scheme for bacteria of the Bacillus cereus group. Appl. Environ. Microbiol. 2004, 70, 191–201. [Google Scholar] [CrossRef]
- Ko, K.S.; Kim, J.-W.; Kim, W.; Chung, S.-I.; Kim, I.J.; Kook, Y.-H.; Kim, J.-M. Population structure of the Bacillus cereus group as determined by sequence analysis of six housekeeping genes and the plcR gene. Infect. Immun. 2004, 72, 5253–5261. [Google Scholar] [CrossRef]
- Sorokin, A.; Candelon, B.; Guilloux, K.; Galleron, N.; Wackerow-Kouzova, N.; Ehrlich, S.D.; Bourguet, D.; Sanchis, V. Multiple-locus sequence typing analysis of Bacillus cereus and Bacillus thuringiensis reveals separate clustering and a distinct population structure of psychrotrophic strains. Appl. Environ. Microbiol. 2006, 72, 1569–1578. [Google Scholar] [CrossRef]
- Ticknor, L.O.; Kolstø, A.-B.; Hill, K.K.; Keim, P.; Laker, M.T.; Tonks, M.; Jackson, P.J. Fluorescent amplified fragment length polymorphism analysis of norwegian Bacillus cereus and Bacillus thuringiensis soil isolates. Appl. Environ. Microbiol. 2001, 67, 4863–4873. [Google Scholar] [CrossRef]
- Rasigade, J.-P.; Hollandt, F.; Wirth, T. Genes under positive selection in the core genome of pathogenic Bacillus cereus group members. Infect. Genet. Evol. 2018, 65, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.; Moravek, M.; Bürk, C.; Granum, P.E.; Märtlbauer, E. Production and characterization of antibodies against each of the three subunits of the Bacillus cereus nonhemolytic enterotoxin complex. Appl. Environ. Microbiol. 2005, 71, 8214–8220. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Bock, S.; Didier, A.; Märtlbauer, E. Bacillus cereus enterotoxins act as major virulence factors and exhibit distinct cytotoxicity to different human cell lines. Toxicon 2014, 77, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Duport, C.; Schmitt, P. Anaerobiosis and low specific growth rates enhance hemolysin BL production by Bacillus cereus F4430/73. Arch. Microbiol. 2004, 182, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Duport, C.; Zigha, A.; Rosenfeld, E.; Schmitt, P. Control of enterotoxin gene expression in Bacillus cereus F4430/73 involves the redox-sensitive ResDE signal transduction system. J. Bacteriol. 2006, 188, 6640–6651. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, A.; Dubois, T.; Økstad, O.-A.; Verplaetse, E.; Gilois, N.; Bennaceur, I.; Perchat, S.; Gominet, M.; Aymerich, S.; Kolstø, A.-B.; et al. SinR controls enterotoxin expression in Bacillus thuringiensis biofilms. PLoS ONE 2014, 9, e87532. [Google Scholar] [CrossRef]
- Glatz, B.A.; Goepfert, J.M. Production of Bacillus cereus enterotoxin in defined media in fermenter-grown cultures. J. Food Prot. 1977, 40, 472–474. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; O’Sullivan, K.; Granum, P.E. Food poisoning potential of Bacillus cereus strains from Norwegian dairies. Int. J. Food Microbiol. 2007, 116, 292–296. [Google Scholar] [CrossRef]
- Van Der Voort, M.; Kuipers, O.P.; Buist, G.; De Vos, W.M.; Abee, T. Assessment of CcpA-mediated catabolite control of gene expression in Bacillus cereus ATCC 14579. BMC Microbiol. 2008, 8, 62. [Google Scholar] [CrossRef]
- Zigha, A.; Rosenfeld, E.; Schmitt, P.; Duport, C. The redox regulator Fnr is required for fermentative growth and enterotoxin synthesis in Bacillus cereus F4430/73. J. Bacteriol. 2007, 189, 2813–2824. [Google Scholar] [CrossRef]
- Esbelin, J.; Armengaud, J.; Zigha, A.; Duport, C. ResDE-dependent regulation of enterotoxin gene expression in Bacillus cereus: Evidence for multiple modes of binding for ResD and interaction with Fnr. J. Bacteriol. 2009, 191, 4419–4426. [Google Scholar] [CrossRef]
- Esbelin, J.; Jouanneau, Y.; Armengaud, J.; Duport, C. ApoFnr binds as a monomer to promoters regulating the expression of enterotoxin genes of Bacillus cereus. J. Bacteriol. 2008, 190, 4242–4251. [Google Scholar] [CrossRef]
- Gohar, M.; Økstad, O.A.; Gilois, N.; Sanchis, V.; Kolstø, A.B.; Lereclus, D. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2002, 2, 784–791. [Google Scholar] [CrossRef]
- Lereclus, D.; Agaisse, H.; Gominet, M.; Salamitou, S.; Sanchis, V. Identification of a Bacillus thuringiensis gene that positively regulates transcription of the phosphatidylinositol-specific phospholipase C gene at the onset of the stationary phase. J. Bacteriol. 1996, 178, 2749–2756. [Google Scholar] [CrossRef]
- Lereclus, D.; Agaisse, H.; Grandvalet, C.; Salamitou, S.; Gominet, M. Regulation of toxin and virulence gene transcription in Bacillus thuringiensis. Int. J. Med Microbiol. 2000, 290, 295–299. [Google Scholar] [CrossRef]
- Brillard, J.; Susanna, K.; Michaud, C.; Dargaignaratz, C.; Gohar, M.; Nielsen-Leroux, C.; Ramarao, N.; Kolstø, A.-B.; Nguyen-The, C.; Lereclus, D.; et al. The YvfTU Two-component System is involved in plcR expression in Bacillus cereus. BMC Microbiol. 2008, 8, 183. [Google Scholar] [CrossRef]
- Gominet, M.; Slamti, L.; Gilois, N.; Rose, M.; Lereclus, D. Oligopeptide permease is required for expression of the Bacillus thuringiensis plcR regulon and for virulence. Mol. Microbiol. 2001, 40, 963–975. [Google Scholar] [CrossRef]
- Slamti, L.; Lereclus, D. A cell-cell signaling peptide activates the PlcR virulence regulon in bacteria of the Bacillus cereus group. EMBO J. 2002, 21, 4550–4559. [Google Scholar] [CrossRef]
- Bouillaut, L.; Perchat, S.; Arold, S.; Zorrilla, S.; Slamti, L.; Henry, C.; Gohar, M.; Declerck, N.; Lereclus, D. Molecular basis for group-specific activation of the virulence regulator PlcR by PapR heptapeptides. Nucleic Acids Res. 2008, 36, 3791–3801. [Google Scholar] [CrossRef]
- Pomerantsev, A.P.; Pomerantseva, O.M.; Camp, A.S.; Mukkamala, R.; Goldman, S.; Leppla, S. PapR peptide maturation: Role of the NprB protease in Bacillus cereus 569 PlcR/PapR global gene regulation. FEMS Immunol. Med. Microbiol. 2009, 55, 361–377. [Google Scholar] [CrossRef]
- Grenha, R.; Slamti, L.; Nicaise, M.; Refes, Y.; Lereclus, D.; Nessler, S. Structural basis for the activation mechanism of the PlcR virulence regulator by the quorum-sensing signal peptide PapR. Proc. Natl. Acad. Sci. USA 2013, 110, 1047–1052. [Google Scholar] [CrossRef]
- Slamti, L.; Perchat, S.; Gominet, M.; Vilas-Boas, G.; Fouet, A.; Mock, M.; Sanchis-Borja, V.; Chaufaux, J.; Gohar, M.; Lereclus, D. Distinct mutations in PlcR explain why some strains of the Bacillus cereus group are nonhemolytic. J. Bacteriol. 2004, 186, 3531–3538. [Google Scholar] [CrossRef] [PubMed]
- Sastalla, I.; Maltese, L.M.; Pomerantseva, O.M.; Pomerantsev, A.P.; Keane-Myers, A.; Leppla, S. Activation of the latent PlcR regulon in Bacillus anthracis. Microbiology 2010, 156, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Gohar, M.; Gilois, N.; Graveline, R.; Garreau, C.; Sanchis, V.; Lereclus, D. A comparative study of Bacillus cereus, Bacillus thuringiensis and Bacillus anthracis extracellular proteomes. Proteomics 2005, 5, 3696–3711. [Google Scholar] [CrossRef] [PubMed]
- Slamti, L.; Lereclus, D. Specificity and polymorphism of the PlcR-PapR quorum-sensing system in the Bacillus cereus group. J. Bacteriol. 2005, 187, 1182–1187. [Google Scholar] [CrossRef]
- Huillet, E.; Bridoux, L.; Barboza, I.; Lemy, C.; André-Leroux, G.; Lereclus, D. The signaling peptide PapR is required for the activity of the quorum-sensor PlcRa in Bacillus thuringiensis. Microbiology 2020, 166, 398–410. [Google Scholar] [CrossRef]
- Huillet, E.; Tempelaars, M.H.; André-Leroux, G.; Wanapaisan, P.; Bridoux, L.; Makhzami, S.; Panbangred, W.; Martin-Verstraete, I.; Abee, T.; Lereclus, D. PlcRa, a new quorum-sensing regulator from Bacillus cereus, plays a role in oxidative stress responses and cysteine metabolism in stationary phase. PLoS ONE 2012, 7, e51047. [Google Scholar] [CrossRef]
- Yehuda, A.; Slamti, L.; Bochnik-Tamir, R.; Malach, E.; Lereclus, D.; Hayouka, Z. Turning off Bacillus cereus quorum sensing system with peptidic analogs. Chem. Commun. 2018, 54, 9777–9780. [Google Scholar] [CrossRef]
- Yehuda, A.; Slamti, L.; Malach, E.; Lereclus, D.; Hayouka, Z. Elucidating the hot spot residues of quorum sensing peptidic autoinducer PapR by multiple amino acid replacements. Front. Microbiol. 2019, 10, 1246. [Google Scholar] [CrossRef]
- Lindbäck, T.; Mols, M.; Basset, C.; Granum, P.E.; Kuipers, O.P.; Kovács, A.T. CodY, a pleiotropic regulator, influences multicellular behaviour and efficient production of virulence factors in Bacillus cereus. Environ. Microbiol. 2012, 14, 2233–2246. [Google Scholar] [CrossRef]
- Barbieri, G.; Albertini, A.M.; Ferrari, E.; Sonenshein, A.L.; Belitsky, B.R. Interplay of CodY and ScoC in the regulation of major extracellular protease genes of Bacillus subtilis. J. Bacteriol. 2016, 198, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Belitsky, B.R. Role of branched-chain amino acid transport in Bacillus subtilis CodY activity. J. Bacteriol. 2015, 197, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Belitsky, B.R.; Brinsmade, S.R.; Sonenshein, A.L. Intermediate levels of Bacillus subtilis CodY activity are required for derepression of the branched-chain amino acid permease, BraB. PLoS Genet. 2015, 11, e1005600. [Google Scholar] [CrossRef] [PubMed]
- Belitsky, B.R.; Sonenshein, A.L. Roadblock repression of transcription by Bacillus subtilis CodY. J. Mol. Biol. 2011, 411, 729–743. [Google Scholar] [CrossRef]
- Brinsmade, S.R. CodY, a master integrator of metabolism and virulence in Gram-positive bacteria. Curr. Genet. 2016, 63, 417–425. [Google Scholar] [CrossRef]
- Brinsmade, S.R.; Alexander, E.L.; Livny, J.; Stettner, A.I.; Segrè, D.; Rhee, K.Y.; Sonenshein, A.L. Hierarchical expression of genes controlled by the Bacillus subtilis global regulatory protein CodY. Proc. Natl. Acad. Sci. USA 2014, 111, 8227–8232. [Google Scholar] [CrossRef]
- Brinsmade, S.R.; Kleijn, R.J.; Sauer, U.; Sonenshein, A.L. Regulation of CodY activity through modulation of intracellular branched-chain amino acid pools. J. Bacteriol. 2010, 192, 6357–6368. [Google Scholar] [CrossRef]
- Handke, L.D.; Shivers, R.P.; Sonenshein, A.L. Interaction of Bacillus subtilis CodY with GTP. J. Bacteriol. 2007, 190, 798–806. [Google Scholar] [CrossRef]
- Ratnayake-Lecamwasam, M. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 2001, 15, 1093–1103. [Google Scholar] [CrossRef]
- Sonenshein, A.L. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 2005, 8, 203–207. [Google Scholar] [CrossRef]
- Belitsky, B.R.; Barbieri, G.; Albertini, A.M.; Ferrari, E.; Strauch, M.A.; Sonenshein, A.L. Interactive regulation by the Bacillus subtilis global regulators CodY and ScoC. Mol. Microbiol. 2015, 97, 698–716. [Google Scholar] [CrossRef] [PubMed]
- Slamti, L.; Lemy, C.; Henry, C.; Guillot, A.; Huillet, E.; Lereclus, D. CodY regulates the activity of the virulence quorum sensor PlcR by controlling the import of the signaling peptide PapR in Bacillus thuringiensis. Front. Microbiol. 2016, 6, 1501. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, K.; Clavel, T.; Schmitt, P.; Duport, C. Fnr mediates carbohydrate-dependent regulation of catabolic and enterotoxin genes in Bacillus cereus F4430/73. Res. Microbiol. 2010, 161, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Esbelin, J.; Jouanneau, Y.; Duport, C. Bacillus cereus Fnr binds a [4Fe-4S] cluster and forms a ternary complex with ResD and PlcR. BMC Microbiol. 2012, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Baldwin, M.L. The molecular basis for the differential regulation of the hlyE-encoded haemolysin of Escherichia coli by FNR and HlyX lies in the improved activating region 1 contact of HlyX. Microbiology 1997, 143, 3785–3793. [Google Scholar] [CrossRef] [PubMed]
- Duport, C.; Jobin, M.; Schmitt, P. Adaptation in Bacillus cereus: From stress to disease. Front. Microbiol. 2016, 7, 1550. [Google Scholar] [CrossRef]
- Stülke, J.; Hillen, W. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 2000, 54, 849–880. [Google Scholar] [CrossRef]
- Ouhib, O.; Clavel, T.; Schmitt, P. The production of Bacillus cereus enterotoxins is influenced by carbohydrate and growth rate. Curr. Microbiol. 2006, 53, 222–226. [Google Scholar] [CrossRef]
- Ouhib-Jacobs, O.; Lindley, N.D.; Schmitt, P.; Clavel, T. Fructose and glucose mediates enterotoxin production and anaerobic metabolism of Bacillus cereus ATCC14579T. J. Appl. Microbiol. 2009, 107, 821–829. [Google Scholar] [CrossRef]
- Newman, J.A.; Rodrigues, C.; Lewis, R.J. Molecular basis of the activity of Sinr protein, the master regulator of biofilm formation in Bacillus subtilis. J. Biol. Chem. 2013, 288, 10766–10778. [Google Scholar] [CrossRef]
- Milton, M.E.; Draughn, G.L.; Bobay, B.G.; Stowe, S.D.; Olson, A.L.; Feldmann, E.A.; Thompson, R.J.; Myers, K.H.; Santoro, M.T.; Kearns, D.B.; et al. The solution structures and interaction of SinR and SinI: Elucidating the mechanism of action of the master regulator switch for biofilm formation in Bacillus subtilis. J. Mol. Biol. 2020, 432, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, N.; Zheng, S.; Yan, F.; Jiang, C.; Yu, Y.; Guo, J.; Chai, Y.; Chen, Y. The spo0A-sinI-sinR regulatory circuit plays an essential role in biofilm formation, nematicidal activities, and plant protection in Bacillus cereus AR156. Mol. Plant Microbe Interact. 2017, 30, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, S.; Faegri, K.; Ghelardi, E.; Kolstø, A.-B.; Senesi, S. Global gene expression profile for swarming Bacillus cereus bacteria. Appl. Environ. Microbiol. 2011, 77, 5149–5156. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, N.; Dietrich, R.; Mohr, A.-K.; Da Riol, C.; Märtlbauer, E. Porcine gastric mucin triggers toxin production of enteropathogenic Bacillus cereus. Infect. Immun. 2019, 87, 00765-18. [Google Scholar] [CrossRef] [PubMed]
- Clair, G.; Lorphelin, A.; Armengaud, J.; Duport, C. OhrRA functions as a redox-responsive system controlling toxinogenesis in Bacillus cereus. J. Proteom. 2013, 94, 527–539. [Google Scholar] [CrossRef]
- Hayrapetyan, H.; Tempelaars, M.; Groot, M.N.; Abee, T. Bacillus cereus ATCC 14579 RpoN (Sigma 54) is a pleiotropic regulator of growth, carbohydrate metabolism, motility, biofilm formation and toxin production. PLoS ONE 2015, 10, e0134872. [Google Scholar] [CrossRef]
- Neuhaus, K.; Landstorfer, R.; Fellner, L.; Simon, S.; Schafferhans, A.; Goldberg, T.; Marx, H.; Ozoline, O.N.; Rost, B.; Kuster, B.; et al. Translatomics combined with transcriptomics and proteomics reveals novel functional, recently evolved orphan genes in Escherichia coli O157:H7 (EHEC). BMC Genom. 2016, 17, 133. [Google Scholar] [CrossRef]
- Wijnands, L.M.; Dufrenne, J.B.; Van Leusden, F.M.; Abee, T. Germination of Bacillus cereus spores is induced by germinants from differentiated Caco-2 cells, a human cell line mimicking the epithelial cells of the small intestine. Appl. Environ. Microbiol. 2007, 73, 5052–5054. [Google Scholar] [CrossRef]
- Fagerlund, A.; Lindbäck, T.; Granum, P. Bacillus cereus cytotoxins Hbl, Nhe and CytK are secreted via the Sec translocation pathway. BMC Microbiol. 2010, 10, 304. [Google Scholar] [CrossRef]
- Tjalsma, H.; Antelmann, H.; Jongbloed, J.D.; Braun, P.G.; Darmon, E.; Dorenbos, R.; Dubois, J.-Y.F.; Westers, H.; Zanen, G.; Quax, W.J.; et al. Proteomics of protein secretion by Bacillus subtilis: Separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 2004, 68, 207–233. [Google Scholar] [CrossRef]
- Vörös, A.; Simm, R.; Slamti, L.; McKay, M.J.; Hegna, I.K.; Nielsen-Leroux, C.; Hassan, K.A.; Paulsen, I.T.; Lereclus, D.; Økstad, O.A.; et al. SecDF as part of the sec-translocase facilitates efficient secretion of Bacillus cereus toxins and cell wall-associated proteins. PLoS ONE 2014, 9, e103326. [Google Scholar] [CrossRef] [PubMed]
- Hueck, C.J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 1998, 62, 379–433. [Google Scholar] [CrossRef] [PubMed]
- Callegan, M.C.; Kane, S.T.; Cochran, D.C.; Gilmore, M.S.; Gominet, M.; Lereclus, D. Relationship of plcR-regulated factors to Bacillus endophthalmitis virulence. Infect. Immun. 2003, 71, 3116–3124. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Beecher, D.J.; Gominet, M.; Lereclus, D.; Wong, A.C.L.; Senesi, S. Requirement of flhA for swarming differentiation, flagellin export, and secretion of virulence-associated proteins in Bacillus thuringiensis. J. Bacteriol. 2002, 184, 6424–6433. [Google Scholar] [CrossRef]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Ceragioli, M.; Beecher, D.J.; Senesi, S.; Wong, A.C.L. Swarming behavior of and hemolysin BL secretion by Bacillus cereus. Appl. Environ. Microbiol. 2007, 73, 4089–4093. [Google Scholar] [CrossRef]
- Salvetti, S.; Ghelardi, E.; Celandroni, F.; Ceragioli, M.; Giannessi, F.; Senesi, S. FlhF, a signal recognition particle-like GTPase, is involved in the regulation of flagellar arrangement, motility behaviour and protein secretion in Bacillus cereus. Microbiology 2007, 153, 2541–2552. [Google Scholar] [CrossRef]
- Bouillaut, L.; Ramarao, N.; Buisson, C.; Gilois, N.; Gohar, M.; Lereclus, D.; Nielsen-Leroux, C. FlhA influences Bacillus thuringiensis PlcR-regulated gene transcription, protein production, and virulence. Appl. Environ. Microbiol. 2005, 71, 8903–8910. [Google Scholar] [CrossRef]
- Mazzantini, D.; Celandroni, F.; Salvetti, S.; Gueye, S.A.; Lupetti, A.; Senesi, S.; Ghelardi, E. FlhF is required for swarming motility and full pathogenicity of Bacillus cereus. Front. Microbiol. 2016, 7, 1644. [Google Scholar] [CrossRef]
- Mazzantini, D.; Fonnesu, R.; Celandroni, F.; Calvigioni, M.; Vecchione, A.; Mrusek, D.; Bange, G.; Ghelardi, E. GTP-dependent FlhF homodimer supports secretion of a hemolysin in Bacillus cereus. Front. Microbiol. 2020, 11, 879. [Google Scholar] [CrossRef]
- Senesi, S.; Salvetti, S.; Celandroni, F.; Ghelardi, E. Features of Bacillus cereus swarm cells. Res. Microbiol. 2010, 161, 743–749. [Google Scholar] [CrossRef]
- Senesi, S.; Celandroni, F.; Salvetti, S.; Beecher, D.J.; Wong, A.C.L.; Ghelardi, E. Swarming motility in Bacillus cereus and characterization of a fliY mutant impaired in swarm cell differentiation. Microbiology 2002, 148, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Senesi, S.; Ghelardi, E. Production, secretion and biological activity of Bacillus cereus enterotoxins. Toxins 2010, 2, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, N.; Kranzler, M.; Da Riol, C.; Schwenk, V.; Buchacher, T.; Dietrich, R.; Ehling-Schulz, M.; Märtlbauer, E. Assessing the toxic potential of enteropathogenic Bacillus cereus. Food Microbiol. 2019, 84, 103276. [Google Scholar] [CrossRef] [PubMed]
- Gilois, N.; Ramarao, N.; Bouillaut, L.; Perchat, S.; Aymerich, S.; Lereclus, D.; Gohar, M.; Nielsen-Leroux, C. Growth-related variations in the Bacillus cereus secretome. Proteom. 2007, 7, 1719–1728. [Google Scholar] [CrossRef]
- Turnbull, P.C.; Jørgensen, K.; Kramer, J.M.; Gilbert, R.J.; Parry, J.M. Severe clinical conditions associated with Bacillus cereus and the apparent involvement of exotoxins. J. Clin. Pathol. 1979, 32, 289–293. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C. Tripartite hemolysin BL from Bacillus cereus. Hemolytic analysis of component interactions and a model for its characteristic paradoxical zone phenomenon. J. Biol. Chem. 1997, 272, 233–239. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Schwemmer, S.; Tausch, F.; Schwenk, V.; Didier, A.; Märtlbauer, E. Binding to the target cell surface is the crucial step in pore formation of hemolysin BL from Bacillus cereus. Toxins 2019, 11, 281. [Google Scholar] [CrossRef]
- Beecher, D.J.; Olsen, T.W.; Somers, E.B.; Wong, A.C.L. Evidence for contribution of tripartite hemolysin BL, phosphatidylcholine-preferring phospholipase C, and collagenase to virulence of Bacillus cereus endophthalmitis. Infect. Immun. 2000, 68, 5269–5276. [Google Scholar] [CrossRef]
- Beecher, D.J.; Pulido, J.S.; Barney, N.P.; Wong, A.C. Extracellular virulence factors in Bacillus cereus endophthalmitis: Methods and implication of involvement of hemolysin BL. Infect. Immun. 1995, 63, 632–639. [Google Scholar] [CrossRef]
- Beecher, D.J.; Schoeni, J.L.; Wong, A.C. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 1995, 63, 4423–4428. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C. Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular permeability factor from Bacillus cereus. Infect. Immun. 1994, 62, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.; Granum, P.E. Comparison of biological effect of the two different enterotoxin complexes isolated from three different strains of Bacillus cereus. Microbiology 1997, 143, 3329–3336. [Google Scholar] [CrossRef]
- Ramarao, N.; Lereclus, D. Adhesion and cytotoxicity of Bacillus cereus and Bacillus thuringiensis to epithelial cells are FlhA and PlcR dependent, respectively. Microbes Infect. 2006, 8, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Tausch, F.; Dietrich, R.; Schauer, K.; Janowski, R.; Niessing, D.; Märtlbauer, E.; Jessberger, N. Evidence for complex formation of the Bacillus cereus haemolysin BL components in solution. Toxins 2017, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Ramm, F.; Dondapati, S.K.; Thoring, L.; Zemella, A.; Wüstenhagen, D.A.; Frentzel, H.; Stech, M.; Kubick, S. Mammalian cell-free protein expression promotes the functional characterization of the tripartite non-hemolytic enterotoxin from Bacillus cereus. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Schoeni, J.L.; Wong, A.C.L. Bacillus cereus food poisoning and its toxins. J. Food Prot. 2005, 68, 636–648. [Google Scholar] [CrossRef]
- Madegowda, M.; Eswaramoorthy, S.; Burley, S.K.; Swaminathan, S. X-ray crystal structure of the B component of Hemolysin BL from Bacillus cereus. Proteins Struct. Funct. Bioinform. 2008, 71, 534–540. [Google Scholar] [CrossRef]
- Phung, D.; Ganash, M.; Sedelnikova, S.E.; Lindbäck, T.; Granum, P.E.; Artymiuk, P.J. Crystallization and preliminary crystallographic analysis of the NheA component of the Nhe toxin from Bacillus cereus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 1073–1076. [Google Scholar] [CrossRef]
- Roderer, D.; Glockshuber, R. Assembly mechanism of the α-pore–forming toxin cytolysin A from Escherichia coli. Philos. Trans. R. Soc. B 2017, 372, 20160211. [Google Scholar] [CrossRef]
- Westermark, M.; Oscarsson, J.; Mizunoe, Y.; Urbonaviciene, J.; Uhlin, B.E. Silencing and activation of ClyA cytotoxin expression in Escherichia coli. J. Bacteriol. 2000, 182, 6347–6357. [Google Scholar] [CrossRef]
- Ludwig, A.; Bauer, S.; Benz, R.; Bergmann, B.; Goebel, W. Analysis of the SlyA-controlled expression, subcellular localization and pore-forming activity of a 34 kDa haemolysin (ClyA) from Escherichia coli K-12. Mol. Microbiol. 1999, 31, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Oscarsson, J.; Mizunoe, Y.; Li, L.; Lai, X.-H.; Wieslander, A.; Uhlin, B.E. Molecular analysis of the cytolytic protein ClyA (SheA) from Escherichia coli. Mol. Microbiol. 1999, 32, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.J.; Stillman, T.J.; Atkins, A.; Jamieson, S.J.; Bullough, P.A.; Green, J.; Artymiuk, P.J. E. coli Hemolysin E (HlyE, ClyA, SheA). Cell 2000, 100, 265–276. [Google Scholar] [CrossRef]
- Eifler, N.; Vetsch, M.; Gregorini, M.; Ringler, P.; Chami, M.; Philippsen, A.; Fritz, A.; Müller, S.A.; Glockshuber, R.; Engel, A.; et al. Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. EMBO J. 2006, 25, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Grauschopf, U.; Maier, T.; Glockshuber, R.; Ban, N. The structure of a cytolytic α-helical toxin pore reveals its assembly mechanism. Nat. Cell Biol. 2009, 459, 726–730. [Google Scholar] [CrossRef]
- Benke, S.; Roderer, D.; Wunderlich, B.; Nettels, D.; Glockshuber, R.; Schuler, B. The assembly dynamics of the cytolytic pore toxin ClyA. Nat. Commun. 2015, 6, 6198. [Google Scholar] [CrossRef]
- Peng, W.; Santos, M.D.S.; Li, Y.; Tomchick, D.R.; Orth, K. High-resolution cryo-EM structures of the E. coli hemolysin ClyA oligomers. PLoS ONE 2019, 14, e0213423. [Google Scholar] [CrossRef]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1865. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C.L. Cooperative, synergistic and antagonistic haemolytic interactions between haemolysin BL, phosphatidylcholine phospholipase C and sphingomyelinase from Bacillus cereus. Microbiology 2000, 146, 3033–3039. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Schauer, K.; Schwemmer, S.; Märtlbauer, E.; Benz, R. Characteristics of the protein complexes and pores formed by Bacillus cereus hemolysin BL. Toxins 2020, 12, 672. [Google Scholar] [CrossRef]
- Mohamed, N.; Li, J.; Ferreira, C.S.; Little, S.F.; Friedlander, A.M.; Spitalny, G.L.; Casey, L.S. Enhancement of anthrax lethal toxin cytotoxicity: A subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect. Immun. 2004, 72, 3276–3283. [Google Scholar] [CrossRef]
- Didier, A.; Dietrich, R.; Gruber, S.; Bock, S.; Moravek, M.; Nakamura, T.; Lindbäck, T.; Granum, P.E.; Märtlbauer, E. Monoclonal antibodies neutralize Bacillus cereus Nhe enterotoxin by inhibiting ordered binding of its three exoprotein components. Infect. Immun. 2011, 80, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Heilkenbrinker, U.; Dietrich, R.; Didier, A.; Zhu, K.; Lindbäck, T.; Granum, P.E.; Märtlbauer, E. Complex formation between NheB and NheC is necessary to induce cytotoxic activity by the three-component Bacillus cereus Nhe enterotoxin. PLoS ONE 2013, 8, e63104. [Google Scholar] [CrossRef] [PubMed]
- Lindbäck, T.; Hardy, S.P.; Dietrich, R.; Sødring, M.; Didier, A.; Moravek, M.; Fagerlund, A.; Bock, S.; Nielsen, C.; Casteel, M.; et al. Cytotoxicity of the Bacillus cereus Nhe enterotoxin requires specific binding order of its three exoprotein components. Infect. Immun. 2010, 78, 3813–3821. [Google Scholar] [CrossRef] [PubMed]
- Didier, A.; Dietrich, R.; Märtlbauer, E. Antibody binding studies reveal conformational flexibility of the Bacillus cereus non-hemolytic enterotoxin (Nhe) A-component. PLoS ONE 2016, 11, e0165135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Didier, A.; Dietrich, R.; Heilkenbrinker, U.; Waltenberger, E.; Jessberger, N.; Märtlbauer, E.; Benz, R. Formation of small transmembrane pores: An intermediate stage on the way to Bacillus cereus non-hemolytic enterotoxin (Nhe) full pores in the absence of NheA. Biochem. Biophys. Res. Commun. 2016, 469, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Beecher, D.J.; Wong, A.C. Identification and analysis of the antigens detected by two commercial Bacillus cereus diarrheal enterotoxin immunoassay kits. Appl. Environ. Microbiol. 1994, 60, 4614–4616. [Google Scholar] [CrossRef] [PubMed]
- Phung, D.; Granum, P.E.; Dietrich, R.; Märtlbauer, E.; Hardy, S.P. Inhibition of cytotoxicity by the Nhe cytotoxin of Bacillus cereus through the interaction of dodecyl maltoside with the NheB component. FEMS Microbiol. Lett. 2012, 330, 98–104. [Google Scholar] [CrossRef]
- Ludwig, A.; Tengel, C.; Bauer, S.; Bubert, A.; Benz, R.; Mollenkopf, H.-J.; Goebel, W. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol. Genet. Genom. 1995, 249, 474–486. [Google Scholar] [CrossRef]
- Sathyanarayana, P.; Maurya, S.; Behera, A.; Ravichandran, M.; Visweswariah, S.S.; Ayappa, K.G.; Roy, R. Cholesterol promotes Cytolysin A activity by stabilizing the intermediates during pore formation. Proc. Natl. Acad. Sci. USA 2018, 115, E7323–E7330. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, Z.; Sastalla, I.; Liu, C.; Jang, J.Y.; Sekine, Y.; Li, Y.; Pirooznia, M.; Leppla, S.H.; Finkel, T.; et al. Sequential CRISPR-based screens identify LITAF and CDIP1 as the Bacillus cereus hemolysin BL toxin host receptors. Cell Host Microbe 2020, 28, 402–410.e5. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Churchill-Angus, A.M.; Davies, S.P.; Sedelnikova, S.E.; Tzokov, S.B.; Rafferty, J.B.; Bullough, P.A.; Bisson, C.; Baker, P.J. Identification and structural analysis of the tripartite α-pore forming toxin of Aeromonas hydrophila. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.; Mathur, A.; Xue, Y.; Liu, Y.; Tan, W.H.; Feng, S.; Pandey, A.; Ngo, C.; Hayward, J.A.; Atmosukarto, I.I.; et al. Bacillus cereus non-haemolytic enterotoxin activates the NLRP3 inflammasome. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Feng, S.; Hayward, J.A.; Ngo, C.; Fox, D.; Atmosukarto, I.I.; Price, J.D.; Schauer, K.; Märtlbauer, E.; Robertson, A.A.B.; et al. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat. Microbiol. 2019, 4, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Peraro, M.D.; Van Der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Genet. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Bravo, A.; Gómez, I.; Porta, H.; García-Gómez, B.I.; Rodriguez-Almazan, C.; Pardo, L.; Soberón, M. Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb. Biotechnol. 2012, 6, 17–26. [Google Scholar] [CrossRef]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Pilo, P.; Frey, J. Pathogenicity, population genetics and dissemination of Bacillus anthracis. Infect. Genet. Evol. 2018, 64, 115–125. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.-C.; Yu, Z.; Sun, M. Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins. Toxins 2014, 6, 2732–2770. [Google Scholar] [CrossRef]
- Los, F.C.O.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef]
- Bischofberger, M.; Iacovache, I.; Van Der Goot, F.G. Pathogenic pore-forming proteins: Function and host response. Cell Host Microbe 2012, 12, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ding, S.; Shi, P.; Dietrich, R.; Märtlbauer, E.; Zhu, K. Non-hemolytic enterotoxin of Bacillus cereus induces apoptosis in Vero cells. Cell Microbiol. 2016, 19, e12684. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Girisch, M.; Ries, M.; Zenker, M.; Carbon, R.; Rauch, R.; Hofbeck, M. Intestinal perforations in a premature infant caused by Bacillus cereus. Infection 2003, 31, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Messelhäusser, U.; Ehling-Schulz, M. Bacillus cereus—A multifaceted opportunistic pathogen. Curr. Clin. Microbiol. Rep. 2018, 5, 120–125. [Google Scholar] [CrossRef]
- Veysseyre, F.; Fourcade, C.; Lavigne, J.-P.; Sotto, A. Bacillus cereus infection: 57 case patients and a literature review. Med. Mal. Infect. 2015, 45, 436–440. [Google Scholar] [CrossRef]
- Hilliard, N.J.; Schelonka, R.L.; Waites, K.B. Bacillus cereus bacteremia in a preterm neonate. J. Clin. Microbiol. 2003, 41, 3441–3444. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-L.; Cheng, S.-N.; Hsieh, K.-H.; Wang, C.-C.; Chen, S.-J.; Lo, W.-T. Bacillus cereus septicemia in a patient with acute lymphoblastic leukemia: A case report and review of the literature. J. Microbiol. Immunol. Infect. 2016, 49, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.S.; Kodera, S.; Nagai, Y.; Suzuki, Y. Fulminant Bacillus cereus septicaemia with multiple organ ischaemic/haemorrhagic complications in a patient undergoing chemotherapy for acute myelogenous leukaemia. BMJ Case Rep. 2017, 2017, bcr2017219996. [Google Scholar] [CrossRef]
- Ikeda, M.; Yagihara, Y.; Tatsuno, K.; Okazaki, M.; Okugawa, S.; Moriya, K. Clinical characteristics and antimicrobial susceptibility of Bacillus cereus blood stream infections. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 1–7. [Google Scholar] [CrossRef]
- Kato, K.; Matsumura, Y.; Yamamoto, M.; Nagao, M.; Ito, Y.; Takakura, S.; Ichiyama, S. Seasonal trend and clinical presentation of Bacillus cereus bloodstream infection: Association with summer and indwelling catheter. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Kiyomizu, K.; Yagi, T.; Yoshida, H.; Minami, R.; Tanimura, A.; Karasuno, T.; Hiraoka, A. Fulminant septicemia of Bacillus cereus resistant to carbapenem in a patient with biphenotypic acute leukemia. J. Infect. Chemother. 2008, 14, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kutsuna, S.; Hayakawa, K.; Kita, K.; Katanami, Y.; Imakita, N.; Kasahara, K.; Seto, M.; Akazawa, K.; Shimizu, M.; Kano, T.; et al. Risk factors of catheter-related bloodstream infection caused by Bacillus cereus: Case-control study in 8 teaching hospitals in Japan. Am. J. Infect. Control. 2017, 45, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Tatara, R.; Nagai, T.; Suzuki, M.; Oh, I.; Fujiwara, S.-I.; Norizuki, M.; Muroi, K.; Ozawa, K. Sepsis and meningoencephalitis caused by Bacillus cereus in a patient with myelodysplastic syndrome. Intern. Med. 2013, 52, 1987–1990. [Google Scholar] [CrossRef]
- Uchino, Y.; Iriyama, N.; Matsumoto, K.; Hirabayashi, Y.; Miura, K.; Kurita, D.; Kobayashi, Y.; Yagi, M.; Kodaira, H.; Hojo, A.; et al. A case series of Bacillus cereus septicemia in patients with hematological disease. Intern. Med. 2012, 51, 2733–2738. [Google Scholar] [CrossRef]
- Yamada, K.; Shigemi, H.; Suzuki, K.; Yasutomi, M.; Iwasaki, H.; Ohshima, Y. Successful management of a Bacillus cereus catheter-related bloodstream infection outbreak in the pediatric ward of our facility. J. Infect. Chemother. 2019, 25, 873–879. [Google Scholar] [CrossRef]
- Barrie, D.; Wilson, J.; Hoffman, P.; Kramer, J. Bacillus cereus meningitis in two neuro surgical patients: An investigation into the source of the organism. J. Infect. 1992, 25, 291–297. [Google Scholar] [CrossRef]
- Lebessi, E.; Dellagrammaticas, H.D.; Antonaki, G.; Foustoukou, M.; Iacovidou, N. Bacillus cereus meningitis in a term neonate. J. Matern. Neonatal Med. 2009, 22, 458–461. [Google Scholar] [CrossRef]
- Turnbull, P.C.; Kramer, J.M. Non-gastrointestinal Bacillus cereus infections: An analysis of exotoxin production by strains isolated over a two-year period. J. Clin. Pathol. 1983, 36, 1091–1096. [Google Scholar] [CrossRef]
- Chu, W.P.; Que, T.L.; Lee, W.K.; Wong, S.N. Meningoencephalitis caused by Bacillus cereus in a neonate. Hong Kong Med. J. 2001, 7, 89–92. [Google Scholar]
- De Almeida, S.M.; Teive, H.A.G.; Brandi, I.; Nabhan, S.K.; Werneck, L.C.; Bittencourt, M.A.; Medeiros, C.R.; Pasquini, R.; De Mello, H.H. Fatal Bacillus cereus meningitis without inflammatory reaction in cerebral spinal fluid after bone marrow transplantation. Transplantation 2003, 76, 1533–1534. [Google Scholar] [CrossRef] [PubMed]
- Drazin, D.; Lehman, D.; Danielpour, M. Successful surgical drainage and aggressive medical therapy in a preterm neonate with Bacillus cereus meningitis. Pediatr. Neurosurg. 2010, 46, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.H.; Patrick, C.C.; McCullers, J.A.; Flynn, P.M.; Pearson, T.A.; Razzouk, B.I.; Thompson, S.J.; Shenep, J.L. Bacillus cereus bacteremia and meningitis in immunocompromised children. Clin. Infect. Dis. 2001, 32, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Haase, R.; Sauer, H.; Dagwadordsch, U.; Foell, J.; Lieser, U. Successful treatment of Bacillus cereus meningitis following allogenic stem cell transplantation. Pediatr. Transplant. 2005, 9, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, Y.; Okuno, T.; Minamiguchi, H.; Hodohara, K.; Mikamo, H.; Andoh, A. Survival of a case of Bacillus cereus meningitis with brain abscess presenting as immune reconstitution syndrome after febrile neutropenia—A case report and literature review. BMC Infect. Dis. 2020, 20, 15. [Google Scholar] [CrossRef]
- Manickam, N.; Knorr, A.; Muldrew, K.L. Neonatal meningoencephalitis caused by Bacillus cereus. Pediatr. Infect. Dis. J. 2008, 27, 843–846. [Google Scholar] [CrossRef]
- Marshman, L. Bacillus cereus meningitis complicating cerebrospinal fluid fistula repair and spinal drainage. Br. J. Neurosurg. 2000, 14, 580–582. [Google Scholar] [CrossRef]
- Stevens, M.P.; Elam, K.; Bearman, G.M. Meningitis due to Bacillus cereus: A case report and review of the literature. Can. J. Infect. Dis. Med Microbiol. 2012, 23, e16–e19. [Google Scholar] [CrossRef]
- Avashia, S.B.; Riggins, W.S.; Lindley, C.; Hoffmaster, A.; Drumgoole, R.; Nekomoto, T.; Jackson, P.J.; Hill, K.K.; Williams, K.; Lehman, L.; et al. Fatal pneumonia among metalworkers due to inhalation exposure to Bacillus cereus containing Bacillus anthracis toxin genes. Clin. Infect. Dis. 2007, 44, 414–416. [Google Scholar] [CrossRef]
- Hoffmaster, A.R.; Hill, K.K.; Gee, J.E.; Marston, C.K.; De, B.K.; Popovic, T.; Sue, D.; Wilkins, P.P.; Avashia, S.B.; Drumgoole, R.; et al. Characterization of Bacillus cereus isolates associated with fatal pneumonias: Strains are closely related to Bacillus anthracis and harbor B. anthracis virulence genes. J. Clin. Microbiol. 2006, 44, 3352–3360. [Google Scholar] [CrossRef]
- Ishida, R.; Ueda, K.; Kitano, T.; Yamamoto, T.; Mizutani, Y.; Tsutsumi, Y.; Imoto, K.; Yamamori, Y. Fatal community-acquired Bacillus cereus pneumonia in an immunocompetent adult man: A case report. BMC Infect. Dis. 2019, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.C.; McFadden, S.; Tremblay, A. An unusual case of massive hemoptysis due to Bacillus cereus necrotizing pneumonia. Respir. Med. Case Rep. 2019, 28, 100943. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, Y.; Umegaki, O.; Ooi, Y.; Agui, T.; Kadono, N.; Minami, T. Bacillus cereus pneumonia in an immunocompetent patient: A case report. JA Clin. Rep. 2017, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Viel-Thériault, I.; Saban, J.; Lewis, A.; Bariciak, E.; Grynspan, D. A case of fulminant Bacillus cereus lung necrosis in a preterm neonate. Pediatr. Dev. Pathol. 2019, 22, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.P.; Lee, W.-S.; Ho, M. Bacillus cereus endocarditis in an addict. Ann. Intern. Med. 1974, 80, 418. [Google Scholar] [CrossRef]
- Barraud, O.; Hidri, N.; Ly, K.; Pichon, N.; Manea, P.; Ploy, M.-C.; Garnier, F. Pacemaker-associated Bacillus cereus endocarditis. Diagn. Microbiol. Infect. Dis. 2012, 74, 313–315. [Google Scholar] [CrossRef]
- Gopinathan, A.; Kumar, A.; Sen, A.C.; Sudha, S.; Varma, P.; Gs, S.; Eapen, M.; Dinesh, K.R. A case series and review of Bacillus cereus endocarditis from India. Open Microbiol. J. 2018, 12, 28–33. [Google Scholar] [CrossRef]
- Nallarajah, J.; Mujahieth, M.I. Bacillus cereus subacute native valve infective endocarditis and its multiple complications. Case Rep. Cardiol. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Ngow, H.; Khairina, W.W. Bacillus cereus endocarditis in native aortic valve. J. Infect. Chemother. 2013, 19, 154–157. [Google Scholar] [CrossRef]
- Ren, B.; Lasam, G. A rare case of native mitral valve Bacillus cereus endocarditis culminating into a cerebrovascular infarction. Cardiol. Res. 2018, 9, 173–175. [Google Scholar] [CrossRef]
- Shah, M.; Patnaik, S.; Wongrakpanich, S.; Alhamshari, Y.; Alnabelsi, T. Infective endocarditis due to Bacillus cereus in a pregnant female: A case report and literature review. IDCases 2015, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Soudet, S.; Becquart, C.; Dezoteux, F.; Faure, K.; Staumont-Salle, D.; Delaporte, E. Endocardite à Bacillus cereus avec probable porte d’entrée cutanée. Ann. Dermatol. Vénéréologie 2017, 144, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Steen, M.K.; Bruno-Murtha, L.A.; Chaux, G.; Lazar, H.; Bernard, S.; Sulis, C. Bacillus cereus endocarditis: Report of a case and review. Clin. Infect. Dis. 1992, 14, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.F. Central venous access device-related Bacillus cereus endocarditis: A case report and review of the literature. Clin. Med. Res. 2016, 14, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Mursalin, H.; Livingston, E.T.; Callegan, M.C. The cereus matter of Bacillus endophthalmitis. Exp. Eye Res. 2020, 193, 107959. [Google Scholar] [CrossRef]
- Moyer, A.L.; Ramadan, R.T.; Novosad, B.D.; Astley, R.; Callegan, M.C. Bacillus cereus–induced permeability of the blood–ocular barrier during experimental endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3783–3793. [Google Scholar] [CrossRef]
- Moyer, A.L.; Ramadan, R.T.; Thurman, J.; Burroughs, A.; Callegan, M.C. Bacillus cereus induces permeability of an in vitro blood-retina barrier. Infect. Immun. 2008, 76, 1358–1367. [Google Scholar] [CrossRef]
- Callegan, M.C.; Kane, S.T.; Cochran, D.C.; Novosad, B.; Gilmore, M.S.; Gominet, M.; Lereclus, D. Bacillus endophthalmitis: Roles of bacterial toxins and motility during infection. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3233–3238. [Google Scholar] [CrossRef]
- Callegan, M.C.; Novosad, B.D.; Ramirez, R.; Ghelardi, E.; Senesi, S. Role of swarming migration in the pathogenesis of Bacillus endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4461–4467. [Google Scholar] [CrossRef]
- Callegan, M.C.; Parkunan, S.M.; Randall, C.B.; Coburn, P.S.; Miller, F.C.; LaGrow, A.L.; Astley, R.A.; Land, C.; Oh, S.-Y.; Schneewind, O. The role of pili in Bacillus cereus intraocular infection. Exp. Eye Res. 2017, 159, 69–76. [Google Scholar] [CrossRef]
- Mursalin, H.; Coburn, P.S.; Livingston, E.; Miller, F.C.; Astley, R.; Fouet, A.; Callegan, M.C. S-layer impacts the virulence of Bacillus in endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3727–3739. [Google Scholar] [CrossRef] [PubMed]
- Callegan, M.C.; Gilmore, M.S.; Gregory, M.; Ramadan, R.T.; Wiskur, B.J.; Moyer, A.L.; Hunt, J.J.; Novosad, B.D. Bacterial endophthalmitis: Therapeutic challenges and host–pathogen interactions. Prog. Retin. Eye Res. 2007, 26, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Coburn, P.S.; Miller, F.C.; LaGrow, A.L.; Parkunan, S.M.; Randall, C.B.; Staats, R.L.; Callegan, M.C. TLR4 modulates inflammatory gene targets in the retina during Bacillus cereus endophthalmitis. BMC Ophthalmol. 2018, 18, 96. [Google Scholar] [CrossRef]
- Rajabli, N.; Williamson, L.; Nimmer, P.S.; Kelly-Worden, M.; Bange, J.S.; Ho, Y.; McKillip, J.L. The dangers of sublethal carvacrol exposure: Increases in virulence of Bacillus cereus during endophthalmitis. Int. J. Biochem. Mol. Biol. 2018, 9, 11–21. [Google Scholar]
- Coburn, P.S.; Miller, F.C.; Enty, M.A.; Land, C.; LaGrow, A.L.; Mursalin, H.; Callegan, M.C. Expression of Bacillus cereus virulence-related genes in an ocular infection-related environment. Microorganisms 2020, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Wang, Q.; Tang, Z.; Wang, Y.; Gu, Y.; Lou, Y.; Zheng, M. Association of genotyping of Bacillus cereus with clinical features of post-traumatic endophthalmitis. PLoS ONE 2016, 11, e0147878. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, Y.-Y.; Xu, Y.; Sun, B.-J.; Shao, J.; Zhang, D.; Li, K.; Fan, D.-D.; Xue, Z.-B.; Chen, W.-H.; et al. Molecular signatures related to the virulence of Bacillus cereus sensu lato, a leading cause of devastating endophthalmitis. mSystems 2019, 4. [Google Scholar] [CrossRef]
- Stenfors, L.P.; Granum, P.E. Psychrotolerant species from the Bacillus cereus group are not necessarily Bacillus weihenstephanensis. FEMS Microbiol. Lett. 2001, 197, 223–228. [Google Scholar] [CrossRef]
- Fagerlund, A.; Ween, O.; Lund, T.; Hardy, S.P.; Granum, P.E. Genetic and functional analysis of the cytK family of genes in Bacillus cereus. Microbiol. 2004, 150, 2689–2697. [Google Scholar] [CrossRef]
- Guinebretière, M.-H.; Fagerlund, A.; Granum, P.E.; Nguyen-The, C. Rapid discrimination of cytK-1 and cytK-2 genes in Bacillus cereus strains by a novel duplex PCR system. FEMS Microbiol. Lett. 2006, 259, 74–80. [Google Scholar] [CrossRef]
- Castiaux, V.; Liu, X.; Delbrassinne, L.; Mahillon, J. Is Cytotoxin K from Bacillus cereus a bona fide enterotoxin? Int. J. Food Microbiol. 2015, 211, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, N.; Sanchis-Borja, V. The pore-forming haemolysins of Bacillus cereus: A review. Toxins 2013, 5, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Contzen, M.; Hailer, M.; Rau, J. Isolation of Bacillus cytotoxicus from various commercial potato products. Int. J. Food Microbiol. 2014, 174, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Heini, N.; Stephan, R.; Ehling-Schulz, M.; Johler, S. Characterization of Bacillus cereus group isolates from powdered food products. Int. J. Food Microbiol. 2018, 283, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Koné, K.M.; Douamba, Z.; De Halleux, M.; Bougoudogo, F.; Mahillon, J. Prevalence and diversity of the thermotolerant bacterium Bacillus cytotoxicus among dried food products. J. Food Prot. 2019, 82, 1210–1216. [Google Scholar] [CrossRef]
- Stevens, M.J.A.; Tasara, T.; Klumpp, J.; Stephan, R.; Ehling-Schulz, M.; Johler, S. Whole-genome-based phylogeny of Bacillus cytotoxicus reveals different clades within the species and provides clues on ecology and evolution. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Cavello, I.; Urbieta, M.S.; Cavalitto, S.F.; Donati, E.R. Bacillus cytotoxicus isolated from a pristine natural geothermal area reveals high keratinolytic activity. Microorganisms 2020, 8, 796. [Google Scholar] [CrossRef]
- Ołtuszak-Walczak, E.; Walczak, P. PCR detection of cytK gene in Bacillus cereus group strains isolated from food samples. J. Microbiol. Methods 2013, 95, 295–301. [Google Scholar] [CrossRef]
- De Jonghe, V.; Coorevits, A.; De Block, J.; Van Coillie, E.; Grijspeerdt, K.; Herman, L.; De Vos, P.; Heyndrickx, M. Toxinogenic and spoilage potential of aerobic spore-formers isolated from raw milk. Int. J. Food Microbiol. 2010, 136, 318–325. [Google Scholar] [CrossRef]
- Brillard, J.; Lereclus, D. Comparison of cytotoxin cytK promoters from Bacillus cereus strain ATCC 14579 and from a B. cereus food-poisoning strain. Microbiology 2004, 150, 2699–2705. [Google Scholar] [CrossRef]
- Ceuppens, S.; Rajkovic, A.; Heyndrickx, M.; Tsilia, V.; Van De Wiele, T.; Boon, N.; Uyttendaele, M. Regulation of toxin production by Bacillus cereus and its food safety implications. Crit. Rev. Microbiol. 2011, 37, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Ceuppens, S.; Timmery, S.; Mahillon, J.; Uyttendaele, M.; Boon, N. Small Bacillus cereus ATCC 14579 subpopulations are responsible for cytotoxin K production. J. Appl. Microbiol. 2013, 114, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.P.; Lund, T.; Granum, P.E. CytK toxin of Bacillus cereus forms pores in planar lipid bilayers and is cytotoxic to intestinal epithelia. FEMS Microbiol. Lett. 2001, 197, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Gouaux, E. α-Hemolysin from Staphylococcus aureus: An archetype of β-barrel, channel-forming toxins. J. Struct. Biol. 1998, 121, 110–122. [Google Scholar] [CrossRef]
- Menestrina, G.; Serra, M.D.; Prévost, G. Mode of action of β-barrel pore-forming toxins of the staphylococcal α-hemolysin family. Toxicon 2001, 39, 1661–1672. [Google Scholar] [CrossRef]
- Popoff, M.R. Clostridial pore-forming toxins: Powerful virulence factors. Anaerobe 2014, 30, 220–238. [Google Scholar] [CrossRef]
- Steinthorsdottir, V.; Halldórsson, H.; Andrésson, O.S. Clostridium perfringens beta-toxin forms multimeric transmembrane pores in human endothelial cells. Microb. Pathog. 2000, 28, 45–50. [Google Scholar] [CrossRef]
- Baida, G.; Budarina, Z.I.; Kuzmin, N.P.; Solonin, A.S. Complete nucleotide sequence and molecular characterization of hemolysin II gene from Bacillus cereus. FEMS Microbiol. Lett. 1999, 180, 7–14. [Google Scholar] [CrossRef]
- Andreeva, Z.I.; Nesterenko, V.F.; Fomkina, M.G.; Ternovsky, V.I.; Suzina, N.E.; Bakulina, A.Y.; Solonin, A.S.; Sineva, E.V. The properties of Bacillus cereus hemolysin II pores depend on environmental conditions. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 253–263. [Google Scholar] [CrossRef]
- Andreeva, Z.I.; Nesterenko, V.F.; Yurkov, I.S.; Budarina, Z.I.; Sineva, E.V.; Solonin, A.S. Purification and cytotoxic properties of Bacillus cereus hemolysin II. Protein Expr. Purif. 2006, 47, 186–193. [Google Scholar] [CrossRef]
- Martínez-Blanch, J.; Sánchez, G.; Garay, E.; Aznar, R. Development of a real-time PCR assay for detection and quantification of enterotoxigenic members of Bacillus cereus group in food samples. Int. J. Food Microbiol. 2009, 135, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Blanch, J.F.; Sánchez, G.; Garay, E.; Aznar, R. Evaluation of phenotypic and PCR-based approaches for routine analysis of Bacillus cereus group foodborne isolates. Antonie van Leeuwenhoek 2010, 99, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, E.; Moravek, M.; Dietrich, R.; Bürk, C.; Didier, A.; Märtlbauer, E. Comparison of multiplex PCR, enzyme immunoassay and cell culture methods for the detection of enterotoxinogenic Bacillus cereus. J. Microbiol. Methods 2009, 78, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Tallent, S.M.; Hait, J.M.; Bennett, R.W. Analysis of Bacillus cereus toxicity using PCR, ELISA and a lateral flow device. J. Appl. Microbiol. 2015, 118, 1068–1075. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Tallent, S.M. Screening food for Bacillus cereus toxins using whole genome sequencing. Food Microbiol. 2019, 78, 164–170. [Google Scholar] [CrossRef]
- Carroll, L.M.; Wiedmann, M.; Mukherjee, M.; Nicholas, D.C.; Mingle, L.A.; Dumas, N.B.; Cole, J.A.; Kovac, J. Characterization of emetic and diarrheal Bacillus cereus strains from a 2016 foodborne outbreak using whole-genome sequencing: Addressing the microbiological, epidemiological, and bioinformatic challenges. Front. Microbiol. 2019, 10, 144. [Google Scholar] [CrossRef]
- Krawczyk, A.O.; De Jong, A.; Eijlander, R.T.; Berendsen, E.M.; Holsappel, S.; Wells-Bennik, M.H.J.; Kuipers, O.P. Next-generation whole-genome sequencing of eight strains of Bacillus cereus, isolated from food. Genome Announc. 2015, 3, e01480-15. [Google Scholar] [CrossRef]
- Thirkell, C.E.; Sloan-Gardner, T.S.; Kaczmarek, M.C.; Polkinghorne, B. An outbreak of Bacillus cereus toxin-mediated emetic and diarrhoeal syndromes at a restaurant in Canberra, Australia 2018. Commun. Dis. Intell. 2019, 43. [Google Scholar] [CrossRef]
- Zwick, M.E.; Kiley, M.P.; Stewart, A.C.; Mateczun, A.; Read, T.D. Genotyping of Bacillus cereus strains by microarray-based resequencing. PLoS ONE 2008, 3, e2513. [Google Scholar] [CrossRef]
- Sergeev, N.; Distler, M.; Vargas, M.; Chizhikov, V.; Herold, K.E.; Rasooly, A. Microarray analysis of Bacillus cereus group virulence factors. J. Microbiol. Methods 2006, 65, 488–502. [Google Scholar] [CrossRef]
- Papazisi, L.; Rasko, D.A.; Ratnayake, S.; Bock, G.R.; Remortel, B.G.; Appalla, L.; Liu, J.; Dracheva, T.; Braisted, J.C.; Shallom, S.; et al. Investigating the genome diversity of B. cereus and evolutionary aspects of B. anthracis emergence. Genomics 2011, 98, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Krause, N.; Moravek, M.; Dietrich, R.; Wehrle, E.; Slaghuis, J.; Märtlbauer, E. Performance characteristics of the Duopath® Cereus Enterotoxins assay for rapid detection of enterotoxinogenic Bacillus cereus strains. Int. J. Food Microbiol. 2010, 144, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Griffiths, M.W. Evidence for increased thermostability of Bacillus cereus enterotoxin in milk. J. Food Prot. 1995, 58, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Ceuppens, S.; Rajkovic, A.; Hamelink, S.; Van De Wiele, T.; Boon, N.; Uyttendaele, M. Enterotoxin production by Bacillus cereus under gastrointestinal conditions and their immunological detection by commercially available kits. Foodborne Pathog. Dis. 2012, 9, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.; Logan, N.A. Improved cytotoxicity assay for Bacillus cereus diarrhoeal enterotoxin. Lett. Appl. Microbiol. 1999, 28, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Odumeru, J.A.; Toner, A.K.; Muckle, C.A.; Griffiths, M.W.; Lynch, J.A. Detection of Bacillus cereus diarrheal enterotoxin in raw and pasteurized milk. J. Food Prot. 1997, 60, 1391–1394. [Google Scholar] [CrossRef]
- Didier, A.; Jessberger, N.; Krey, V.M.; Dietrich, R.; Scherer, S.; Märtlbauer, E. The mutation Glu151Asp in the B-component of the Bacillus cereus non-hemolytic enterotoxin (Nhe) leads to a diverging reactivity in antibody-based detection systems. Toxins 2015, 7, 4655–4667. [Google Scholar] [CrossRef]
- Rusul, G.; Yaacob, N.H. Prevalence of Bacillus cereus in selected foods and detection of enterotoxin using TECRA-VIA and BCET-RPLA. Int. J. Food Microbiol. 1995, 25, 131–139. [Google Scholar] [CrossRef]
- Banerjee, M.; Nair, G.B.; Ramamurthy, T. Phenotypic & genetic characterization of Bacillus cereus isolated from the acute diarrhoeal patients. Indian J. Med Res. 2011, 133, 88–95. [Google Scholar]
- Kaminska, P.S.; Yernazarova, A.; Murawska, E.; Swiecicki, J.; Fiedoruk, K.; Bideshi, D.K.; Swiecicka, I. Comparative analysis of quantitative reverse transcription real-time PCR and commercial enzyme imunoassays for detection of enterotoxigenic Bacillus thuringiensis isolates. FEMS Microbiol. Lett. 2014, 357, 34–39. [Google Scholar] [CrossRef]
- Dietrich, R.; Fella, C.; Strich, S.; Märtlbauer, E. Production and characterization of monoclonal antibodies against the hemolysin BL enterotoxin complex produced by Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4470–4474. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.D.; Urs, R.M.; Balakrishna, K.; Murali, H.S.; Batra, H.; Bawa, A.S. Monoclonal antibodies against recombinant hemolysin BL complex of Bacillus cereus. Hybridoma 2010, 29, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.; Wegscheider, M.; Schulz, A.; Dietrich, R.; Bürk, C.; Märtlbauer, E. Colony immunoblot assay for the detection of hemolysin BL enterotoxin producing Bacillus cereus. FEMS Microbiol. Lett. 2004, 238, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Tsilia, V.; Devreese, B.; De Baenst, I.; Mesuere, B.; Rajkovic, A.; Uyttendaele, M.; Van De Wiele, T.; Heyndrickx, M. Application of MALDI-TOF mass spectrometry for the detection of enterotoxins produced by pathogenic strains of the Bacillus cereus group. Anal. Bioanal. Chem. 2012, 404, 1691–1702. [Google Scholar] [CrossRef]
- Glatz, B.A.; Spira, W.M.; Goepfert, J.M. Alteration of vascular permeability in rabbits by culture filtrates of Bacillus cereus and related species. Infect. Immun. 1974, 10, 299–303. [Google Scholar] [CrossRef]
- Spira, W.M.; Goepfert, J.M. Bacillus cereus-induced fluid accumulation in rabbit ileal loops. Appl. Microbiol. 1972, 24, 341–348. [Google Scholar] [CrossRef]
- Melling, J.; Capel, B.J.; Turnbull, P.C.; Gilbert, R.J. Identification of a novel enterotoxigenic activity associated with Bacillus cereus. J. Clin. Pathol. 1976, 29, 938–940. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Messelhäusser, U.; Granum, P.E. Bacillus cereus in milk and dairy production. In Rapid Detection, Characterization, and Enumeration of Foodborne Pathogens; Hoorfar, J., Ed.; ASM Press: Washington, DC, USA, 2011; pp. 275–289. [Google Scholar]
- Fermanian, C.; Lapeyre, C.; Fremy, J.-M.; Claisse, M. Diarrhoeal toxin production at low temperature by selected strains of Bacillus cereus. J. Dairy Res. 1997, 64, 551–559. [Google Scholar] [CrossRef]
- Rowan, N.J.; Deans, K.; Anderson, J.G.; Gemmell, C.G.; Hunter, I.S.; Chaithong, T. Putative virulence factor expression by clinical and food isolates of Bacillus spp. after growth in reconstituted infant milk formulae. Appl. Environ. Microbiol. 2001, 67, 3873–3881. [Google Scholar] [CrossRef]
- Shinagawa, K.; Sugiyama, J.; Terada, T.; Matsusaka, N.; Sugii, S. Improved methods for purification of an enterotoxin produced by Bacillus cereus. FEMS Microbiol. Lett. 1991, 80, 1–5. [Google Scholar] [CrossRef]
- Thompson, N.E.; Ketterhagen, M.J.; Bergdoll, M.S.; Schantz, E.J. Isolation and some properties of an enterotoxin produced by Bacillus cereus. Infect. Immun. 1984, 43, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.I.; Nukumizu, Y.; Bando, H.; Iizuka, T.; Yamamoto, T. Cloning of novel enterotoxin genes from Bacillus cereus and Bacillus thuringiensis. Appl. Environ. Microbiol. 1997, 63, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.-L.; Guillemet, E.; Gohar, M.; Lereclus, D.; Ramarao, N. CwpFM (EntFM) is a Bacillus cereus potential cell wall peptidase implicated in adhesion, biofilm formation, and virulence. J. Bacteriol. 2010, 192, 2638–2642. [Google Scholar] [CrossRef]
- Boonchai, N.; Asano, S.-I.; Bando, H.; Wiwat, C. Study on cytotoxicity and nucleotide sequences of enterotoxin FM of Bacillus cereus isolated from various food sources. J. Med Assoc. Thai. 2008, 91, 1425–1432. [Google Scholar]
- Kreft, J.; Berger, H.; Härtlein, M.; Müller, B.; Weidinger, G.; Goebel, W. Cloning and expression in Escherichia coli and Bacillus subtilis of the hemolysin (cereolysin) determinant from Bacillus cereus. J. Bacteriol. 1983, 155, 681–689. [Google Scholar] [CrossRef]
- Tran, S.-L.; Guillemet, E.; Ngo-Camus, M.; Clybouw, C.; Puhar, A.; Moris, A.; Gohar, M.; Lereclus, D.; Ramarao, N. Haemolysin II is a Bacillus cereus virulence factor that induces apoptosis of macrophages. Cell. Microbiol. 2010, 13, 92–108. [Google Scholar] [CrossRef]
- Budarina, Z.I.; Nikitin, D.V.; Zenkin, N.; Zakharova, M.; Semenova, E.; Shlyapnikov, M.G.; Rodikova, E.A.; Masyukova, S.; Ogarkov, O.; Baida, G.E.; et al. A new Bacillus cereus DNA-binding protein, HlyIIR, negatively regulates expression of B. cereus haemolysin II. Microbiology 2004, 150, 3691–3701. [Google Scholar] [CrossRef]
- Guillemet, E.; Tran, S.-L.; Cadot, C.; Rognan, D.; Lereclus, D.; Ramarao, N. Glucose 6P binds and activates HlyIIR to repress Bacillus cereus haemolysin hlyII gene expression. PLoS ONE 2013, 8, e55085. [Google Scholar] [CrossRef]
- Harvie, D.R.; Vílchez, S.; Steggles, J.R.; Ellar, D.J. Bacillus cereus Fur regulates iron metabolism and is required for full virulence. Microbiology 2005, 151, 569–577. [Google Scholar] [CrossRef]
- Sineva, E.V.; Shadrin, A.; Rodikova, E.A.; Andreeva-Kovalevskaya, Z.I.; Protsenko, A.S.; Mayorov, S.G.; Galaktionova, D.Y.; Magelky, E.; Solonin, A.S. Iron regulates expression of Bacillus cereus hemolysin II via global regulator Fur. J. Bacteriol. 2012, 194, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Budarina, Z.I.; Sinev, M.A.; Mayorov, S.G.; Tomashevski, A.Y.; Shmelev, I.V.; Kuzmin, N.P. Hemolysin II is more characteristic of Bacillus thuringiensis than Bacillus cereus. Arch. Microbiol. 1994, 161, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.R.; Kaus, K.; De, S.; Olson, R.; Alexandrescu, A.T. NMR structure of the Bacillus cereus hemolysin II C-terminal domain reveals a novel fold. Sci. Rep. 2017, 7, 3277. [Google Scholar] [CrossRef] [PubMed]
- Miles, G.; Bayley, H.; Cheley, S. Properties of Bacillus cereus hemolysin II: A heptameric transmembrane pore. Protein Sci. 2009, 11, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.R.; Maciejewski, M.W.; Olson, R.; Alexandrescu, A.T. NMR assignments for the cis and trans forms of the hemolysin II C-terminal domain. Biomol. NMR Assign. 2013, 8, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Baida, G.E.; Kuzmin, N.P. Cloning and primary structure of a new hemolysin gene from Bacillus cereus. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1995, 1264, 151–154. [Google Scholar] [CrossRef]
- Baida, G.E.; Kuzmin, N.P. Mechanism of action of hemolysin III from Bacillus cereus. Biochim. Biophys. Acta (BBA) Biomembr. 1996, 1284, 122–124. [Google Scholar] [CrossRef]
- Cadot, C.; Tran, S.-L.; Vignaud, M.-L.; De Buyser, M.-L.; Kolstø, A.-B.; Brisabois, A.; Nguyen-Thé, C.; Lereclus, D.; Guinebretière, M.-H.; Ramarao, N. InhA1, NprA, and HlyII as candidates for markers to differentiate pathogenic from nonpathogenic Bacillus cereus strains. J. Clin. Microbiol. 2010, 48, 1358–1365. [Google Scholar] [CrossRef]
- Fedhila, S.; Nel, P.; Lereclus, D. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 2002, 184, 3296–3304. [Google Scholar] [CrossRef]
- Guillemet, E.; Cadot, C.; Tran, S.-L.; Guinebretière, M.-H.; Lereclus, D.; Ramarao, N. The InhA metalloproteases of Bacillus cereus contribute concomitantly to virulence. J. Bacteriol. 2009, 192, 286–294. [Google Scholar] [CrossRef]
- Ramarao, N.; Lereclus, D. The InhA1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell. Microbiol. 2005, 7, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Haydar, A.; Tran, S.-L.; Guillemet, E.; Darrigo, C.; Perchat, S.; Lereclus, D.; Coquet, L.; Jouenne, T.; Ramarao, N. InhA1-mediated cleavage of the metalloprotease NprA allows Bacillus cereus to escape from macrophages. Front. Microbiol. 2018, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Kuppe, A.; Evans, L.M.; McMillen, D.A.; Griffith, O.H. Phosphatidylinositol-specific phospholipase C of Bacillus cereus: Cloning, sequencing, and relationship to other phospholipases. J. Bacteriol. 1989, 171, 6077–6083. [Google Scholar] [CrossRef] [PubMed]
- Abfalter, C.M.; Schönauer, E.; Ponnuraj, K.; Huemer, M.; Gadermaier, G.; Regl, C.; Briza, P.; Ferreira, F.; Huber, C.G.; Brandstetter, H.; et al. Cloning, purification and characterization of the collagenase ColA expressed by Bacillus cereus ATCC 14579. PLoS ONE 2016, 11, e0162433. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.; Granum, P.E. The 105-kDa protein component of Bacillus cereus non-haemolytic enterotoxin (Nhe) is a metalloprotease with gelatinolytic and collagenolytic activity. FEMS Microbiol. Lett. 1999, 178, 355–361. [Google Scholar] [CrossRef]
- Lövgren, A.; Carlson, C.R.; Eskils, K.; Kolstø, A.-B. Localization of putative virulence genes on a physical map of the Bacillus thuringiensis subsp. Gelechiae Chromosome. Curr. Microbiol. 1998, 37, 245–250. [Google Scholar] [CrossRef]
- Candela, T.; Fagerlund, A.; Buisson, C.; Gilois, N.; Kolstø, A.; Økstad, O.A.; Aymerich, S.; Nielsen-Leroux, C.; Lereclus, D.; Gohar, M. CalY is a major virulence factor and a biofilm matrix protein. Mol. Microbiol. 2019, 111, 1416–1429. [Google Scholar] [CrossRef]
- Fricke, B.; Drößler, K.; Willhardt, I.; Schierhorn, A.; Menge, S.; Rücknagel, P. The cell envelope-bound metalloprotease (camelysin) from Bacillus cereus is a possible pathogenic factor. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2001, 1537, 132–146. [Google Scholar] [CrossRef]
- Grass, G.; Schierhorn, A.; Sorkau, E.; Müller, H.; Rücknagel, P.; Nies, D.H.; Fricke, B. Camelysin is a novel surface metalloproteinase from Bacillus cereus. Infect. Immun. 2004, 72, 219–228. [Google Scholar] [CrossRef]
- Caro-Astorga, J.; Peréz-García, A.; de Vicente, A.; Romero, D. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front. Microbiol. 2015, 5, 745. [Google Scholar] [CrossRef]
- Eomer, H.; Ealpha-Bazin, B.; Ebrunet, J.-L.; Earmengaud, J.; Duport, C. Proteomics identifies Bacillus cereus EntD as a pivotal protein for the production of numerous virulence factors. Front. Microbiol. 2015, 6, 1004. [Google Scholar] [CrossRef] [PubMed]
- Doll, V.M.; Ehling-Schulz, M.; Vogelmann, R. Concerted action of sphingomyelinase and non-hemolytic enterotoxin in pathogenic Bacillus cereus. PLoS ONE 2013, 8, e61404. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Fujita, A.; Okui, K.; Miyamoto, K.; Shibutani, M.; Takagishi, T.; Nagahama, M. Bacillus cereus sphingomyelinase recognizes ganglioside GM3. Biochem. Biophys. Res. Commun. 2013, 431, 164–168. [Google Scholar] [CrossRef]
- Oda, M.; Hashimoto, M.; Takahashi, M.; Ohmae, Y.; Seike, S.; Kato, R.; Fujita, A.; Tsuge, H.; Nagahama, M.; Ochi, S.; et al. Role of sphingomyelinase in infectious diseases caused by Bacillus cereus. PLoS ONE 2012, 7, e38054. [Google Scholar] [CrossRef]
- Oda, M.; Takahashi, M.; Matsuno, T.; Uoo, K.; Nagahama, M.; Sakurai, J. Hemolysis induced by Bacillus cereus sphingomyelinase. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 1073–1080. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).