A New Peritoneal Dialysis Solution Containing L-Carnitine and Xylitol for Patients on Continuous Ambulatory Peritoneal Dialysis: First Clinical Experience
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
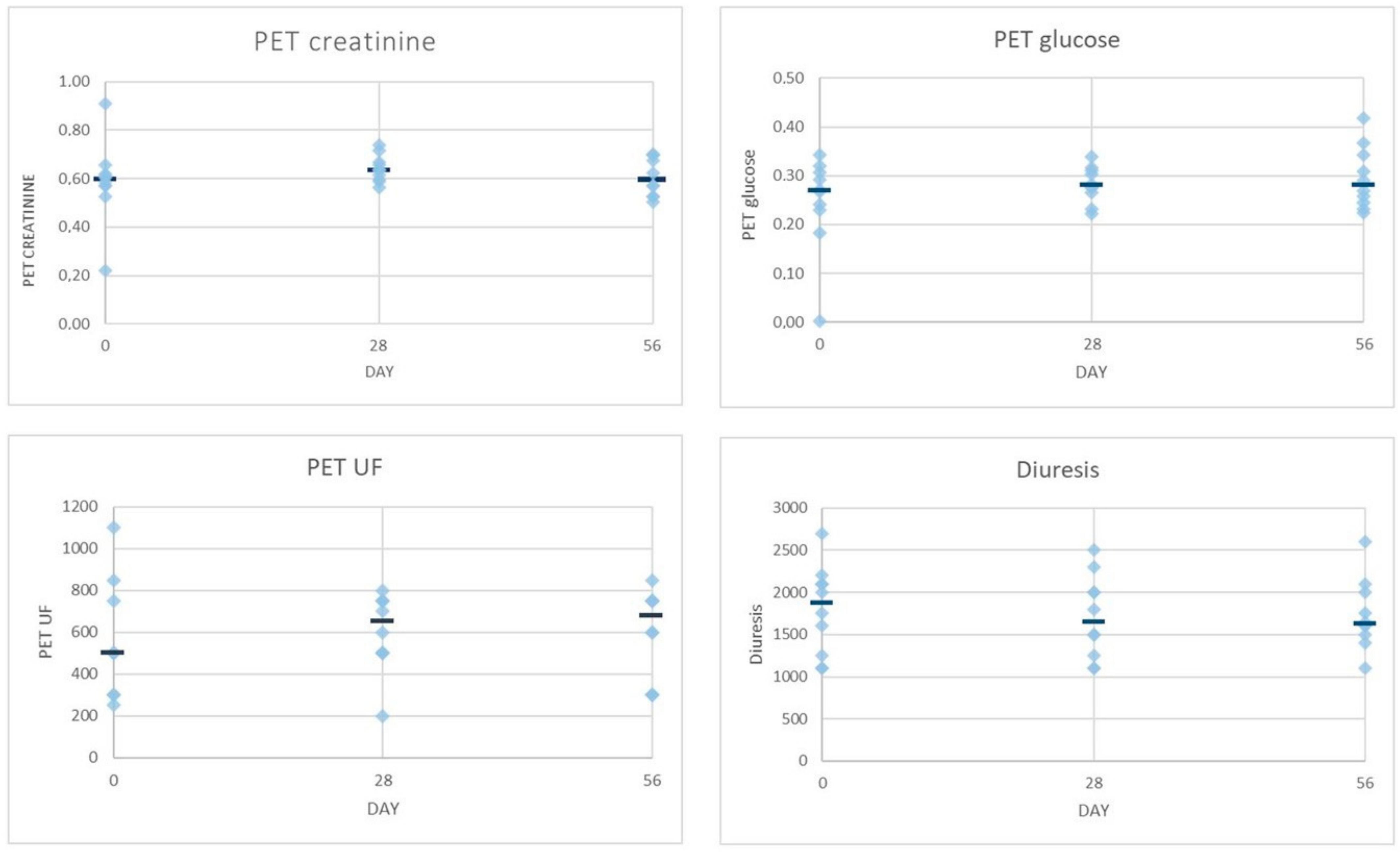
2.2. Dialysis Efficiency Parameters
2.3. Safety Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Design
4.3. Study Solutions
4.4. Study Procedures
4.5. Data Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Howell, M.; Walker, R.C.; Howard, K. Cost effectiveness of dialysis modalities: A systemic review of 723 economic evaluations. Appl. Health Econ. Health Policy 2019, 17, 315–330. [Google Scholar] [CrossRef]
- Gokal, R.; Mallick, R.P. Peritoneal dialysis. Lancet 1999, 353, 823–828. [Google Scholar] [CrossRef]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The current state of peritoneal dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef]
- Rippe, B. A three-pore model of peritoneal transport. Perit. Dial. Int. 1993, 13, S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Afentakis, N.; Ambühl, P.M.; Andrusev, A.M.; Fuster, E.A.; Monzón, F.E.A.; Åsberg, A.; et al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA)—Registry Annual Report 739 2015: A summary. Clin. Kidney J. 2018, 11, 108–122. [Google Scholar] [CrossRef]
- Bartosova, M.; Schmitt, C.P. Biocompatible peritoneal dialysis: The target is still way off. Front. Physiol. 2019, 9, 1853. [Google Scholar] [CrossRef]
- Schmitt, C.P.; Aufricht, C. Is there such a thing as biocompatible peritoneal dialysis fluid? Pediatr. Nephrol. 2016, 32, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.G. Is the peritoneal dialysis biocompatibility hypothesis dead? Kidney Int. 2018, 94, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Bajo, M.A.; del Peso, G.; Teitelbaum, I. Peritoneal membrane preservation. Semin. Nephrol. 2017, 37, 77–92. [Google Scholar] [CrossRef]
- Balzer, M.S. Molecular pathways in peritoneal fibrosis. Cell. Signal. 2020, 75, 109778. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Phillips, L.; Griffiths, A.; Russell, L.H.; Naish, P.F.; Russell, G.I. What really happens to people on long-term peritoneal dialysis? Kidney Int. 1998, 54, 2207–2217. [Google Scholar] [CrossRef]
- Holmes, C.J. Glucotoxicity in peritoneal dialysis—Solutions for the solution! Adv. Chronic Kidney Dis. 2007, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-K.; Lin, C.-L.; Chen, H.-C.; Lin, S.-Y.; Chang, C.-T.; Yen, T.-H.; Sung, F.-C. Risk of new-onset diabetes in end-stage renal disease patients undergoing dialysis: Analysis from registry data of Taiwan. Nephrol. Dial. Transplant. 2018, 33, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Dousdampanis, P.; Musso, C.; Trigka, K. Icodextrin and peritoneal dialysis: Advantages and new applications. Int. Urol. Nephrol. 2018, 50, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Goossen, K.; Becker, M.; Marshall, M.R.; Buhn, S.; Breuing, J.; Firanek, C.A.; Hess, S.; Nariai, H.; Sloand, J.A.; Yao, Q.; et al. Icodextrin versus glucose solutions for the once-daily long dwell in peritoneal dialysis: An enriched systematic review and meta-analysis of randomized controlled trials. Am. J. Kidney Dis. 2020, 75, 830–846. [Google Scholar] [CrossRef]
- Li, F.K.; Chan, L.Y.; Woo, J.C.; Ho, S.K.; Lo, W.K.; Lai, K.N.; Chan, T.M. A 3-year, prospective, randomized, controlled study on amino acid dialysate in patients on CAPD. Am. J. Kidney Dis. 2003, 42, 173–183. [Google Scholar] [CrossRef]
- Jones, M.; Hagen, T.; Boyle, C.A.; Vonesh, E.; Hamburger, R.; Charytan, C.; Sandroni, S.; Bernard, D.; Piraino, B.; Schreiber, M.; et al. Treatment of malnutrition with 1.1% amino acid peritoneal dialysis solution: Results of a multicenter outpatient study. Am. J. Kidney Dis. 1998, 32, 761–769. [Google Scholar] [CrossRef]
- Johnson, D.W.; Agar, J.; Collins, J.; Disney, A.; Harris, D.C.; Ibels, L.; Irish, A.; Saltissi, D.; Suranyi, M. Recommendations for the use of icodextrin in peritoneal dialysis patients. Nephrology 2003, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.; Culleton, B.F.; Ariza, A.; Do, J.Y.; Johnson, D.W.; Sanabria, M.; Shockley, T.R.; Story, K.; Vatazin, A.; Verrelli, M.; et al. Randomized, controlled trial of glucose-sparing peritoneal dialysis in diabetic patients. J. Am. Soc. Nephrol. 2013, 24, 1889–1900. [Google Scholar] [CrossRef]
- Bonomini, M.; Zammit, V.; Divino-Filho, J.C.; Davies, S.J.; Di Liberato, L.; Arduini, A.; Lambie, M. The osmo-metabolic approach: A novel and tantalizing glucose-sparing strategy in peritoneal dialysis. J. Nephrol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Di Liberato, L.; Zammit, V.; Arduini, A. Current opinion on usage of L-carnitine in end-stage renal disease patients on peritoneal dialysis. Molecules 2019, 24, 3449. [Google Scholar] [CrossRef]
- Bonomini, M.; Di Silvestre, S.; Di Tomo, P.; Di Pietro, N.; Mandatori, D.; Di Liberato, L.; Sirolli, V.; Chiarelli, F.; Indiveri, C.; Pandolfi, A.; et al. Effect of peritoneal dialysis fluid containing osmo-metabolic agents on human endothelial cells. Drug Des. Dev. Ther. 2016, 10, 3925–3932. [Google Scholar] [CrossRef]
- Budavari, S.K.; Smith, K.A.; Heckelman, P. (Eds.) The Merck Index: An Encyclopedia of Drugs, Chemical, and Biologicals, 12th ed.; Merck & CO: Whitehouse Station, NJ, USA, 1996; pp. 302–303. [Google Scholar]
- Bonomini, M.; Pandolfi, A.; Di Liberato, L.; Di Silvestre, S.; Cnops, Y.; Di Tomo, P.; D’arezzo, M.; Monaco, M.P.; Giardinelli, A.; Di Pietro, N.; et al. L-carnitine is an osmotic agent suitable for peritoneal dialysis. Kidney Int. 2011, 80, 645–654. [Google Scholar] [CrossRef]
- Bonomini, M.; Di Liberato, L.; Del Rosso, G.; Stingone, A.; Marinangeli, G.; Consoli, A.; Bertoli, S.; De Vecchi, A.; Bosi, E.; Russo, R.; et al. Effect of an L-carnitine-containing peritoneal dialysate on insulin sensitivity in patients treated with CAPD: A 4-month prospective, multicenter randomized trial. Am. J. Kidney Dis. 2013, 62, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Bazzato, G.; Coli, U.; Landini, S.; Fracasso, A.; Morachiello, P.; Righetto, F.; Scanferla, F.; Onesti, G. Xylitol as osmotic agent in CAPD: An alternative to glucose for uremic diabetic patients? Trans. Am. Soc. Artif. Intern. Organs 1982, 28, 280–286. [Google Scholar]
- Seo, E.Y.; An, S.H.; Cho, J.H.; Suh, H.S.; Park, S.H.; Gwak, H.; Kim, Y.L.; Ha, H. Effect of biocompatible peritoneal dialysis solution on residual renal function: A systematic review of randomized controlled trials. Perit. Dial. Int. 2014, 34, 724–731. [Google Scholar] [CrossRef]
- Piccapane, F.; Bonomini, M.; Castellano, G.; Gerbino, A.; Carmosino, M.; Svelto, M.; Arduini, A.; Procino, G. A novel formulation of glucose-sparing peritoneal dialysis solutions with L-carnitine improves biocompatibility on human mesothelial cells. Int. J. Mol. Sci. 2021, 22, 123. [Google Scholar] [CrossRef]
- Lau, W.L.; Vaziri, N.D. Urea, a true uremic toxin: The empire strikes back. Clin. Sci. 2017, 131, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Pietrement, C.; Toure, F. Reconsidering the lack of urea toxicity in dialysis patients. Semin. Dial. 2016, 29, 333–337. [Google Scholar] [CrossRef]
- Twardowski, Z.J.; Nolph, K.D.; Khanna, R. Peritoneal equilibration test. Perit. Dial. Bull. 1987, 7, 138–147. [Google Scholar]
- Ladwig, P.M.; Liedtke, R.R.; Larson, T.S.; Lieske, J.C. Sensitive spectrophotometric assay for plasma oxalate. Clin. Chem. 2005, 51, 2377–2380. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Cree, M.G.; Zhang, X.; Boersheim, E.; Wolfe, R.R. Measurement of stable isotopic enrichment and concentration of long-chain acyl-carnitines in tissue by HPLC-MS. J. Lipid Res. 2006, 47, 431–439. [Google Scholar] [CrossRef] [PubMed]



| Group A | Group B | |
|---|---|---|
| Number of patients | 6 | 4 |
| Age (years) | 69.8 ± 5.2 | 55.7 ± 1 2.4 |
| Gender (male/female) | 3/3 | 4/0 |
| Body mass index (kg/m2) | 28.8 ± 5.6 | 28.3 ± 1.2 |
| Systolic blood pressure (mm Hg) | 134 ± 22 | 135 ± 17 |
| Diastolic blood pressure (mm Hg) | 82 ± 8 | 81 ± 10 |
| Heart rate (beats/min) | 65 ± 10 | 77 ± 15 |
| Time on dialysis (months) | 6.7 ± 2.6 | 6.3 ± 0.5 |
| Group A | |||
|---|---|---|---|
| Day 0 | Day 28 | Day 56 | |
| Urea Kt/V (weekly) | 1.34 (1.12–1.52) | 1.42 (1.08–1.57) | 1.32 (1.14–1.49) |
| Net peritoneal UF (mL/day) | 175 (0–300) | 200 (100–300) | 350 (300–500) |
| Residual kidney function (L/week) * | 60.5 (39.0–76.2) | 64.8 (46.3–85.9) | 48.0 (42.8–78.8) |
| Creatinine clearance (L/week) * | 77.9 (55.5–85.5) | 79.9 (55.5–95.8) | 62.5 (58.0–91.5) |
| Solute transport (D/P creatinine) | 0.59 (0.57–0.62) | 0.62 (0.60–0.66) | 0.57 (0.53–0.62) |
| Solute transport (D/D0 glucose) | 0.26 (0.23–0.31) | 0.28 (0.27–0.28) | 0.30 (0.27–0.37) |
| Urine output (mL/day) | 1425 (1100–2000) | 1500 (1100–2000) | 1550 (1400–1750) |
| Group B | |||
| Day 0 | Day 28 | Day 56 | |
| Urea Kt/V (weekly) | 1.41 (1.06–1.52) | 1.53 (1.49–1.64) | 1.53 (1.49–1.64) |
| Net peritoneal UF (mL/day) | 350 (300–400) | 350 (300–400) | 400 (350–425) |
| Residual kidney function (L/week) * | 43.0 (38.3–58.3) | 45.2 (36.0–65.4) | 44.7 (42.1–55.4) |
| Creatinine clearance (L/week) * | 67.4 (62.0–79.6) | 67.9 (65.7–84.0) | 69.6 (65.3–75.8) |
| Solute transport (D/P creatinine) | 0.60 (0.56–0.64) | 0.65 (0.60–0.70) | 0.69 (0.60–0.70) |
| Solute transport (D/D0 glucose) | 0.28 (0.23–0.32) | 0.31 (0.27–0.33) | 0.25 (0.24–0.30) |
| Urine output (mL/day) | 2100 (1925–2150) | 1900 (1525–2250) | 1825 (1625–2050) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rago, C.; Lombardi, T.; Di Fulvio, G.; Di Liberato, L.; Arduini, A.; Divino-Filho, J.C.; Bonomini, M. A New Peritoneal Dialysis Solution Containing L-Carnitine and Xylitol for Patients on Continuous Ambulatory Peritoneal Dialysis: First Clinical Experience. Toxins 2021, 13, 174. https://doi.org/10.3390/toxins13030174
Rago C, Lombardi T, Di Fulvio G, Di Liberato L, Arduini A, Divino-Filho JC, Bonomini M. A New Peritoneal Dialysis Solution Containing L-Carnitine and Xylitol for Patients on Continuous Ambulatory Peritoneal Dialysis: First Clinical Experience. Toxins. 2021; 13(3):174. https://doi.org/10.3390/toxins13030174
Chicago/Turabian StyleRago, Carmela, Teresa Lombardi, Giorgia Di Fulvio, Lorenzo Di Liberato, Arduino Arduini, José C. Divino-Filho, and Mario Bonomini. 2021. "A New Peritoneal Dialysis Solution Containing L-Carnitine and Xylitol for Patients on Continuous Ambulatory Peritoneal Dialysis: First Clinical Experience" Toxins 13, no. 3: 174. https://doi.org/10.3390/toxins13030174
APA StyleRago, C., Lombardi, T., Di Fulvio, G., Di Liberato, L., Arduini, A., Divino-Filho, J. C., & Bonomini, M. (2021). A New Peritoneal Dialysis Solution Containing L-Carnitine and Xylitol for Patients on Continuous Ambulatory Peritoneal Dialysis: First Clinical Experience. Toxins, 13(3), 174. https://doi.org/10.3390/toxins13030174






