Hypoglycin A in Cow’s Milk—A Pilot Study
Abstract
1. Introduction
2. Results
2.1. HGA Analysis in Raw Milk Samples
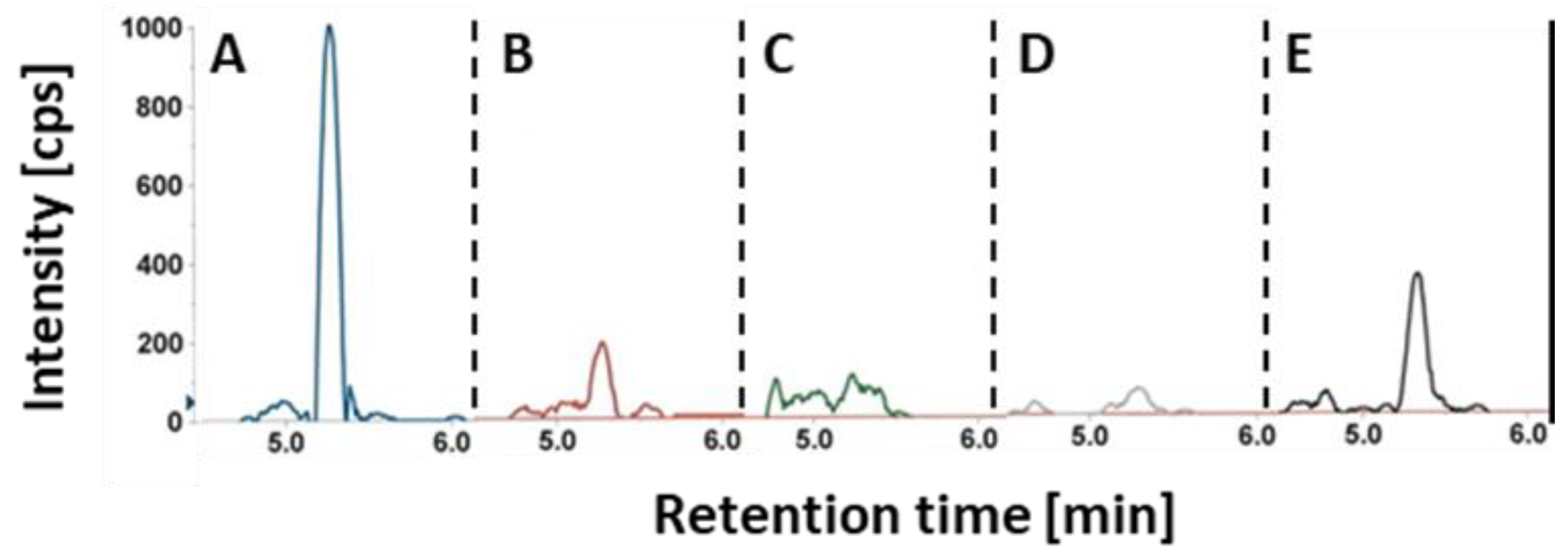
2.2. Extracted Ion Chromatograms for HGA
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Farms, Cows and Milk Sampling
5.2. Storage, Preparation and Analytical Procedure for HGA Detection in Milk Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, K.; Ikeda, Y. Hypoglycin and Jamaican Vomiting Sickness. Prog. Clin. Biol. Res. 1990, 321, 167–184. [Google Scholar] [PubMed]
- Sherratt, H.S.A. Hypoglycin, the famous toxin of the unripe Jamaican ackee fruit. Trends Pharmacol. Sci. 1986, 7, 186–191. [Google Scholar] [CrossRef]
- McTague, J.A.; Forney, R. Jamaican Vomiting Sickness in Toledo, Ohio. Ann. Emerg. Med. 1994, 23, 1116–1118. [Google Scholar] [CrossRef]
- Tanaka, K.; Kean, E.A.; Johnson, B. Jamaican Vomiting Sickness: Biochemical investigation of two cases. N. Engl. J. Med. 1976, 295, 461–467. [Google Scholar] [CrossRef]
- Gaillard, Y.; Carlier, J.; Berscht, M.; Mazoyer, C.; Bevalot, F.; Guitton, J.; Fanton, L. Fatal intoxication due to ackee (Blighia sapida) in Suriname and French Guyana. GC-MS detection and quantification of Hypoglycin-A. Forensic Sci. Int. 2011, 206, e103–e107. [Google Scholar] [CrossRef]
- Joskow, R.; Belson, M.; Vesper, H.; Backer, L.; Rubin, C. Ackee fruit poisoning: An outbreak investigation in Haiti 2000−2001, and review of the literature. Clin. Toxicol. 2006, 44, 267–273. [Google Scholar] [CrossRef]
- Henry, S.H.; Page, S.W.; Bolger, P.M. Hazard assessment of ackee fruit (Blighia sapida). Hum. Ecol. Risk Assess. 1998, 4, 1175–1187. [Google Scholar] [CrossRef]
- Bochnia, M.; Ziegler, J.; Sander, J.; Uhlig, A.; Schaefer, S.; Vollstedt, S.; Glatter, M.; Abel, S.; Recknagel, S.; Schusser, G.F.; et al. Hypoglycin A Content in Blood and Urine Discriminates Horses with Atypical Myopathy from Clinically Normal Horses Grazing on the Same Pasture. PLoS ONE 2015, 10, e0136785. [Google Scholar] [CrossRef]
- Valberg, S.J.; Sponseller, B.T.; Hegeman, A.D.; Earing, J.; Bender, J.B.; Martinson, K.L.; Patterson, S.E.; Sweetman, L. Seasonal Pasture Myopathy/Atypical Myopathy in North America associated with ingestion of hypoglycin A within seeds of the box elder tree. Equine Vet. J. 2013, 45, 419–426. [Google Scholar] [CrossRef]
- Unger, L.; Nicholson, A.; Jewitt, E.M.; Gerber, V.; Hegemann, A.; Sweetman, L.; Valberg, S. Hypoglycin A concentration in seeds of Acer pseudoplatanus trees growing on atypical myopathy-affected and control pastures. J. Vet. Intern. Med. 2014, 28, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Srikantiah, P.; Kumar, A.; Bhushan, G.; Goel, K.; Kumar, S.; Kumar, T.; Mohankumar, R.; Pandey, R.; Pathan, P.; et al. Outbreaks of unexplained neurologic illness—Muzaf farpur, India, 2013−2014. MMWR 2015, 64, 49–53. [Google Scholar]
- Van Galen, G.; Pitel, C.M.; Saegerman, C.; Patarin, F.; Amory, H.; Baily, J.D.; Cassart, D.; Gerber, V.; Hahn, C.; Harris, P.; et al. European outbreaks of atypical myopaythy in grazing equids (2006–2009): Spatiotemporal distribution, history and clinical features. Equine Vet. J. 2012, 44, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, S.L.; Carter, M.D.; Graham, L.A.; Mathews, T.P.; Johnson, D.; Thomas, J.D.; Pirkle, J.L.; Johnson, R.C. Quantifica tion of metabolites for assessing human exposure to soapberry toxins hypoglycin A and methylenecyclopropylglycine. Chem. Res. Toxicol. 2015, 28, 1753–1759. [Google Scholar] [CrossRef]
- Bochnia, M.; Sander, J.; Ziegler, J.; Terhardt, M.; Sander, S.; Janzen, N.; Cavalleri, J.; Zuraw, A.; Wensch-Dorendorf, M.; Zeyner, A. Detection of MCPG metabolites in horses with atypical myopathy. PLoS ONE 2019, 14, e0211698. [Google Scholar] [CrossRef]
- Bunert, C.; Langer, S.; Votion, D.M.; Boemer, F.; Müller, A.; Ternes, K.; Liesegang, A. Atypical myopathy in Père David’s deer (Elaphurus davidianus) associated with ingestion of hypoglycin A. J. Anim. Sci. 2018, 96, 3537–3547. [Google Scholar] [CrossRef]
- Bochnia, M.; Ziemssen, E.; Sander, J.; Stief, B.; Zeyner, A. Methylenecyclopropylglycine and hypoglycin A intoxication in three Pére David´s Deers (Elaphurus davidianus) with Atypical Myopathy. 2020, Vet. Med. Sci. Vet. Med. Sci. 2020, 1–8. [Google Scholar] [CrossRef]
- González-Medina, S.; Hyde, C.; Lovera, I.; Piercy, R.J. Detection of hypoglycin A and MCPA-carnitine in equine serum and muscle tissue: Optimization and validation of a LC-MS-based method without derivatization. Equine Vet. J. 2020, 53, 558–568. [Google Scholar] [CrossRef]
- Rudolph, W.; Remane, D.; Wissenbach, D.K.; Peters, F.T. Liquid chromatography-mass spectrometry-based determination of ergocristine, ergocryptine, ergotamine, ergovaline, hypoglycin A, lolitrem B, methylene cyclopropyl acetic acid carnitine, N- acetylloline, N-formylloline, paxilline, and peramine in equine hair. J. Chromatography B 2019, 1117, 127–135. [Google Scholar]
- González-Medina, S.; Bevin, W.; Alzalo-Domingo, R.; Cheng, Y.-M.; Piercy, R.J. Hypoglycin A absorption in sheep without concurrent clinical or biochemical evidence of disease. J. Vet. Intern. Med. 2021, 35, 1170–1176. [Google Scholar] [CrossRef]
- Krägeloh, T.; Cavalleri, M.J.M.V.; Terhardt, M.; Sander, J.; Janzen, N.; Ziegler, J.; Breves, G.; Cehak, A. Die Atypische Myopathie des Pferdes—Spielt enterale Mobilisierung des Hypoglycin A eine Rolle im Hinblick auf die toxische Wirkung? Prakt. Tierarzt 2017, 98, 1262–1270. [Google Scholar]
- Sander, J.; Terhardt, M.; Janzen, N. Detection of maple toxins in Mare’s milk. J. Vet. Intern. Med. 2020, 35, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Renaud, B.; François, A.-C.; Boemer, F.; Kruse, C.; Stern, D.; Piot, A.; Petitjean, T.; Gustin, P.; Votion, D.-M. Grazing mares on pasture with sycamore maples: A potential threat to suckling foals and food safety through milk contamination. Animals 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Votion, D.M.; François, A.C.; Kruse, C.; Benaud, B.; Farinelle, A.; Bouquieaux, M.C.; Marcillaud-Pitel, C.; Gustin, P. Answers to the frequently asked questions regarding horse feeding and management practices to reduce the risk of atypical myopathy. Animals 2020, 10, 365. [Google Scholar] [CrossRef]
- González-Medina, S.; Montesso, F.; Chang, Y.M.; Hyde, C.; Piercy, R.J. Atypical myopathy-associated hypoglycin A toxin remains in sycamore seedlings despite mowing, herbicidal spraying or storage in hay and silage. Equine Vet. J. 2019, 51, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Hussain, H.; Neubert, R.H.H.; Abel, S. Sensitive and Selective Amino Acid Profiling of Minute Tissue Amounts by HPLC/Electrospray Negative Tandem Mass Spectrometry Using 9-Fluorenylmethoxycarbonyl (Fmoc-Cl) Derivatization. Methods Mol. Biol. 2019, 2030, 365–379. [Google Scholar] [PubMed]
| Milk Sample 1 | Fraction | HGA (µg/L) | HGA (nmol/L) |
|---|---|---|---|
| 1A | raw milk | 69 | 489 |
| 1B | raw milk | 17 | 120 |
| 2A | raw milk | <LOD | <LOD |
| 2B | raw milk | <LOD | <LOD |
| 3A | raw milk | <LOD | <LOD |
| 3B | raw milk | <LOD | <LOD |
| 4A | raw milk | <LOD | <LOD |
| 4B | raw milk | <LOD | <LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochnia, M.; Ziegler, J.; Glatter, M.; Zeyner, A. Hypoglycin A in Cow’s Milk—A Pilot Study. Toxins 2021, 13, 381. https://doi.org/10.3390/toxins13060381
Bochnia M, Ziegler J, Glatter M, Zeyner A. Hypoglycin A in Cow’s Milk—A Pilot Study. Toxins. 2021; 13(6):381. https://doi.org/10.3390/toxins13060381
Chicago/Turabian StyleBochnia, Mandy, Jörg Ziegler, Maren Glatter, and Annette Zeyner. 2021. "Hypoglycin A in Cow’s Milk—A Pilot Study" Toxins 13, no. 6: 381. https://doi.org/10.3390/toxins13060381
APA StyleBochnia, M., Ziegler, J., Glatter, M., & Zeyner, A. (2021). Hypoglycin A in Cow’s Milk—A Pilot Study. Toxins, 13(6), 381. https://doi.org/10.3390/toxins13060381





