Mimosa tenuiflora Aqueous Extract: Role of Condensed Tannins in Anti-Aflatoxin B1 Activity in Aspergillus flavus
Abstract
1. Introduction
2. Results and Discussion
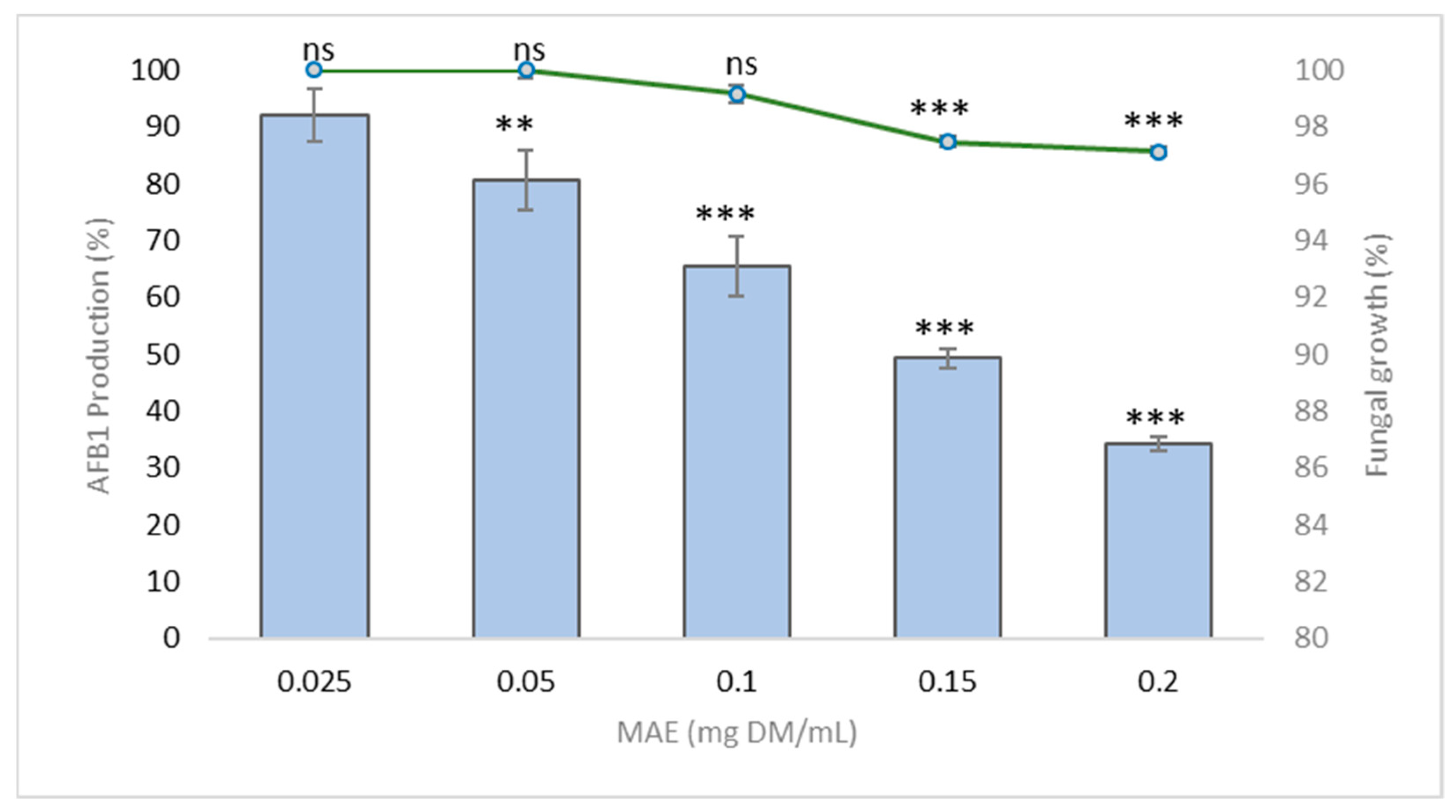
2.1. Effect of M. tenuiflora Stem Bark Aqueous Extract on Fungal Growth and Aflatoxin B1 Production
2.1.1. Impact on Fungal Growth
2.1.2. Reduced Production of Aflatoxin B1
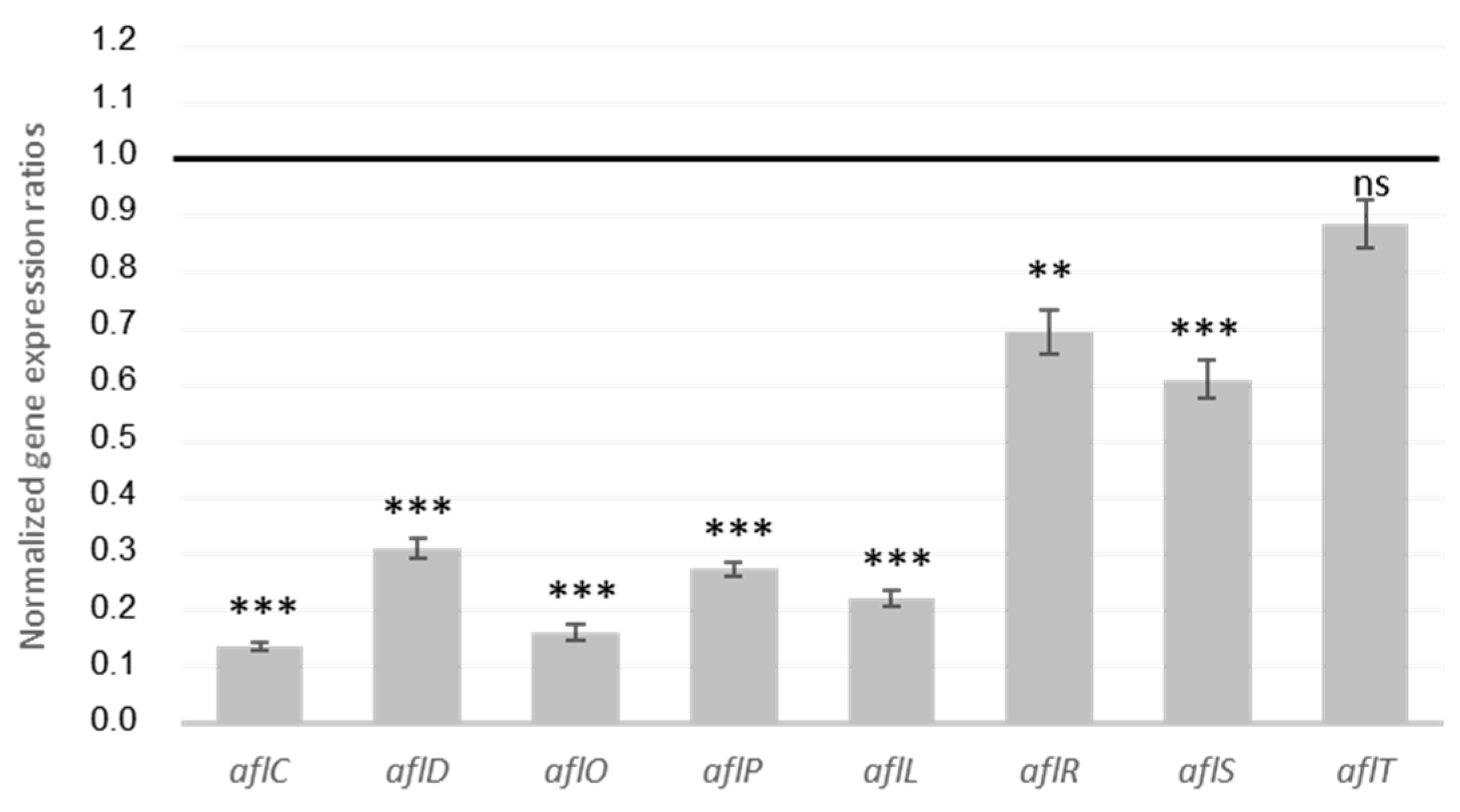
2.1.3. MAE Inhibition of Genes from AFB1 Cluster
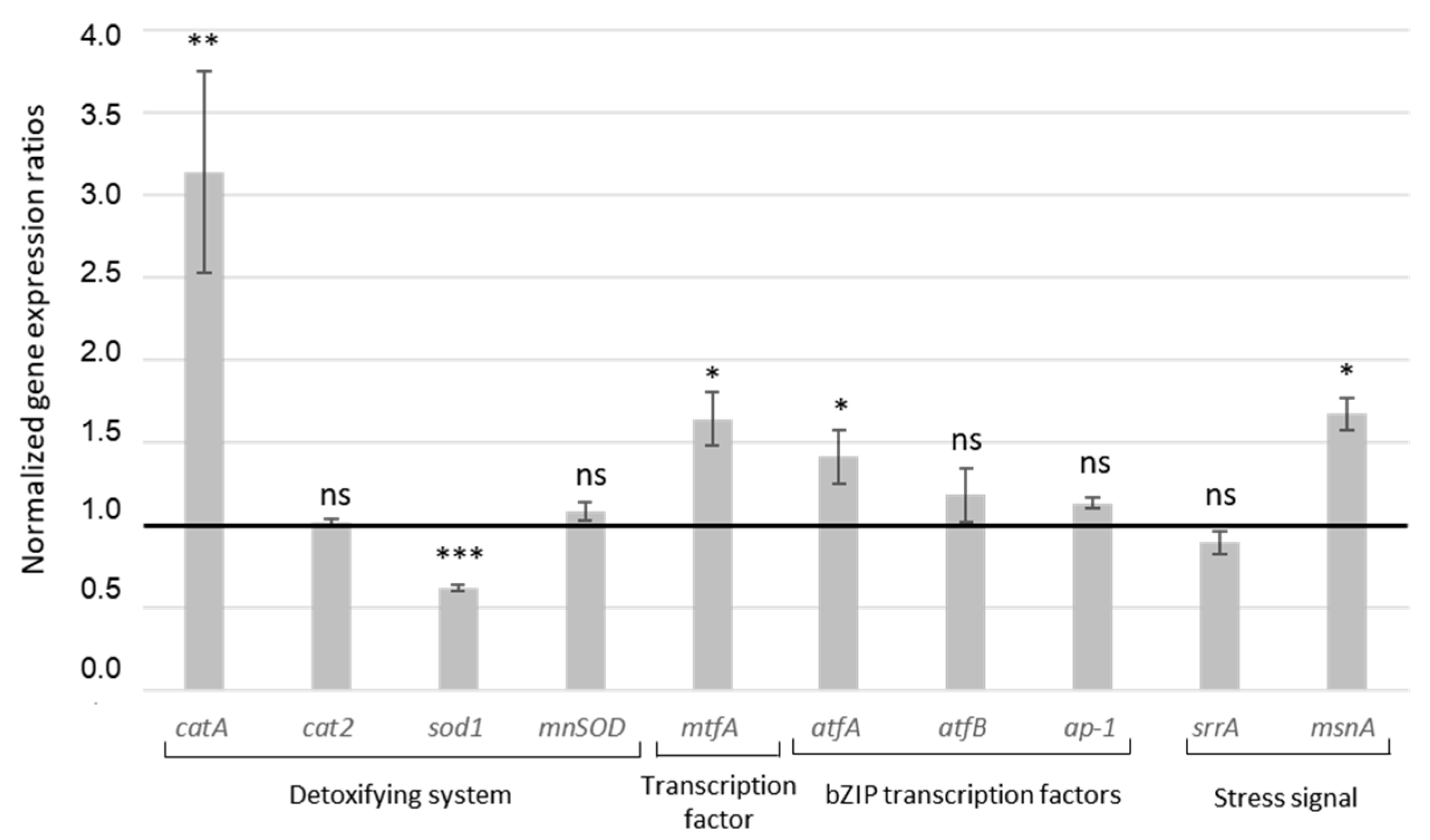
2.1.4. Effect of MAE on Expression of Genes Involved in Oxidative Stress Response
2.2. Fractionation and Characterization of Fractions
2.2.1. Composition
2.2.2. Characterization by High-Performance Liquid Chromatography
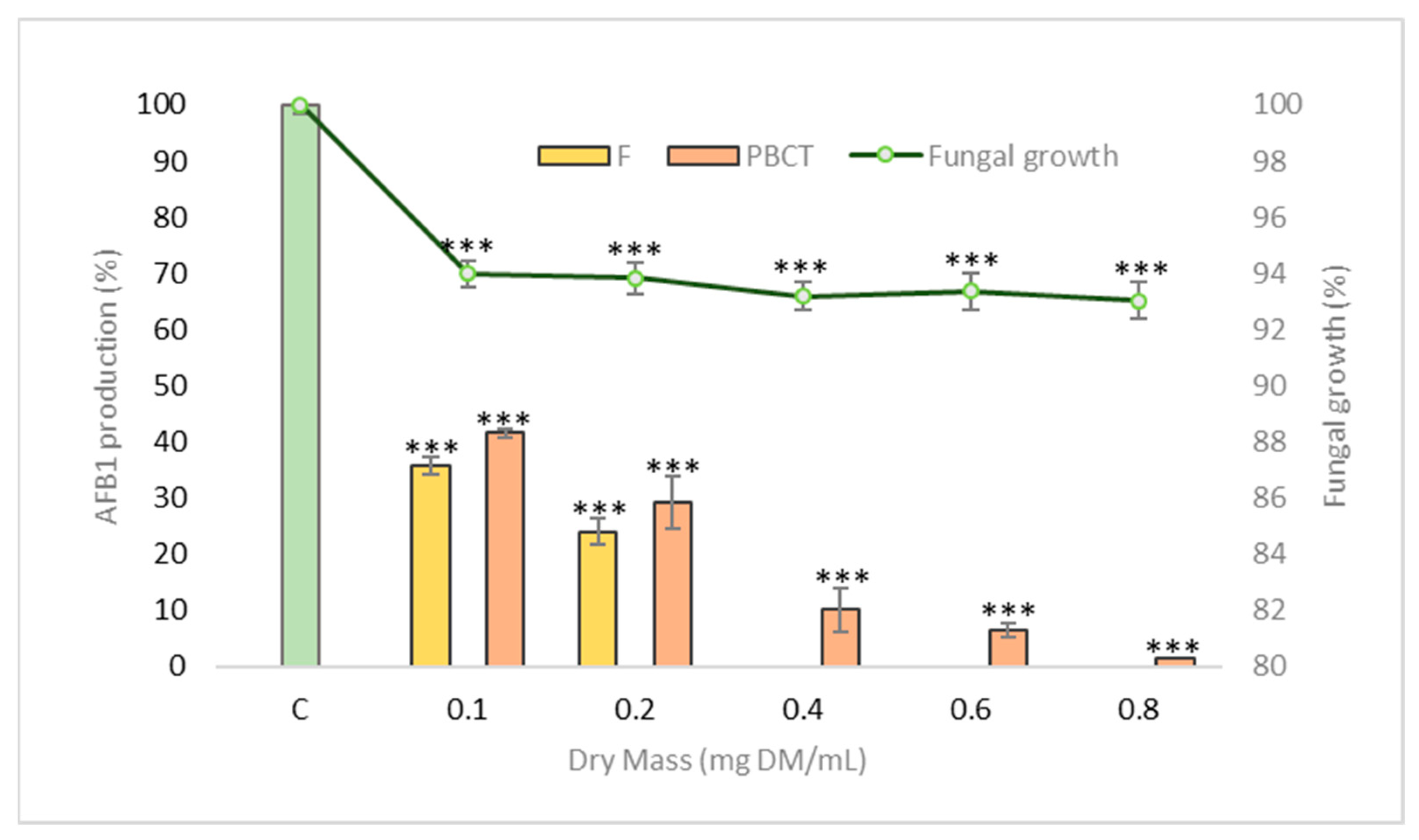
2.3. Effect of Fractions on Fungal Growth and Aflatoxin B1 Production
2.3.1. Effect of Fractions on Fungal Growth
2.3.2. Decreased Production of Aflatoxin B1
2.4. Effect of Condensed Tannins on Fungal Growth and Aflatoxin B1 Production
3. Conclusions
4. Materials and Methods
4.1. Materials
4.1.1. Solvents and Standards
4.1.2. Plant Materials
4.2. Plant Characterization
4.3. Preparation of MAE, F, and Sf
4.3.1. Preparation of M. tenuiflora Aqueous Extract
4.3.2. Fractionation of MAE
MAR Fractionation
PVPP Fractionation
4.4. Characterization of MAE and Fractions F and Sf
4.4.1. Dry Matter Content
4.4.2. Total Phenolic Content
4.4.3. Condensed Tannin Content
4.4.4. Radical-Scavenging Activity
4.5. Effect of M. tenuiflora Extract and Fractions on Aspergillus flavus Growth and Aflatoxin B1 Synthesis
4.5.1. Fungal Strain and Culture Conditions
4.5.2. Extraction and Quantification of Aflatoxin B1 by HPLC
4.6. Effect of M. tenuiflora Extract on Gene Expression
4.6.1. Fungal RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction
4.6.2. q-PCR Conditions and Analysis of Gene Expression
4.7. Characterization of Compounds Present in M. tenuiflora Extract and Fractions
4.8. Statistics
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marasas, W.F.O.; Gelderblom, W.; Shephard, G.; Vismer, H. Mycotoxins: A Global Problem. In Mycotoxins Detection Methods, Management, Public Health and Agricultural Trade; CAB Internationa: London, UK, 2008; pp. 29–39. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus Species and Mycotoxins: Occurrence and Importance in Major Food Commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus . Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative Technologies to Manage Aflatoxins in Foods and Feeds and the Profitability of Application—A Review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef] [PubMed]
- IARC. Fungi Producing Significant Mycotoxins; IARC: Lyon, France, 2012; pp. 1–30. [Google Scholar]
- Caceres, I.; Khoury, A.A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; Khoury, A.E.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Kemboi, D.C.; Ochieng, P.E.; Antonissen, G.; Croubels, S.; Scippo, M.-L.; Okoth, S.; Kangethe, E.K.; Faas, J.; Doupovec, B.; Lindahl, J.F.; et al. Multi-Mycotoxin Occurrence in Dairy Cattle and Poultry Feeds and Feed Ingredients from Machakos Town, Kenya. Toxins 2020, 12, 762. [Google Scholar] [CrossRef]
- Meissonnier, G.M.; Pinton, P.; Laffitte, J.; Cossalter, A.-M.; Gong, Y.Y.; Wild, C.P.; Bertin, G.; Galtier, P.; Oswald, I.P. Immunotoxicity of Aflatoxin B1: Impairment of the Cell-Mediated Response to Vaccine Antigen and Modulation of Cytokine Expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; de Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in Foodstuffs: Occurrence and Recent Advances in Decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Groopman, J.D.; Kensler, T.W.; Wild, C.P. Protective Interventions to Prevent Aflatoxin-Induced Carcinogenesis in Developing Countries. Annu. Rev. Public Health 2008, 29, 187–203. [Google Scholar] [CrossRef]
- Sarma, U.P.; Bhetaria, P.J.; Devi, P.; Varma, A. Aflatoxins: Implications on Health. Indian J. Clin. Biochem. 2017, 32, 124–133. [Google Scholar] [CrossRef]
- Bailly, S.; Mahgubi, A.; Carvajal-Campos, A.; Lorber, S.; Puel, O.; Oswald, I.; Bailly, J.-D.; Orlando, B. Occurrence and Identification of Aspergillus Section Flavi in the Context of the Emergence of Aflatoxins in French Maize. Toxins 2018, 10, 525. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A Review of Novel Physical and Chemical Decontamination Technologies for Aflatoxin in Food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A Three-Year Survey on the Worldwide Occurrence of Mycotoxins in Feedstuffs and Feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating Techniques on Controlling Mycotoxins—A Review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Moral, J.; Garcia-Lopez, M.T.; Camiletti, B.X.; Jaime, R.; Michailides, T.J.; Bandyopadhyay, R.; Ortega-Beltran, A. Present Status and Perspective on the Future Use of Aflatoxin Biocontrol Products. Agronomy 2020, 10, 491. [Google Scholar] [CrossRef]
- Onaran, A.; Yanar, Y. In Vivo and In Vitro Antifungal Activities of Five Plant Extracts Against Various Plant Pathogens. Egypt. J. Biol. Pest Control 2016, 26, 405–411. [Google Scholar]
- El Khoury, R.; Caceres, I.; Puel, O.; Bailly, S.; Atoui, A.; Oswald, I.P.; El Khoury, A.; Bailly, J.-D. Identification of the Anti-Aflatoxinogenic Activity of Micromeria Graeca and Elucidation of Its Molecular Mechanism in Aspergillus flavus. Toxins 2017, 9, 87. [Google Scholar] [CrossRef]
- Thippeswamy, S.; Mohana, D.; Umesh, A.; Kiragandur, M. Inhibitory Activity of Plant Extracts on Aflatoxin B1 Biosynthesis by Aspergillus Flavus. J. Agric. Sci. Technol. 2014, 16, 1123–1132. [Google Scholar]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef]
- Mithöfer, A.; Maffei, M. General Mechanisms of Plant Defense and Plant Toxins. In Plant Toxins; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-94-007-6463-7. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Camargo-Ricalde, S.L. Descripción, distribución, anatomía, composición química y usos de Mimosa tenuiflora (Fabaceae-Mimosoideae) en México. Rev. Biol. Trop. 2000, 48, 939–954. [Google Scholar]
- de Souza, R.S.O.; de Albuquerque, U.P.; Monteiro, J.M.; de Amorim, E.L.C. Jurema-Preta (Mimosa Tenuiflora [Willd.] Poir.): A Review of Its Traditional Use, Phytochemistry and Pharmacology. Braz. Arch. Biol. Technol. 2008, 51, 937–947. [Google Scholar] [CrossRef]
- Lozoya, X.; Navarro, V.; Arnason, J.T.; Kourany, E. Experimental Evaluation of Mimosa Tenuiflora (Willd.) Poir. (Tepescohuite) I. Screening of the Antimicrobial Properties of Bark Extracts. Arch. Investig. Med. 1989, 20, 87–93. [Google Scholar]
- Rivera-Arce, E.; Chávez-Soto, M.A.; Herrera-Arellano, A.; Arzate, S.; Agüero, J.; Feria-Romero, I.A.; Cruz-Guzmán, A.; Lozoya, X. Therapeutic Effectiveness of a Mimosa Tenuiflora Cortex Extract in Venous Leg Ulceration Treatment. J. Ethnopharmacol. 2007, 109, 523–528. [Google Scholar] [CrossRef]
- Shrivastava, R. Clinical Evidence to Demonstrate That Simultaneous Growth of Epithelial and Fibroblast Cells Is Essential for Deep Wound Healing. Diabetes Res. Clin. Pract. 2011, 92, 92–99. [Google Scholar] [CrossRef]
- Padilha, I.Q.M.; Pereira, A.V.; Rodrigues, O.G.; Siqueira-Júnior, J.P.; do Socorro, V. Pereira, M. Antimicrobial Activity of Mimosa Tenuiflora (Willd.) Poir. from Northeast Brazil against Clinical Isolates of Staphylococcus Aureus. Rev. Bras. Farmacogn. 2010, 20, 45–47. [Google Scholar] [CrossRef]
- Martel-Estrada, S.A.; Olivas-Armendáriz, I.; Santos-Rodríguez, E.; Martínez-Pérez, C.A.; García-Casillas, P.E.; Hernández-Paz, J.; Rodríguez-González, C.A.; Chapa-González, C. Evaluation of in Vitro Bioactivity of Chitosan/Mimosa Tenuiflora Composites. Mater. Lett. 2014, 119, 146–149. [Google Scholar] [CrossRef]
- Cruz, M.P.; Andrade, C.M.F.; Silva, K.O.; de Souza, E.P.; Yatsuda, R.; Marques, L.M.; David, J.P.; David, J.M.; Napimoga, M.H.; Clemente-Napimoga, J.T. Antinoceptive and Anti-Inflammatory Activities of the Ethanolic Extract, Fractions and Flavones Isolated from Mimosa Tenuiflora (Willd.) Poir (Leguminosae). PLoS ONE 2016, 11, e0150839. [Google Scholar] [CrossRef]
- Vepsäläinen, J.; Auriola, S.; Tukiainen, M.; Ropponen, N.; Callaway, J. Isolation and Characterization of Yuremamine, a New Phytoindole. Planta Med. 2005, 71, 1053–1057. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Paiva-Souza, I.O.; Fernandes, X.A.; da C. de Moraes, S.Z.; Araujo, S.S.; Shan, A.Y.K.V.; Camargo, E.A.; Santana, A.E.G.; Araujo, B.S.; Estevam, C.S. Anti-Inflammatory and Antioxidant Activities of the Hydroethanol Extract and Fractions of the Bark of Mimosa Tenuiflora (Willd.) Poir. Afr. J. Pharm. Pharmacol. 2016, 10, 823–831. [Google Scholar] [CrossRef]
- Caceres, I.; El Khoury, R.; Bailly, S.; Oswald, I.P.; Puel, O.; Bailly, J.-D. Piperine Inhibits Aflatoxin B1 Production in Aspergillus Flavus by Modulating Fungal Oxidative Stress Response. Fungal Genet. Biol. 2017, 107, 77–85. [Google Scholar] [CrossRef]
- Ben Miri, Y.; Djenane, D. Evaluation of Protective Impact of Algerian Cuminum Cyminum L. and Coriandrum Sativum L. Essential Oils on Aspergillus Flavus Growth and Aflatoxin B1 Production. Pak. J. Biol. Sci. 2018, 21, 67–77. [Google Scholar] [CrossRef]
- Chang, P.-K.; Yu, J.; Yu, J.-H. AflT, a MFS Transporter-Encoding Gene Located in the Aflatoxin Gene Cluster, Does Not Have a Significant Role in Aflatoxin Secretion. Fungal Genet. Biol. 2004, 41, 911–920. [Google Scholar] [CrossRef]
- Kong, Q.; Chi, C.; Yu, J.; Shan, S.; Li, Q.; Li, Q.; Guan, B.; Nierman, W.C.; Bennett, J.W. The Inhibitory Effect of Bacillus Megaterium on Aflatoxin and Cyclopiazonic Acid Biosynthetic Pathway Gene Expression in Aspergillus Flavus. Appl. Microbiol. Biotechnol. 2014, 98, 5161–5172. [Google Scholar] [CrossRef]
- Mo, H.Z.; Zhang, H.; Wu, Q.H.; Hu, L.B. Inhibitory Effects of Tea Extract on Aflatoxin Production by Aspergillus Flavus. Lett. Appl. Microbiol. 2013, 56, 462–466. [Google Scholar] [CrossRef]
- Grintzalis, K.; Vernardis, S.I.; Klapa, M.I.; Georgiou, C.D. Role of Oxidative Stress in Sclerotial Differentiation and Aflatoxin B1 Biosynthesis in Aspergillus Flavus. Appl. Environ. Microbiol. 2014, 80, 5561–5571. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Roze, L.V.; Wee, J.; Linz, J.E. Evidence That a Transcription Factor Regulatory Network Coordinates Oxidative Stress Response and Secondary Metabolism in Aspergilli. Microbiologyopen 2013, 2, 144–160. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, Y.; Liu, X.; Shang, B.; Xing, F.; Zhou, L.; Wang, Y.; Zhang, C.; Bhatnagar, D.; Liu, Y. The BZIP Transcription Factor Afap1 Mediates the Oxidative Stress Response and Aflatoxin Biosynthesis in Aspergillus Flavus. Rev. Argent. Microbiol. 2019, 51, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Lohmar, J.M.; Satterlee, T.; Cary, J.W.; Calvo, A.M. The Master Transcription Factor MtfA Governs Aflatoxin Production, Morphological Development and Pathogenicity in the Fungus Aspergillus Flavus. Toxins 2016, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Balázs, A.; Pócsi, I.; Hamari, Z.; Leiter, E.; Emri, T.; Miskei, M.; Oláh, J.; Tóth, V.; Hegedus, N.; Prade, R.A.; et al. AtfA BZIP-Type Transcription Factor Regulates Oxidative and Osmotic Stress Responses in Aspergillus Nidulans. Mol. Genet. Genom. MGG 2010, 283, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Takahashi, H.; Kusuya, Y.; Kawamoto, S.; Kamei, K.; Gonoi, T. Comparative Transcriptome Analysis Revealing Dormant Conidia and Germination Associated Genes in Aspergillus Species: An Essential Role for AtfA in Conidial Dormancy. BMC Genom. 2016, 17, 358. [Google Scholar] [CrossRef]
- Chang, P.-K.; Scharfenstein, L.L.; Luo, M.; Mahoney, N.; Molyneux, R.J.; Yu, J.; Brown, R.L.; Campbell, B.C. Loss of MsnA, a Putative Stress Regulatory Gene, in Aspergillus Parasiticus and Aspergillus Flavus Increased Production of Conidia, Aflatoxins and Kojic Acid. Toxins 2011, 3, 82–104. [Google Scholar] [CrossRef]
- Caceres, I.; El Khoury, R.; Medina, Á.; Lippi, Y.; Naylies, C.; Atoui, A.; Khoury, A.E.; Oswald, I.P.; Bailly, J.-D.; Puel, O. Deciphering the Anti-Aflatoxinogenic Properties of Eugenol Using a Large-Scale q-PCR Approach. Toxins 2016, 8, 123. [Google Scholar] [CrossRef]
- Meira, C.L.C.; Novaes, C.G.; Novais, F.C.; De Jesus, V.D.; De Oliveira, D.M.; Aguiar, R.M. Application of Principal Component Analysis for the Evaluation of the Chemical Constituents of Mimosa Tenuiflora Methanolic Extract by DLLME/GC–MS. Microchem. J. 2020, 152, 104284. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mahey, S.; Arora, R.; Mahajan, J.; Kumar, V.; Arora, S. Insights into Biological Properties of Less Explored Bark of Industrially Important Acacia Catechu Willd. Ind. Crops Prod. 2019, 138, 111486. [Google Scholar] [CrossRef]
- Ullah, C.; Unsicker, S.B.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Accumulation of Catechin and Proanthocyanidins in Black Poplar Stems After Infection by Plectosphaerella Populi: Hormonal Regulation, Biosynthesis and Antifungal Activity. Front. Plant Sci. 2019, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Oliff, H. Scientific and Clinical Monograph for Pycnogenol. Am. Bot. Counc. 2010, 1, 2–23. [Google Scholar]
- Muñoz-Acevedo, A.; Méndez, L.Y.V.; Stashenko, E.E.; Kouznetsov, V.V. Improved Trolox® Equivalent Antioxidant Capacity Assay for Efficient and Fast Search of New Antioxidant Agents. Anal. Chem. Lett. 2011, 1, 86–102. [Google Scholar] [CrossRef]
- De la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy Properties of Proanthocyanidins. BioFactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Jerez, M.; Tourino, S.; Sineiro, J.; Torres, J.; Núñez, M. Procyanidins from Pine Bark: Relationships between Structure, Composition and Antiradical Activity. Food Chem. 2007, 104, 518–527. [Google Scholar] [CrossRef]
- Azaizeh, H.A.; Pettit, R.E.; Sarr, B.A.; Phillips, T.D. Effect of Peanut Tannin Extracts on Growth of Aspergillus Parasiticus and Aflatoxin Production. Mycopathologia 1990, 110, 125–132. [Google Scholar] [CrossRef]
- Hasan, H.A. Aflatoxin in Detannin Coffee and Tea and Its Destruction. J. Nat. Toxins 2002, 11, 133–138. [Google Scholar]
- Rajput, S.A.; Zhang, C.; Feng, Y.; Wei, X.T.; Khalil, M.M.; Rajput, I.R.; Baloch, D.M.; Shaukat, A.; Rajput, N.; Qamar, H.; et al. Proanthocyanidins Alleviates AflatoxinB1;-Induced Oxidative Stress and Apoptosis through Mitochondrial Pathway in the Bursa of Fabricius of Broilers. Toxins 2019, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Narasaiah, K.V.; Sashidhar, R.B.; Subramanyam, C. Biochemical Analysis of Oxidative Stress in the Production of Aflatoxin and Its Precursor Intermediates. Mycopathologia 2006, 162, 179–189. [Google Scholar] [CrossRef]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Soest, P.J.V.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Buran, T.J.; Sandhu, A.K.; Li, Z.; Rock, C.R.; Yang, W.W.; Gu, L. Adsorption/Desorption Characteristics and Separation of Anthocyanins and Polyphenols from Blueberries using Macroporous Adsorbent Resins. J. Food Eng. 2014, 128, 167–173. [Google Scholar] [CrossRef]
- Peng, Z.; Hayasaka, Y.; Iland, P.G.; Sefton, M.; Høj, P.; Waters, E.J. Quantitative Analysis of Polymeric Procyanidins (Tannins) from Grape (Vitis Vinifera) Seeds by Reverse Phase High-Performance Liquid Chromatography. J. Agric. Food Chem. 2001, 49, 26–31. [Google Scholar] [CrossRef]
- Mbacke DIENG, S.I.; Mathieu, C.; Sarr, A.; Diatta-Badji, K.; Fall, A.D. Condensed Tannins Content and Their Influence on the Antioxidant Activity of Bark Hydroethanol Extract of Piliostigma Reticulatum (Dc) Hochst and Its Fractions. Pharmacogn. J. 2020, 12, 361–368. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific: Oxford, UK; Boston, MA, USA, 1994; ISBN 978-0-632-02969-3. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- El Mahgubi, A.; Puel, O.; Bailly, S.; Tadrist, S.; Querin, A.; Ouadia, A.; Oswald, I.P.; Bailly, J.D. Distribution and Toxigenicity of Aspergillus Section Flavi in Spices Marketed in Morocco. Food Control 2013, 32, 143–148. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]


| Purification Step | Mass Proportion of Total Dry Plant (%) | Fraction Step Yield (%DM) | Polyphenols (mg GAE/g DM Extract) 1 | Condensed Tannins (mg/g DM) | Anti-Oxidant Activity on DPPH IC50 (mg/L) 2 |
|---|---|---|---|---|---|
| Aqueous Extract (MAE) | 11.15 | 11 | 397 ± 22 | 171.6 ± 2.8 | 10 |
| Fraction (F) | 1.82 | 74 | 551 ± 11 | 332.3 ± 0.6 | 9 |
| Subfraction (Sf) | 0.10 | 28 | 96 ± 2 | 2.8 ± 0.2 | >400 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, C.; Cadenillas, L.; Maghubi, A.E.; Caceres, I.; Durrieu, V.; Mathieu, C.; Bailly, J.-D. Mimosa tenuiflora Aqueous Extract: Role of Condensed Tannins in Anti-Aflatoxin B1 Activity in Aspergillus flavus. Toxins 2021, 13, 391. https://doi.org/10.3390/toxins13060391
Hernandez C, Cadenillas L, Maghubi AE, Caceres I, Durrieu V, Mathieu C, Bailly J-D. Mimosa tenuiflora Aqueous Extract: Role of Condensed Tannins in Anti-Aflatoxin B1 Activity in Aspergillus flavus. Toxins. 2021; 13(6):391. https://doi.org/10.3390/toxins13060391
Chicago/Turabian StyleHernandez, Christopher, Laura Cadenillas, Anwar El Maghubi, Isaura Caceres, Vanessa Durrieu, Céline Mathieu, and Jean-Denis Bailly. 2021. "Mimosa tenuiflora Aqueous Extract: Role of Condensed Tannins in Anti-Aflatoxin B1 Activity in Aspergillus flavus" Toxins 13, no. 6: 391. https://doi.org/10.3390/toxins13060391
APA StyleHernandez, C., Cadenillas, L., Maghubi, A. E., Caceres, I., Durrieu, V., Mathieu, C., & Bailly, J.-D. (2021). Mimosa tenuiflora Aqueous Extract: Role of Condensed Tannins in Anti-Aflatoxin B1 Activity in Aspergillus flavus. Toxins, 13(6), 391. https://doi.org/10.3390/toxins13060391







