Sphingomyelinase D Activity in Sicarius tropicus Venom: Toxic Potential and Clues to the Evolution of SMases D in the Sicariidae Family
Abstract
1. Introduction
2. Results
2.1. Sicarius tropicus Venom Biochemical Characterization
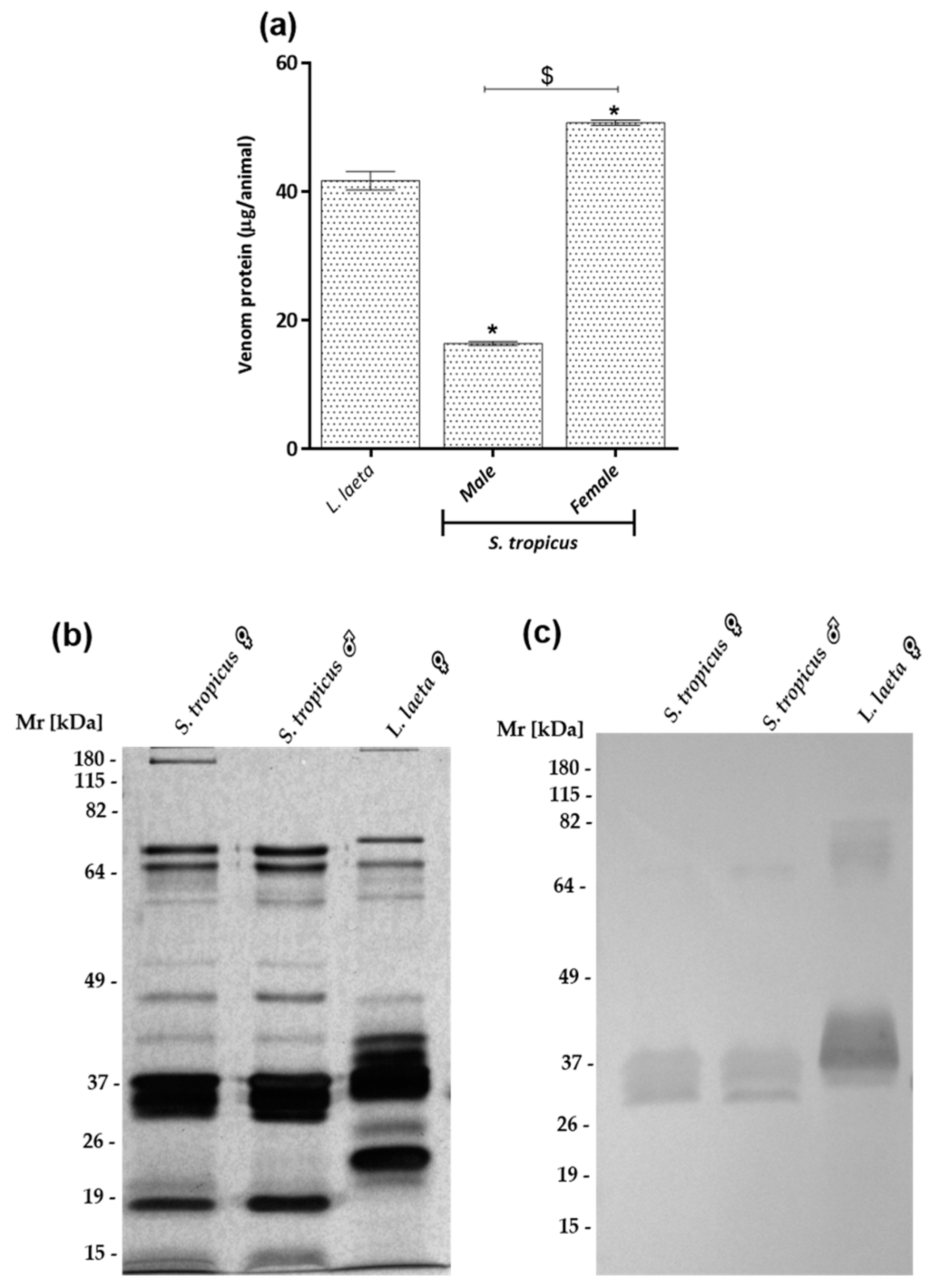
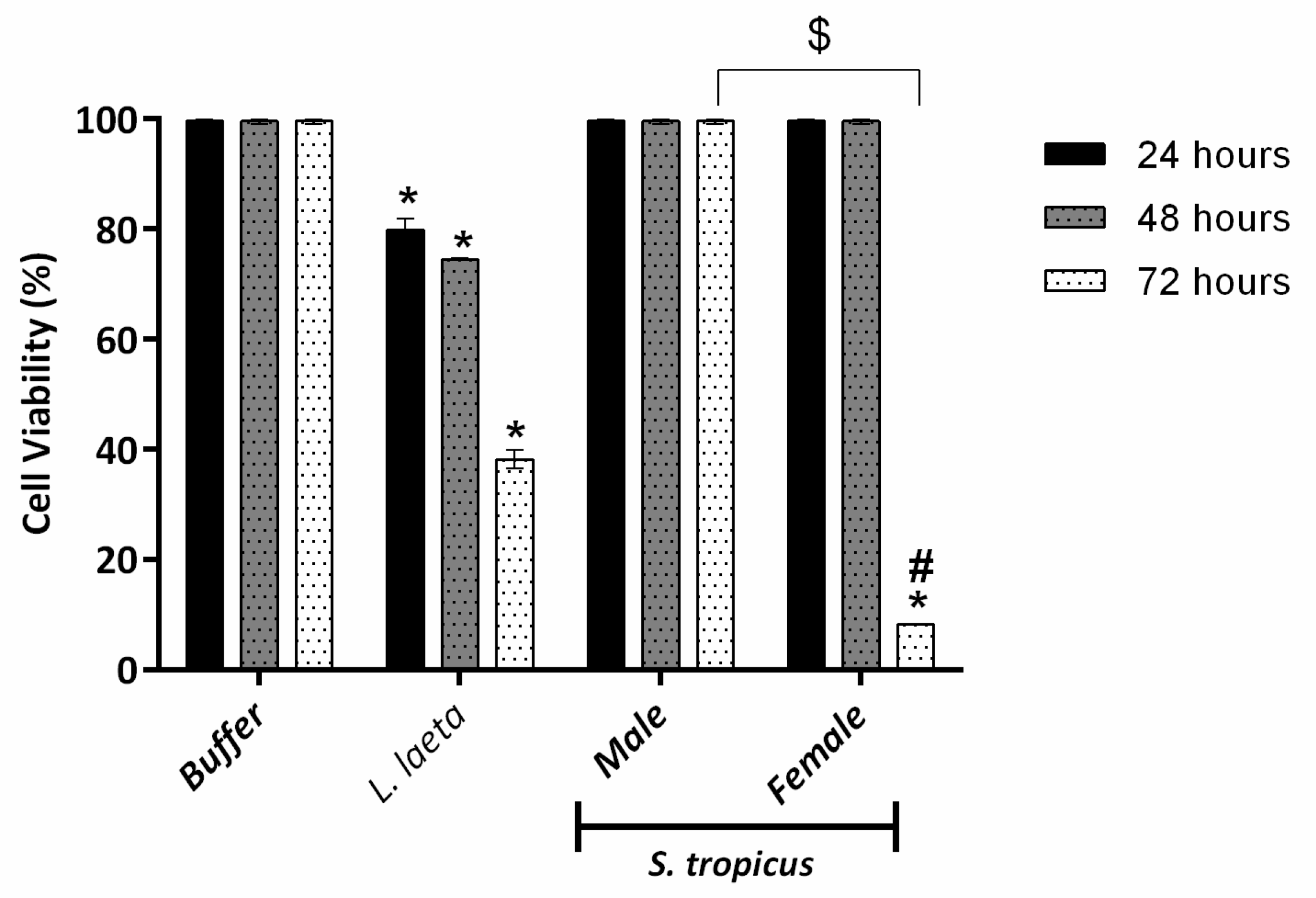
2.2. Female S. tropicus and L. laeta Venoms Reduce the Viability of Human Keratinocytes
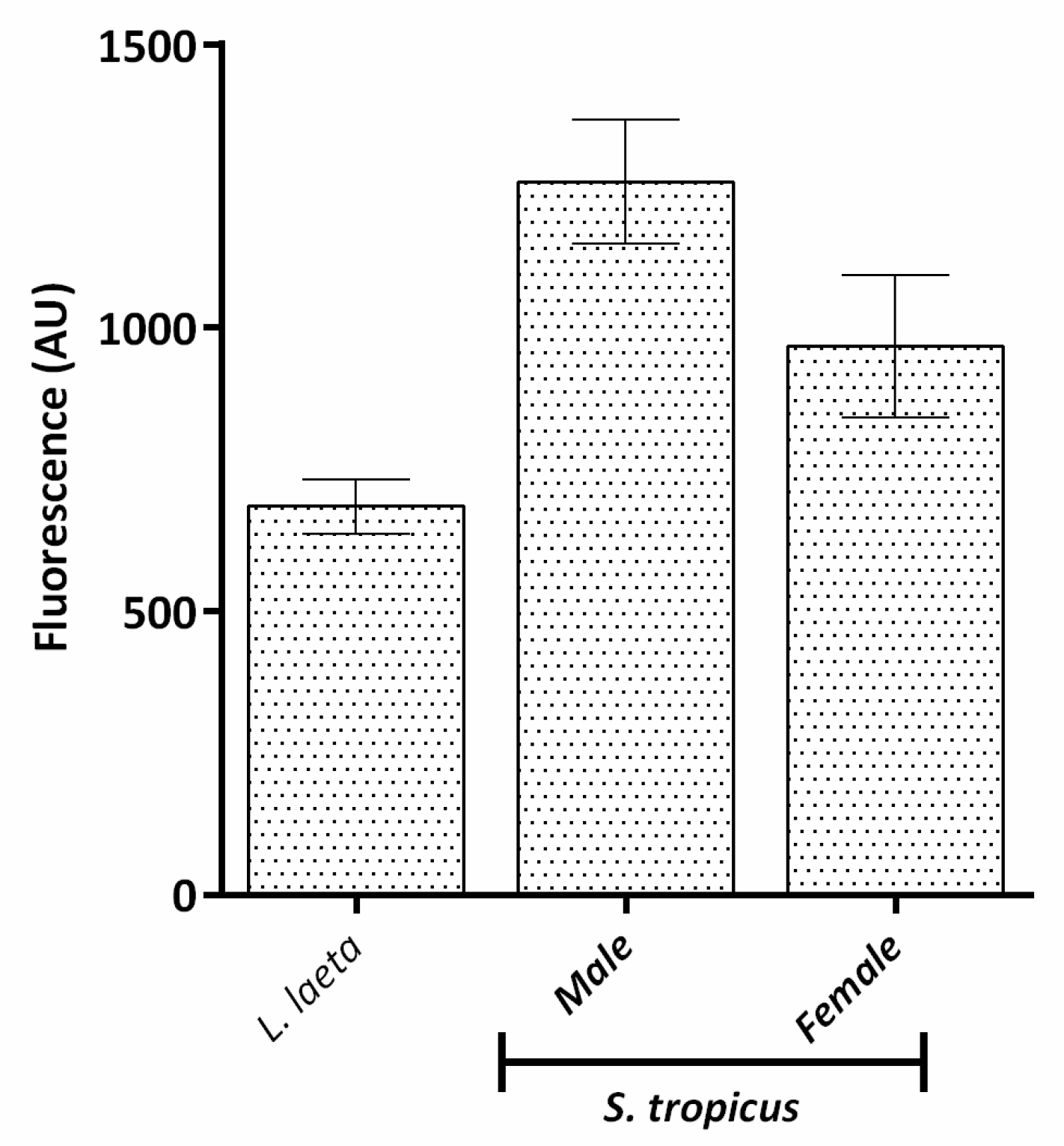
2.3. Sicarius tropicus Venom Induces Complement-Dependent Hemolysis in Human Erythrocytes by Different Mechanisms Than L. laeta Venom
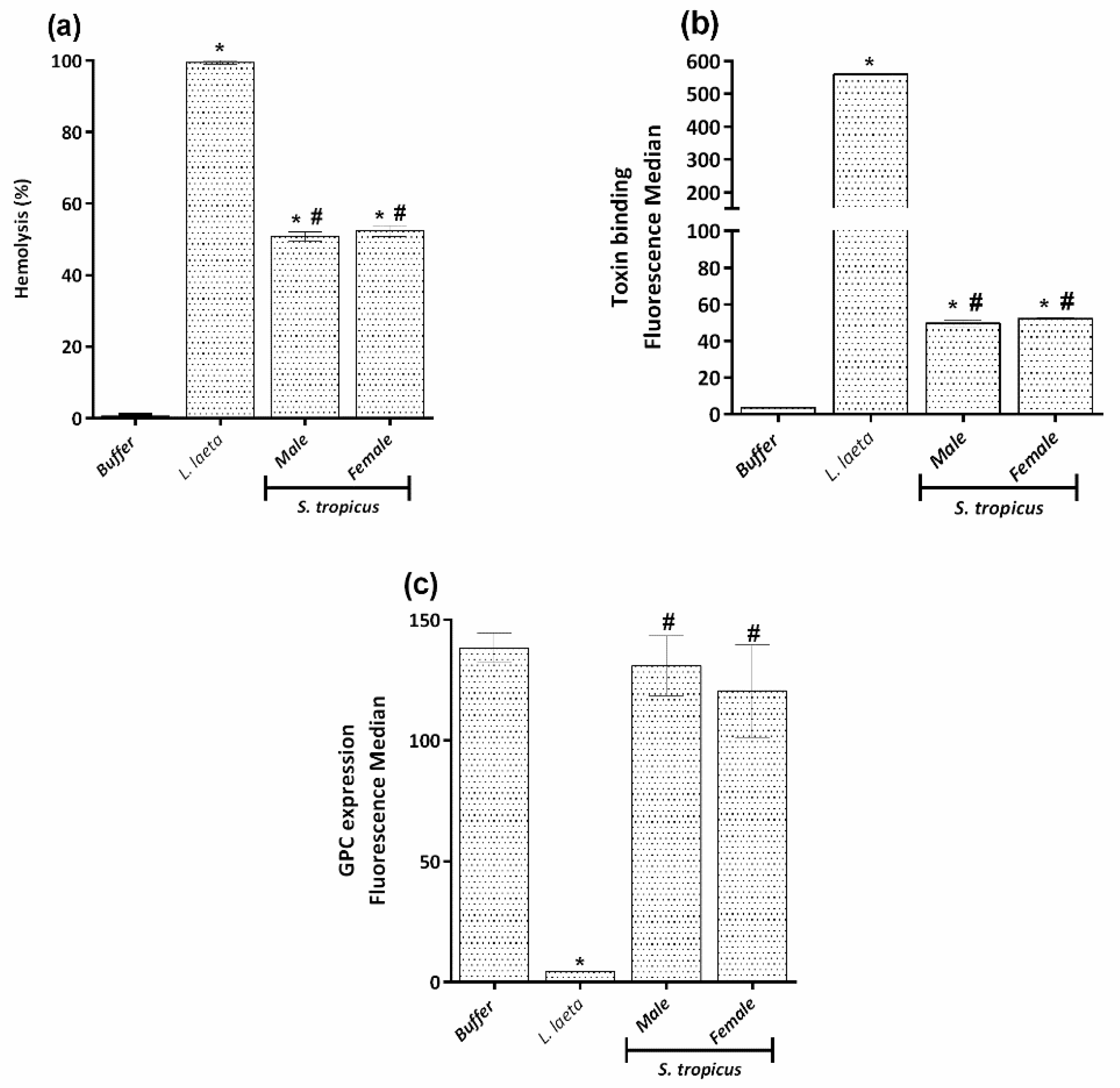
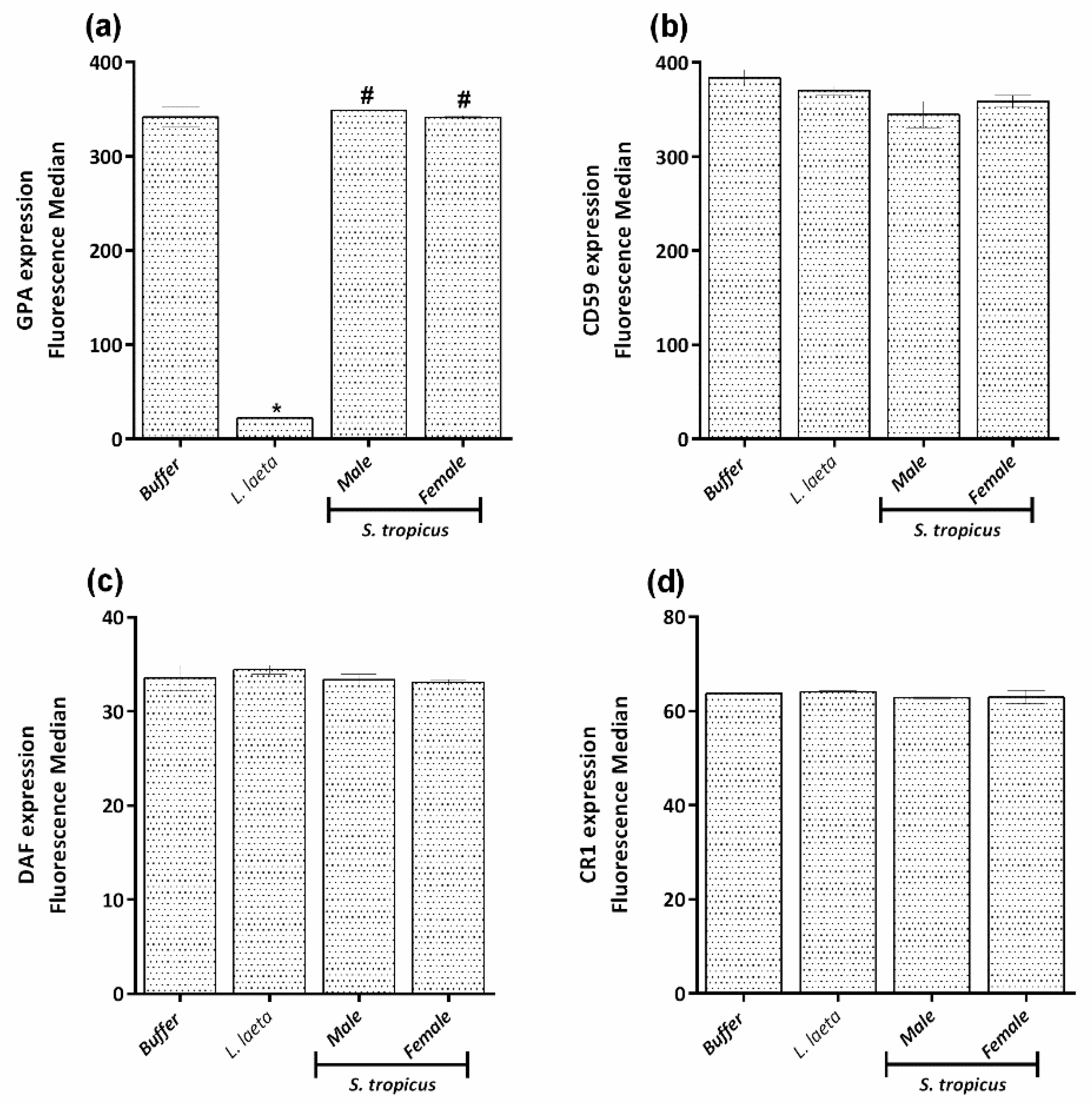
2.4. PLA2 and PLC Activities Are Not Involved in the Hemolysis Induced by the Venom of S. tropicus
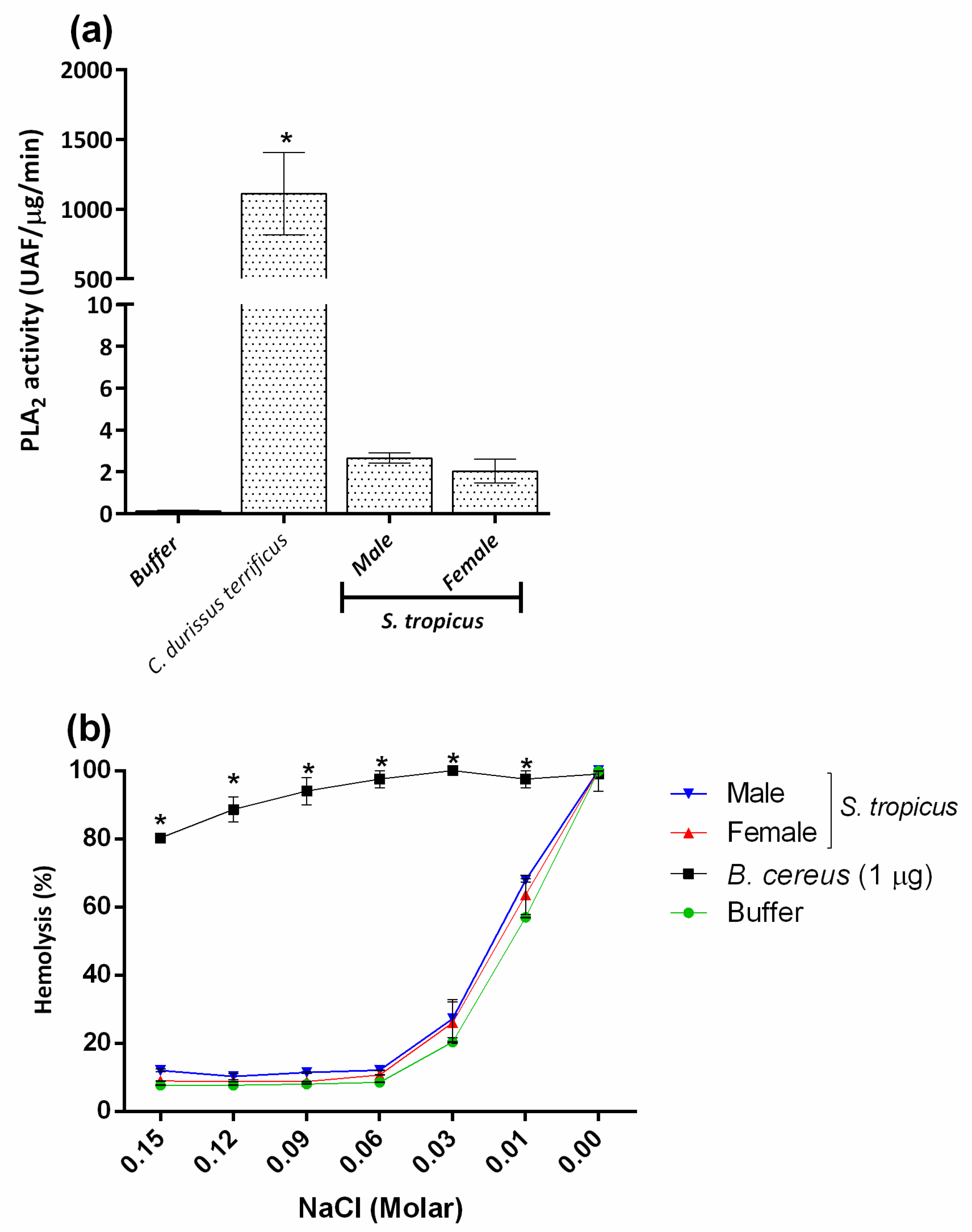
2.5. Sicarius tropicus Venom Induces Phosphatidylserine Exposition in the Erythrocytes Outer Membrane Leading to Complement-Dependent Lysis
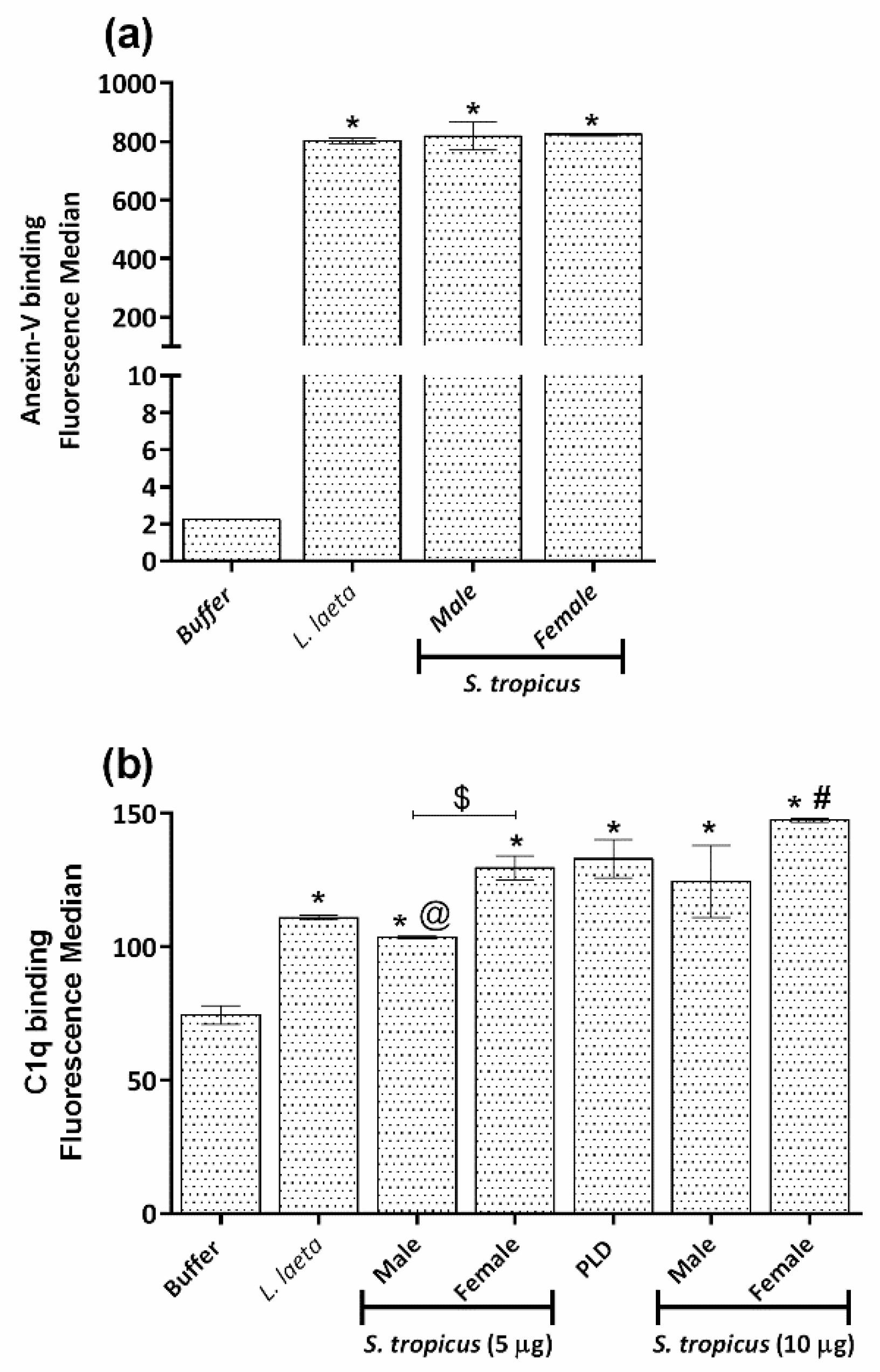
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Reagents, Antibodies and Buffers
5.2. Spiders and Venoms
5.3. Electrophoresis and Western Blotting
5.4. Enzymatic Activity
5.5. Normal Human Serum and Erythrocytes
5.5.1. Treatment of Erythrocytes with Venoms
5.5.2. Flow Cytometry
5.5.3. Spontaneous Osmotic Lysis
5.6. Human Keratinocytes Cultures
Viability Assay
5.7. Phospholipase A2 Activity
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WSC. World Spider Catalog Version 22.0. Available online: http://wsc.nmbe.ch (accessed on 1 March 2021).
- Magalhaes, I.L.; Brescovit, A.D.; Santos, A. Phylogeny of Sicariidae spiders (Araneae: Haplogynae), with a monograph on Neotropical Sicarius. Zool. J. Linn. Soc. 2017, 179, 767–864. [Google Scholar] [CrossRef]
- Gonçalves-De-Andrade, R.M.; Tambourgi, D.V. First record on Loxosceles laeta (Nicolet, 1849) (Araneae, Sicariidae) in the West Zone of São Paulo City, São Paulo, Brazil, and considerations regarding its geographic distribution. Rev. Soc. Bras. Med. Trop. 2003, 36, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.L.; Vasconcellos-Neto, J. Microhabitats Occupied by Loxosceles intermedia and Loxosceles laeta (Araneae: Sicariidae) in Curitiba, Paraná, Brazil. J. Med. Èntomol. 2005, 42, 756–765. [Google Scholar] [CrossRef]
- Newlands, G.; Atkinson, P. Review of southern African spiders of medical importance, with notes on the signs and symptoms of envenomation. S. Afr. Med. J. 1988, 73, 235–239. [Google Scholar] [PubMed]
- Lopes, P.H.; Squaiella-Baptistão, C.C.; Marques, M.O.T.; Tambourgi, D.V. Clinical aspects, diagnosis and management of Loxosceles spider envenomation: Literature and case review. Arch. Toxicol. 2020, 94, 1461–1477. [Google Scholar] [CrossRef]
- Dos-Santos, M.; Cardoso, J. Lesão dermonecrótica por Sicarius tropicus, simulando loxoscelismo cutâneo. Rev. Soc. Bras. Med. Trop. 1992, 25, 115–123. [Google Scholar]
- Filmer, M.; Newlands, G. Araneism in Africa south of the equator with key to clinical diagnosis. Dis. Skin 1990, 8, 4–10. [Google Scholar]
- Newlands, G.; Atkinson, P. A key for the clinical diagnosis of araneism in Africa south of the equator. S. Afr. Med. J. 1990, 77, 96–97. [Google Scholar]
- Newlands, G. Arthropods that sting and bite man—Their recognition and treatment of patient. JCME 1989, 7, 773–784. [Google Scholar]
- Newlands, G.; Isaacson, C.; Martindale, C. Loxoscelism in the Transvaal, South Africa. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 610–615. [Google Scholar] [CrossRef]
- Van Aswegen, G.; Van Rooyen, J.; Van Der Nest, D.; Veldman, F.; De Villiers, T.; Oberholzer, G. Venom of a six-eyed crab spider, Sicarius testaceus (Purcell, 1908), causes necrotic and haemorrhagic lesions in the rabbit. Toxicon 1997, 35, 1149–1152. [Google Scholar] [CrossRef]
- Binford, G.J.; Wells, M.A. The phylogenetic distribution of sphingomyelinase D activity in venoms of Haplogyne spiders. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 25–33. [Google Scholar] [CrossRef]
- Binford, G.J.; Callahan, M.S.; Bodner, M.R.; Rynerson, M.R.; Núñez, P.B.; Ellison, C.E.; Duncan, R.P. Phylogenetic relationships of Loxosceles and Sicarius spiders are consistent with Western Gondwanan vicariance. Mol. Phylogenet. Evol. 2008, 49, 538–553. [Google Scholar] [CrossRef]
- Lopes, P.H.; Bertani, R.; Gonçalves-De-Andrade, R.M.; Nagahama, R.H.; Berg, C.W.V.D.; Tambourgi, D.V. Venom of the Brazilian Spider Sicarius ornatus (Araneae, Sicariidae) Contains Active Sphingomyelinase D: Potential for Toxicity after Envenomation. PLoS Negl. Trop. Dis. 2013, 7, e2394. [Google Scholar] [CrossRef] [PubMed]
- Forrester, L.; Barrett, J.; Campbell, B. Red blood cell lysis induced by the venom of the brown recluse spider. Arch. Biochem. Biophys. 1978, 187, 355–365. [Google Scholar] [CrossRef]
- Kurpiewski, G.; Forrester, L.J.; Barrett, J.T.; Campbell, B.J. Platelet aggregation and sphingomyelinase D activity of a purified toxin from the venom of Loxosceles reclusa. Biochim. Biophys. Acta (BBA) Gen. Subj. 1981, 678, 467–476. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; Magnoli, F.C.; Von Eickstedt, V.R.; Benedetti, Z.C.; Petricevich, V.L.; da Silva, W.D. Incorporation of a 35-kilodalton purified protein from Loxosceles intermedia spider venom transforms human erythrocytes into activators of autolo-gous complement alternative pathway. J. Immunol. 1995, 155, 4459–4466. [Google Scholar]
- Tambourgi, D.V.; Magnoli, F.C.; Berg, C.W.V.D.; Morgan, B.; De Araujo, P.S.; Alves, E.W.; Da Silva, W. Sphingomyelinases in the Venom of the Spider Loxosceles intermedia are Responsible for both Dermonecrosis and Complement-Dependent Hemolysis. Biochem. Biophys. Res. Commun. 1998, 251, 366–373. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; Morgan, B.P.; De Andrade, R.M.G.; Magnoli, F.C.; Berg, C.W.V.D. Loxosceles intermedia spider envenomation induces activation of an endogenous metalloproteinase, resulting in cleavage of glycophorins from the erythrocyte surface and facilitating complement-mediated lysis. Blood 2000, 95, 683–691. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; Gonçalves-De-Andrade, R.M.; Berg, C.W.V.D. Loxoscelism: From basic research to the proposal of new therapies. Toxicon 2010, 56, 1113–1119. [Google Scholar] [CrossRef]
- McNamara, P.J.; Cuevas, W.A.; Songer, J. Toxic phospholipases D of Corynebacterium pseudotuberculosis, C. ulcerans and Arcanobacterium haemolyticum: Cloning and sequence homology. Gene 1995, 156, 113–118. [Google Scholar] [CrossRef]
- Alarcon-Chaidez, F.J.; Boppana, V.D.; Hagymasi, A.; Adler, A.J.; Wikel, S.K. A novel sphingomyelinase-like enzyme in Ixodes scapularis tick saliva drives host CD4+T cells to express IL-4. Parasite Immunol. 2009, 31, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Dias-Lopes, C.; Neshich, I.A.P.; Neshich, G.; Ortega, J.M.; Granier, C.; Chávez-Olórtegui, C.; Molina, F.; Felicori, L. Identification of new Sphingomyelinases D in Pathogenic Fungi and other pathogenic organisms. PLoS ONE 2013, 8, e79240. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Campbell, B.J.; Forrester, L.J. Comparative toxinology of Loxosceles reclusa and Corynebacterium pseudotuberculosis. Science 1985, 228, 590–591. [Google Scholar] [CrossRef]
- Murakami, M.T.; Fernandes-Pedrosa, M.F.; De Andrade, S.A.; Gabdoulkhakov, A.; Betzel, C.; Tambourgi, D.V.; Arni, R.K. Structural insights into the catalytic mechanism of sphingomyelinases D and evolutionary relationship to glycerophosphodiester phosphodiesterases. Biochem. Biophys. Res. Commun. 2006, 342, 323–329. [Google Scholar] [CrossRef]
- Cordes, M.H.J.; Binford, G.J. Lateral gene transfer of a dermonecrotic toxin between spiders and bacteria. Bioinformatics 2005, 22, 264–268. [Google Scholar] [CrossRef]
- Binford, G.J.; Bodner, M.R.; Cordes, M.H.; Baldwin, K.L.; Rynerson, M.R.; Burns, S.N.; Zobel-Thropp, P.A. Molecular Evolution, Functional Variation, and Proposed Nomenclature of the Gene Family That Includes Sphingomyelinase D in Sicariid Spider Venoms. Mol. Biol. Evol. 2008, 26, 547–566. [Google Scholar] [CrossRef]
- Pedroso, A.; Matioli, S.R.; Murakami, M.T.; Pidde-Queiroz, G.; Tambourgi, D.V. Adaptive evolution in the toxicity of a spider’s venom enzymes. BMC Evol. Biol. 2015, 15, 1–16. [Google Scholar] [CrossRef]
- Paixão-Cavalcante, D.; Berg, C.W.V.D.; Fernandes-Pedrosa, M.D.F.; Gon, R.M.; Tambourgi, D.V. Role of Matrix Metalloproteinases in HaCaT Keratinocytes Apoptosis Induced by Loxosceles Venom Sphingomyelinase D. J. Investig. Dermatol. 2006, 126, 61–68. [Google Scholar] [CrossRef]
- Roelofsen, B.; Zwaal, R.F.A. The use of phospholipases in the determination of asymmetric phospholipid distribution in membranes. In Methods in Membrane Biology; Metzler, J.B., Ed.; Springer: Boston, MA, USA, 1976; pp. 147–177. [Google Scholar]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, H.; Mori, M.; Taguchi, R. Studies on sphingomyelinase of Bacillus cereus: Hydrolytic and hemolytic actions on erythrocyte membranes. Arch. Biochem. Biophys. 1980, 199, 572–578. [Google Scholar] [CrossRef]
- Oda, M.; Takahashi, M.; Matsuno, T.; Uoo, K.; Nagahama, M.; Sakurai, J. Hemolysis induced by Bacillus cereus sphingomyelinase. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 1073–1080. [Google Scholar] [CrossRef][Green Version]
- Zwaal, R.F.; Schroit, A.J. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 1997, 89, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Tambourgi, D.V.; Silva, M.D.S.D.; Billington, S.J.; De Andrade, R.M.G.; Magnoli, F.C.; Songer, J.G.; Berg, C.W.V.D. Mechanism of induction of complement susceptibility of erythrocytes by spider and bacterial sphingomyelinases. Immunology 2002, 107, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tambourgi, D.; Pedrosa, M.F.; De Andrade, R.G.; Billington, S.; Griffiths, M.; Berg, C.V.D. Sphingomyelinases D induce direct association of C1q to the erythrocyte membrane causing complement mediated autologous haemolysis. Mol. Immunol. 2007, 44, 576–582. [Google Scholar] [CrossRef]
- Lotz, L. An update on the spider genus Hexophthalma (Araneae: Sicariidae) in the Afrotropical region, with descriptions of new species. Eur. J. Taxon. 2018, 475–494. [Google Scholar] [CrossRef]
- Arán-Sekul, T.; Perčić-Sarmiento, I.; Valencia, V.; Olivero, N.; Rojas, J.M.; Araya, J.E.; Taucare-Ríos, A.; Catalán, A. Toxicological Characterization and Phospholipase D Activity of the Venom of the Spider Sicarius thomisoides. Toxins 2020, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, K.C.; De Andrade, R.M.G.; Giusti, A.L.; Da Silva, W.D.; Tambourgi, D.V. Sex-linked variation of Loxosceles intermedia spider venoms. Toxicon 1999, 37, 217–221. [Google Scholar] [CrossRef]
- Zobel-Thropp, P.A.; Bodner, M.R.; Binford, G.J. Comparative analyses of venoms from American and African Sicarius spiders that differ in sphingomyelinase D activity. Toxicon 2010, 55, 1274–1282. [Google Scholar] [CrossRef]
- Pedrosa, M.D.F.F.; Azevedo, I.D.L.J.D.; Gonçalves-De-Andrade, R.M.; Berg, C.W.V.D.; Ramos, C.R.; Ho, P.L.; Tambourgi, D.V. Molecular cloning and expression of a functional dermonecrotic and haemolytic factor from Loxosceles laeta venom. Biochem. Biophys. Res. Commun. 2002, 298, 638–645. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; Paixão-Cavalcante, D.; De Andrade, R.M.G.; Fernandes-Pedrosa, M.D.F.; Magnoli, F.C.; Morgan, B.P.; Berg, C.W.V.D.; Fernandes-Pedrosa, M.D.F. Loxosceles Sphingomyelinase Induces Complement-Dependent Dermonecrosis, Neutrophil Infiltration, and Endogenous Gelatinase Expression. J. Investig. Dermatol. 2005, 124, 725–731. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, K.C.; De Andrade, R.M.G.; Piazza, R.M.; Ferreira, J.M.; Berg, C.V.D.; Tambourgi, D.V. Variations in Loxosceles spider venom composition and toxicity contribute to the severity of envenomation. Toxicon 2005, 45, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, M.A.; Okamoto, C.K.; Gonçalves-De-Andrade, R.M.; Berg, C.W.V.D.; Tambourgi, D.V. Sphingomyelinase D from Loxosceles laeta Venom Induces the Expression of MMP7 in Human Keratinocytes: Contribution to Dermonecrosis. PLoS ONE 2016, 11, e0153090. [Google Scholar] [CrossRef]
- Lopes, P.H.; Murakami, M.T.; Portaro, F.C.V.; Pasqualoto, K.F.M.; Berg, C.V.D.; Tambourgi, D.V. Targeting Loxosceles spider Sphingomyelinase D with small-molecule inhibitors as a potential therapeutic approach for loxoscelism. J. Enzym. Inhib. Med. Chem. 2019, 34, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.H.; Berg, C.W.V.D.; Tambourgi, D.V. Sphingomyelinases D From Loxosceles Spider Venoms and Cell Membranes: Action on Lipid Rafts and Activation of Endogenous Metalloproteinases. Front. Pharmacol. 2020, 11, 636. [Google Scholar] [CrossRef]
- Estrada-Gomez, S.; Muñoz, L.J.V.; Lanchero, P.; Latorre, C.S. Partial Characterization of Venom from the Colombian Spider Phoneutria boliviensis (Aranae:Ctenidae). Toxins 2015, 7, 2872–2887. [Google Scholar] [CrossRef]
- Cuevas, W.A.; Songer, J.G. Arcanobacterium haemolyticum phospholipase D is genetically and functionally similar to Coryne-bacterium pseudotuberculosis phospholipase D. Infect. Immun. 1993, 61, 4310–4316. [Google Scholar] [CrossRef]
- Ferrara, G.I.D.S.; Fernandes-Pedrosa, M.D.F.; Junqueira-De-Azevedo, I.D.L.; Gonçalves-De-Andrade, R.M.; Portaro, F.C.; Manzoni-De-Almeida, D.; Murakami, M.T.; Arni, R.K.; Berg, C.W.V.D.; Ho, P.L.; et al. SMase II, a new sphingomyelinase D from Loxosceles laeta venom gland: Molecular cloning, expression, function and structural analysis. Toxicon 2009, 53, 743–753. [Google Scholar] [CrossRef]
- Manzoni de Almeida, D.; Fernandes-Pedrosa, M.F.; Gonçalves De Andrade, R.M.; Marcelino, J.R.; Gondo-Higashi, H.; Jun-queira De Azevedo, I.L.M.; Ho, P.L.; van den Berg, C.; Tambourgi, D.V. A new anti-loxoscelic serum produced against re-combinant sphingomyelinase D: Results of preclinical trials. Am. J. Trop. Med. Hyg. 2008, 79, 463–470. [Google Scholar] [CrossRef]
- Bucherl, W. Biology and Venoms of the Most Important Soutli American Spiders of the Genera Phoneutria, Loxosceles, Lycosa, and Latrodectus. Am. Zool. 1969, 9, 157–159. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.H. Silver stain for proteins in polyacrylamide gels: A modified procedure with enhanced uniform sensitivity. Anal. Biochem. 1981, 117, 307–310. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Tokumura, A.; Kanaya, Y.; Miyake, M.; Yamano, S.; Irahara, M.; Fukuzawa, K. Increased production of bioactive lysophosphatidic acid by serum lysophospholipase D in human pregnancy. Biol. Reprod. 2002, 67, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, P.H.; Fukushima, C.S.; Shoji, R.; Bertani, R.; Tambourgi, D.V. Sphingomyelinase D Activity in Sicarius tropicus Venom: Toxic Potential and Clues to the Evolution of SMases D in the Sicariidae Family. Toxins 2021, 13, 256. https://doi.org/10.3390/toxins13040256
Lopes PH, Fukushima CS, Shoji R, Bertani R, Tambourgi DV. Sphingomyelinase D Activity in Sicarius tropicus Venom: Toxic Potential and Clues to the Evolution of SMases D in the Sicariidae Family. Toxins. 2021; 13(4):256. https://doi.org/10.3390/toxins13040256
Chicago/Turabian StyleLopes, Priscila Hess, Caroline Sayuri Fukushima, Rosana Shoji, Rogério Bertani, and Denise V. Tambourgi. 2021. "Sphingomyelinase D Activity in Sicarius tropicus Venom: Toxic Potential and Clues to the Evolution of SMases D in the Sicariidae Family" Toxins 13, no. 4: 256. https://doi.org/10.3390/toxins13040256
APA StyleLopes, P. H., Fukushima, C. S., Shoji, R., Bertani, R., & Tambourgi, D. V. (2021). Sphingomyelinase D Activity in Sicarius tropicus Venom: Toxic Potential and Clues to the Evolution of SMases D in the Sicariidae Family. Toxins, 13(4), 256. https://doi.org/10.3390/toxins13040256




