CE-MS-Based Identification of Uremic Solutes Specific to Hemodialysis Patients
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of HD Patients and Non-HD CKD Patients
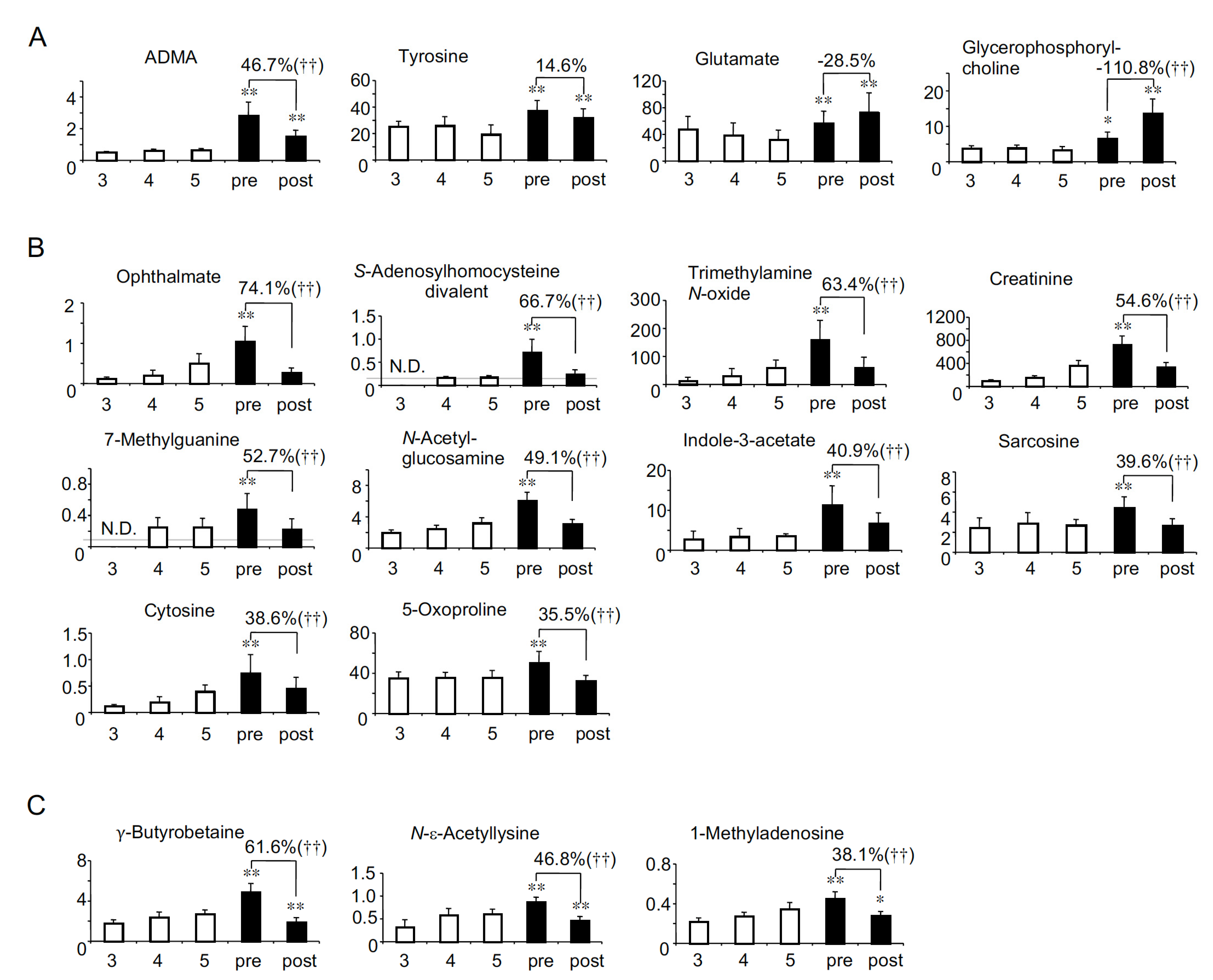
2.2. Solutes Whose Serum Levels Were Higher in HD Patients Than in Non-HD CKD Patients
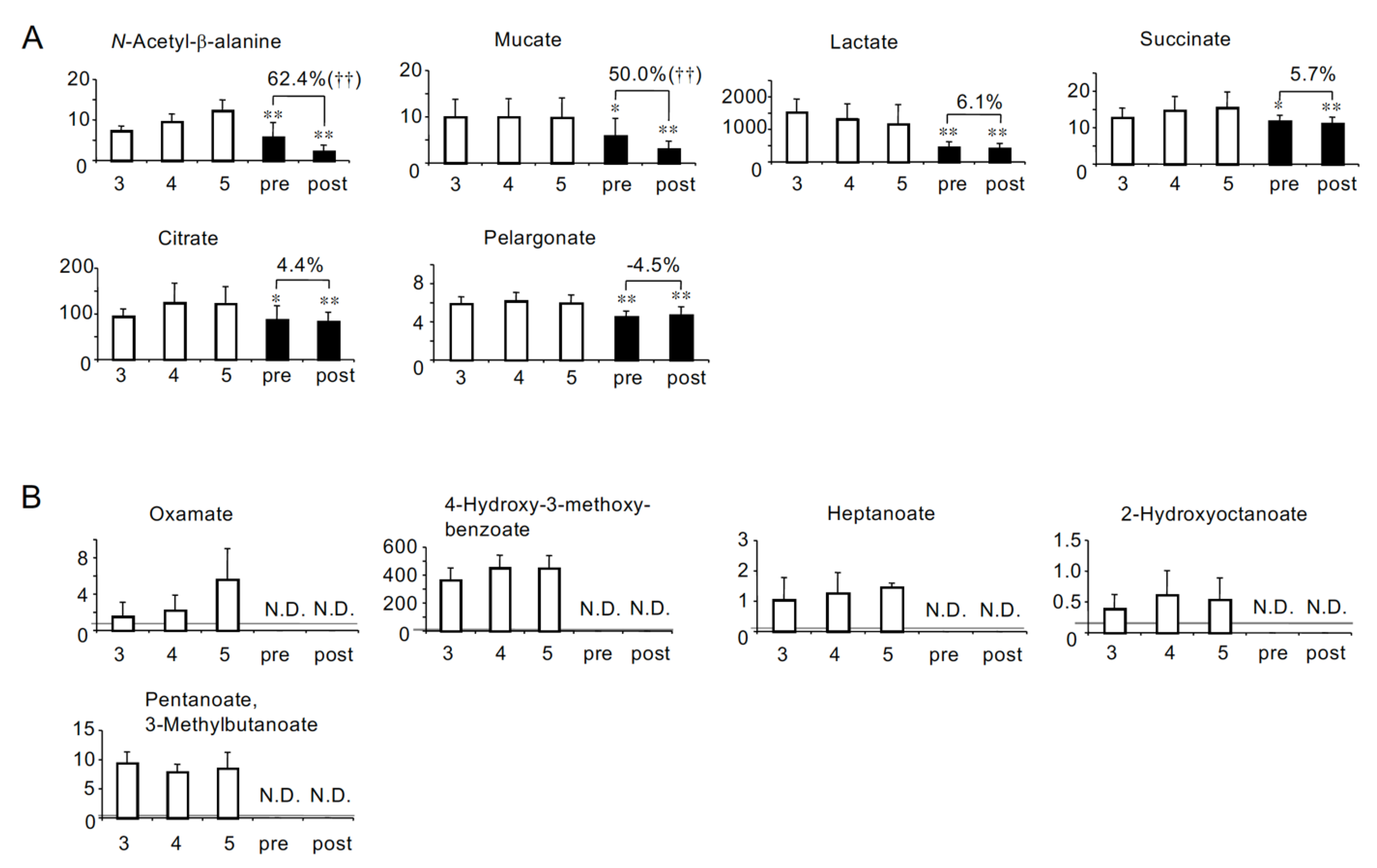
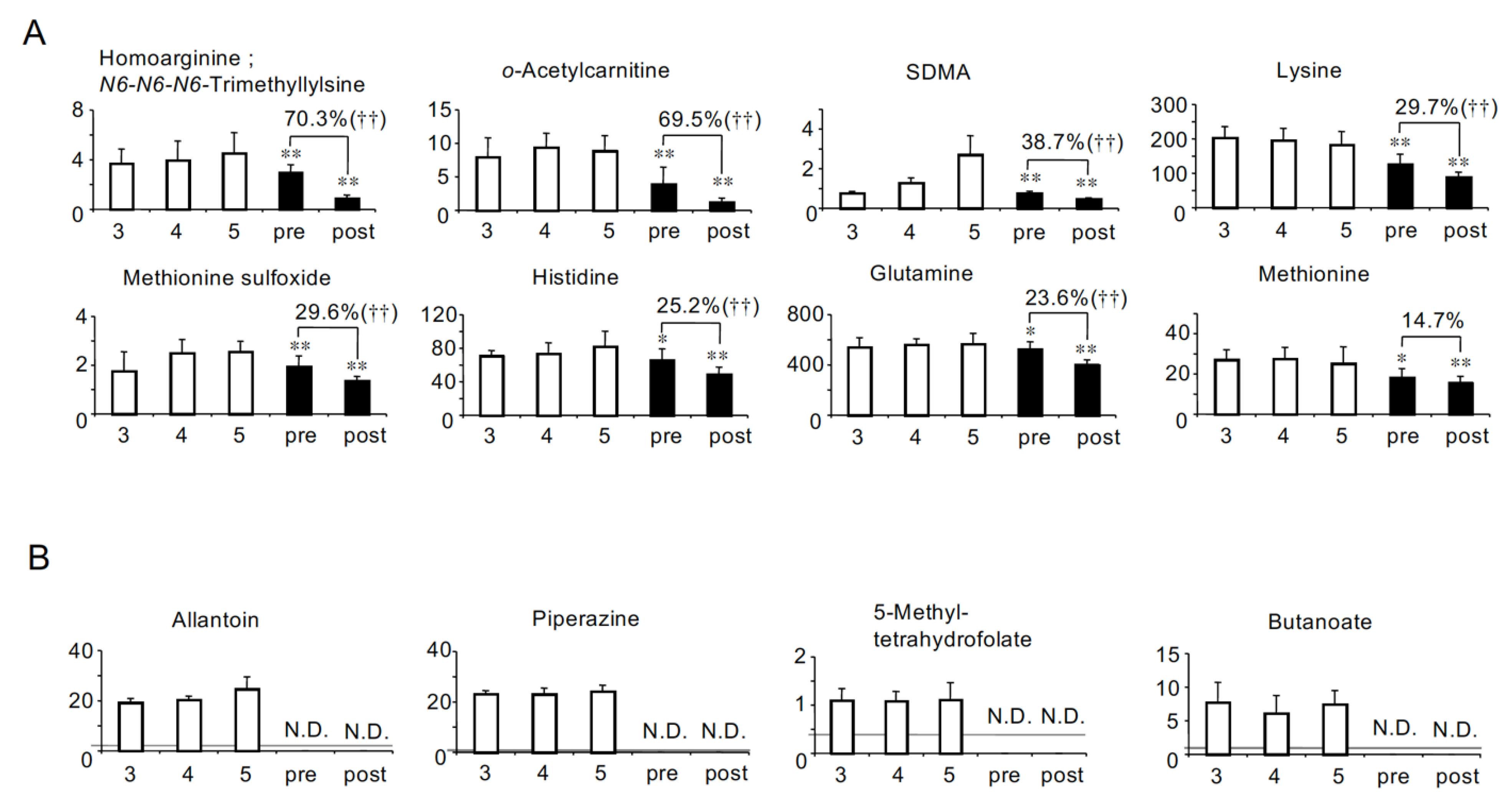
2.3. Solutes the Serum Levels of Which Were Lower in HD Patients Than in Non-HD CKD Patients
2.4. Profiling of HD Patient-Specific Uremic Solutes
2.5. Removal Rate
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. CE-MS Measurement for Metabolome Analysis
5.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levey, A.S.; Atkins, R.; Coresh, J.; Cohen, E.P.; Collins, A.J.; Eckardt, K.U.; Nahas, M.E.; Jaber, B.L.; Jadoul, M.; Levin, A.; et al. Chronic kidney disease as a global public health problem: Approaches and initiatives-a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Uremic Toxins, 1st ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Tanaka, H.; Sirich, T.L.; Plummer, N.S.; Weaver, D.S.; Meyer, T.W. An Enlarged Profile of Uremic Solutes. PLoS ONE 2015, 10, e0135657. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Kohno, M.; Yamamoto, M.; Fujisawa, T.; Fujiwara, K.; Tanaka, N. Metabolomic analysis of human plasma from haemodialysis patients. Eur. J. Clin. Investig. 2011, 41, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Souza, A.; Farrell, L.; Pollak, M.R.; Lewis, G.D.; Steele, D.J.; Thadhani, R.; Clish, C.B.; Greka, A.; Gerszten, R.E. Metabolite profiling identifies markers of uremia. J. Am. Soc. Nephrol. 2010, 21, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Itoh, Y.; Tateoka, R.; Ezawa, A.; Murakami, K.; Niwa, T. Metabolomic analysis of uremic toxins by liquid chromatography/electrospray ionization-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, T.; Akiyama, Y.; Suzuki, T.; Takeuchi, Y.; Mishima, E.; Tanemoto, M.; Momose, A.; Toki, N.; Sato, H.; Nakayama, M.; et al. Metabolomic profiling of uremic solutes in CKD patients. Hypertens. Res. 2010, 33, 944–952. [Google Scholar] [CrossRef]
- Grassmann, A.; Gioberge, S.; Moeller, S.; Brown, G. ESRD patients in 2004: Global overview of patient numbers, treatment modalities and associated trends. Nephrol. Dial. Transplant. 2005, 20, 2587–2593. [Google Scholar] [CrossRef]
- Anand, S.; Bitton, A.; Gaziano, T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS ONE 2013, 8, e72860. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Lindholm, B.; Stenvinkel, P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—The heart of the matter. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 11), 28–31. [Google Scholar] [CrossRef]
- Depner, T.A. Uremic toxicity: Urea and beyond. Semin. Dial. 2001, 14, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Aronov, P.A.; Fullman, J.; Nguyen, K.; Plummer, N.S.; Meyer, T.W. Untargeted mass spectrometry discloses plasma solute levels poorly controlled by hemodialysis. PLoS ONE 2017, 12, e0188315. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.; Hu, C.; Huang, X.; Liu, H.; Xuan, Q.; Lin, X.; Peng, X.; Lu, X.; Chang, M.; et al. Plasma metabolomics profiling of maintenance hemodialysis based on capillary electrophoresis-time of flight mass spectrometry. Sci. Rep. 2017, 7, 8150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, P.; Yang, J.; Niu, J. Plasma Metabolomics Profiling in Maintenance Hemodialysis Patients Based on Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Kidney Dis. 2020, 6, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Clark, W.R.; Gao, D. Determinants of small solute clearance in hemodialysis. Semin. Dial. 2005, 18, 30–35. [Google Scholar]
- Ceballos, I.; Chauveau, P.; Guerin, V.; Bardet, J.; Parvy, P.; Kamoun, P.; Jungers, P. Early alterations of plasma free amino acids in chronic renal failure. Clin. Chim. Acta 1990, 188, 101–108. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Neirynck, N.; Glorieux, G. An update on protein-bound uremic retention solutes. J. Ren. Nutr. 2012, 22, 90–94. [Google Scholar] [CrossRef]
- Niwa, T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010, 20, S2–S6. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Brunet, P. Vascular incompetence in dialysis patients--protein-bound uremic toxins and endothelial dysfunction. Semin. Dial. 2011, 24, 327–337. [Google Scholar] [CrossRef]
- Toyohara, T.; Suzuki, T.; Morimoto, R.; Akiyama, Y.; Souma, T.; Shiwaku, H.O.; Takeuchi, Y.; Mishima, E.; Abe, M.; Tanemoto, M.; et al. SLCO4C1 transporter eliminates uremic toxins and attenuates hypertension and renal inflammation. J. Am. Soc. Nephrol. 2009, 20, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Dhondt, A.; Leybaert, L.; Vanholder, R. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol. Dial. Transplant. 2009, 24, 1429–1435. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Kim, J.S.; Lee, H.J.; Yoon, D.S.; Han, B.G.; Shim, Y.H.; Choi, S.O. Do dialysis patients need extra folate supplementation? Adv. Perit. Dial. 1999, 15, 247–250. [Google Scholar]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Piceno, Y.M.; Desantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Rogachev, B.; Ohayon, S.; Saad, A.; Vorobiovsky, V.; Gruenbaum, B.F.; Leibowitz, A.; Boyko, M.; Shapira, Y.; Shnaider, A.; Zlotnik, M.; et al. The effects of hemodialysis on blood glutamate levels in chronic renal failure: Implementation for neuroprotection. J. Crit. Care 2012, 27, 743–e1. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Boger, R.H.; Bode-Boger, S.M.; Schaffer, J.; Barbey, M.; Koch, K.M.; Frolich, J.C. Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: Relationship to treatment method and atherosclerotic disease. J. Am. Soc. Nephrol. 1999, 10, 594–600. [Google Scholar] [CrossRef]
- Soga, T.; Ohashi, Y.; Ueno, Y.; Naraoka, H.; Tomita, M.; Nishioka, T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef]
- Akiyama, Y.; Takeuchi, Y.; Kikuchi, K.; Mishima, E.; Yamamoto, Y.; Suzuki, C.; Toyohara, T.; Suzuki, T.; Hozawa, A.; Ito, S.; et al. A metabolomic approach to clarifying the effect of AST-120 on 5/6 nephrectomized rats by capillary electrophoresis with mass spectrometry (CE-MS). Toxins 2012, 4, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Baran, R.; Suematsu, M.; Ueno, Y.; Ikeda, S.; Sakurakawa, T.; Kakazu, Y.; Ishikawa, T.; Robert, M.; Nishioka, T.; et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006, 281, 16768–16776. [Google Scholar] [CrossRef] [PubMed]

| HD (n = 11) | Non-HD CKD Stage 5 (n = 13) | |
|---|---|---|
| Gender (female) | 6 (55%) | 9 (69%) |
| Age (years) | 61.8 ± 6.3 | 59.5 ± 14.0 |
| Duration of HD (years) | 8.1 ± 8.2 | Not Applicable |
| Underlying renal disease | ||
| Non-diabetes | 7 (64%) | 8 (62%) |
| Diabetes | 4 (36%) | 5 (38%) |
| Previous history | ||
| Cardiovascular disease | 3 (27%) | 3 (23%) |
| Medication | ||
| Anti-hypertensive agents | 6 (55%) | 13 (100%) |
| Statins | 0 (0%) | 2 (15%) |
| Concentrations in HD Patients Compared to CKD Stage G5 Patients | |||
|---|---|---|---|
| Higher | Lower | ||
| Correlations with eGFR in non-HD CKD patients [8] | Negative (Uremic) | Group A: Uremic solutes more accumulated in HD patients (26 solutes) Adipate *, Malonate *, ADMA *, Phthalate, N-Acetylglutamate, Gluconate, trans-Aconitate, Pimelate, Hippurate, 2-Isopropylmalate, N-Acetylaspartate, Glutarate, Citramalate, cis-Aconitate, 3-Indoxyl sulfate, Isethionate, Ophthalmate, Trimetylamine N-oxide, Creatininte, 7-Methylguanine, N-Acetylglucosamine, Indole-3-acetate, Cytosine, γ-Butyrobetaine, N-ε-Acetyllysine, 1-Methyladenosine | Group D: Uremic solutes less accumulated in HD patients (8 solutes) N-Acetyl-β-alanine, Succinate, Citrate, SDMA, Methionine sulfoxide, Oxamate †, 4-Hydroxy-3-methoxybenzoate †, Allantoin † |
| No correlation (Not uremic) | Group B: HD patients-specific uremic solutes (10 solutes) N-Acetylneuraminate *, Benzoate *, Fumarate *, Decanoate *, Glycerophosphorylcholine *, Glycerophosphate, Saccharate, S-adenosylhomocysteine divalent, Sarcosine, 5-Oxoproline | Group E: Solutes specifically lost in HD patients (14 solutes) Mucate, Pelargonate, Homoarginine/N6-N6-N6-Trimethyllysine, o-Acetylcarnitine, Lysine, Histidine, Glutamine, Methionine, Heptanoate †, 2-Hydroxyoctanoate †, Pentanoate/3-Methylbutanoate †, Piperazine †, 5-Methyltetrahydrofolate †, Butanoate † | |
| Positive (Not uremic) | Group C: Solutes decreased less significantly in HD patients (2 solutes) Tyrosine *, Glutamate * | Group F: Solute decreased more significantly in HD patients (1 solute) Lactate | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyama, Y.; Kikuchi, K.; Toyohara, T.; Mishima, E.; Suzuki, C.; Suzuki, T.; Nakayama, M.; Tomioka, Y.; Soga, T.; Abe, T. CE-MS-Based Identification of Uremic Solutes Specific to Hemodialysis Patients. Toxins 2021, 13, 324. https://doi.org/10.3390/toxins13050324
Akiyama Y, Kikuchi K, Toyohara T, Mishima E, Suzuki C, Suzuki T, Nakayama M, Tomioka Y, Soga T, Abe T. CE-MS-Based Identification of Uremic Solutes Specific to Hemodialysis Patients. Toxins. 2021; 13(5):324. https://doi.org/10.3390/toxins13050324
Chicago/Turabian StyleAkiyama, Yasutoshi, Koichi Kikuchi, Takafumi Toyohara, Eikan Mishima, Chitose Suzuki, Takehiro Suzuki, Masaaki Nakayama, Yoshihisa Tomioka, Tomoyoshi Soga, and Takaaki Abe. 2021. "CE-MS-Based Identification of Uremic Solutes Specific to Hemodialysis Patients" Toxins 13, no. 5: 324. https://doi.org/10.3390/toxins13050324
APA StyleAkiyama, Y., Kikuchi, K., Toyohara, T., Mishima, E., Suzuki, C., Suzuki, T., Nakayama, M., Tomioka, Y., Soga, T., & Abe, T. (2021). CE-MS-Based Identification of Uremic Solutes Specific to Hemodialysis Patients. Toxins, 13(5), 324. https://doi.org/10.3390/toxins13050324







