Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review
Abstract
:1. Introduction
2. Binding Competition for PBUT Removal
3. Evidence from Bench Studies
4. Evidence from Pre-Clinical Studies
5. Clinical Evidence
6. In Silico Evidence
7. Treatment of Drug Intoxications
8. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMPF | 3-carboxy-4-methyl-5-propyl-2-furanpropionate |
| DHA | Docosahexaenoic acid |
| HA | Hippuric acid |
| HD | Hemodialysis |
| HSA | Human serum albumin |
| IAA | Indole-3-acetic acid |
| IS | Indoxyl sulfate |
| pCS | p-cresyl sulfate |
| TRP | Tryptophan |
References
- Vanholder, R.; Schepers, E.; Pletinck, A.; Neirynck, N.; Glorieux, G. An Update on Protein-Bound Uremic Retention Solutes. J. Ren. Nutr. 2012, 22, 90–94. [Google Scholar] [CrossRef]
- Rosner, M.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, CJN.02660221. [Google Scholar] [CrossRef]
- Niwa, T. Removal of Protein-Bound Uraemic Toxins by Haemodialysis. Blood Purif. 2013, 35, 20–25. [Google Scholar] [CrossRef]
- Krieter, D.H.; Hackl, A.; Rodriguez, A.; Chenine, L.; Moragues, H.L.; Lemke, H.-D.; Wanner, C.; Canaud, B. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol. Dial. Transplant. 2009, 25, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, M.K.; Middel, I.R.; Vernooij, R.W.M.; Bots, M.L.; Verhaar, M.C.; Masereeuw, R.; Grooteman, M.P.; Nubé, M.J.; Dorpel, M.A.V.D.; Blankestijn, P.J.; et al. Protein-Bound Uremic Toxins in Hemodialysis Patients Relate to Residual Kidney Function, Are Not Influenced by Convective Transport, and Do Not Relate to Outcome. Toxins 2020, 12, 234. [Google Scholar] [CrossRef] [Green Version]
- Sirich, T.L.; Meyer, T.W.; Gondouin, B.; Brunet, P.; Niwa, T. Protein-Bound Molecules: A Large Family with a Bad Character. Semin. Nephrol. 2014, 34, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Villain, C.; Massy, Z.A. Protein-bound toxins: Has the Cinderella of uraemic toxins turned into a princess? Clin. Sci. 2016, 130, 2209–2216. [Google Scholar] [CrossRef]
- Sirich, T.L.; Funk, B.A.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Prominent Accumulation in Hemodialysis Patients of Solutes Normally Cleared by Tubular Secretion. J. Am. Soc. Nephrol. 2013, 25, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Magnani, S.; Atti, M. Uremic Toxins and Blood Purification: A Review of Current Evidence and Future Perspectives. Toxins 2021, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Kotanko, P.K.; Martin, K.; Chen, C.; Levin, N.W. Hemodialysis: Principles and techniques. In Comprehensive Clinical Nephrology; Feehally, J.F., Tonelli, M., Johnson, R.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- De Smet, R.; Dhondt, A.; Eloot, S.; Galli, F.; Waterloos, M.A.; Vanholder, R. Effect of the super-flux cellulose triacetate dialyser membrane on the removal of non-protein-bound and protein-bound uraemic solutes. Nephrol. Dial. Transplant. 2007, 22, 2006–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotanko, P.; Levin, N.W. Method of Removing Protein-Bound Deleterious Substances during Extracorporeal Renal Replacement Treatment. In World Intelectual Prperty Organization WO2010/045474; World Intelectual Prperty Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Tao, X.; Thijssen, S.; Levin, N.; Kotanko, P.; Handelman, G. Enhanced Indoxyl Sulfate Dialyzer Clearance with the Use of Binding Competitors. Blood Purif. 2015, 39, 323–330. [Google Scholar] [CrossRef]
- Jenkins, H. Le Chatelier’s principle. In Chemical Thermodynamics at a Glance; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 160–163. [Google Scholar]
- Maheshwari, V.; Thijssen, S.; Tao, X.; Fuertinger, D.; Kappel, F.; Kotanko, P. A novel mathematical model of protein-bound uremic toxin kinetics during hemodialysis. Sci. Rep. 2017, 7, 10371. [Google Scholar] [CrossRef] [Green Version]
- Varshney, A.; Sen, P.; Ahmad, E.; Rehan, M.; Subbarao, N.; Khan, R.H. Ligand binding strategies of human serum albumin: How can the cargo be utilized? Chirality 2010, 22, 77–87. [Google Scholar] [CrossRef]
- Tao, X.; Thijssen, S.; Kotanko, P.; Ho, C.-H.; Henrie, M.; Stroup, E.; Handelman, G. Improved dialytic removal of protein-bound uraemic toxins with use of albumin binding competitors: An in vitro human whole blood study. Sci. Rep. 2016, 6, 23389. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Y.; Xu, X.; Cao, W.; Shen, Z.; Wang, N.; Leng, J.; Zou, N.; Shang, E.; Zhu, Z.; et al. Improved dialysis removal of protein-bound uremic toxins by salvianolic acids. Phytomedicine 2019, 57, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Y.; Tian, H.; Wang, Y.; Shen, Y.; Zhu, Q.; Ding, F. Improved dialytic removal of protein-bound uremic toxins by intravenous lipid emulsion in chronic kidney disease rats. Nephrol. Dial. Transplant. 2019, 34, 1842–1852. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, H.; Wang, Y.; Shen, Y.; Zhu, Q.; Ding, F. Improved Dialysis Removal of Protein-Bound Uraemic Toxins with a Combined Displacement and Adsorption Technique. Blood Purif. 2021, 1–11. [Google Scholar] [CrossRef]
- Madero, M.; Cano, K.B.; Campos, I.; Tao, X.; Maheshwari, V.; Brown, J.; Cornejo, B.; Handelman, G.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins during Hemodialysis Using a Binding Competitor. Clin. J. Am. Soc. Nephrol. 2019, 14, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, V.; Thijssen, S.; Tao, X.; Fuertinger, D.H.; Kappel, F.; Kotanko, P. In silico comparison of protein-bound uremic toxin removal by hemodialysis, hemodiafiltration, membrane adsorption, and binding competition. Sci. Rep. 2019, 9, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, V.; Hoffman, R.; Thijssen, S.; Tao, X.; Fuertinger, D.H.; Kotanko, P. A model-based analysis of phenytoin and carbamazepine toxicity treatment using binding-competition during hemodialysis. Sci. Rep. 2020, 10, 11294. [Google Scholar] [CrossRef] [PubMed]
- Anseeuw, K.; Mowry, J.B.; Burdmann, E.; Ghannoum, M.; Hoffman, R.; Gosselin, S.; Lavergne, V.; Nolin, T.D. Extracorporeal Treatment in Phenytoin Poisoning: Systematic Review and Recommendations from the EXTRIP (Extracorporeal Treatments in Poisoning) Workgroup. Am. J. Kidney Dis. 2016, 67, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghannoum, M.; Yates, C.; Galvao, T.F.; Sowinski, K.M.; Vo, T.H.V.; Coogan, A.; Gosselin, S.; Lavergne, V.; Nolin, T.D.; Hoffman, R.S.; et al. Extracorporeal treatment for carbamazepine poisoning: Systematic review and recommendations from the EXTRIP workgroup. Clin. Toxicol. 2014, 52, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.; Fedecostante, M.; Wilmer, M.J.; Peters, J.G.; Kreuser, U.M.; Broek, P.H.V.D.; Mensink, R.; Boltje, T.J.; Stamatialis, D.; Wetzels, J.F.; et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep. 2016, 6, 26715. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Magagnoli, L.; Ciceri, P.; Conte, F.; Galassi, A. Effects of a medium cut-off (Theranova®) dialyser on haemodialysis patients: A prospective, cross-over study. Clin. Kidney J. 2021, 14, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Takkavatakarn, K.; Wuttiputinun, T.; Phannajit, J.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Protein-bound uremic toxin lowering strategies in chronic kidney disease: A systematic review and meta-analysis. J. Nephrol. 2021, 1–13. [Google Scholar] [CrossRef]
- Jankovic, S.M.; Aleksic, J.; Rakovic, S.; Aleksic, A.; Stevanovic, I.; Stefanovic-Stoimenov, N.; Radosavljevic, M.; Kostic, M.; Tesic, D.; Petrovic, B. Nonsteroidal antiinflammatory drugs and risk of gastrointestinal bleeding among patients on hemodialysis. J. Nephrol. 2009, 22, 502. [Google Scholar]

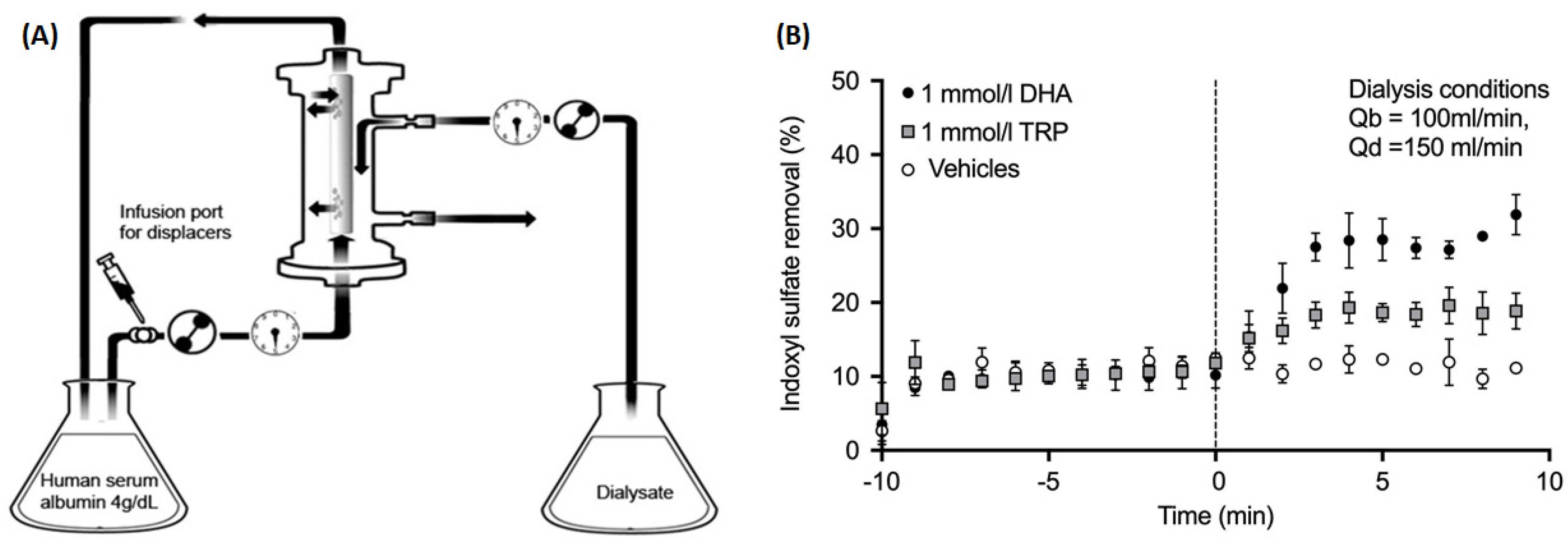


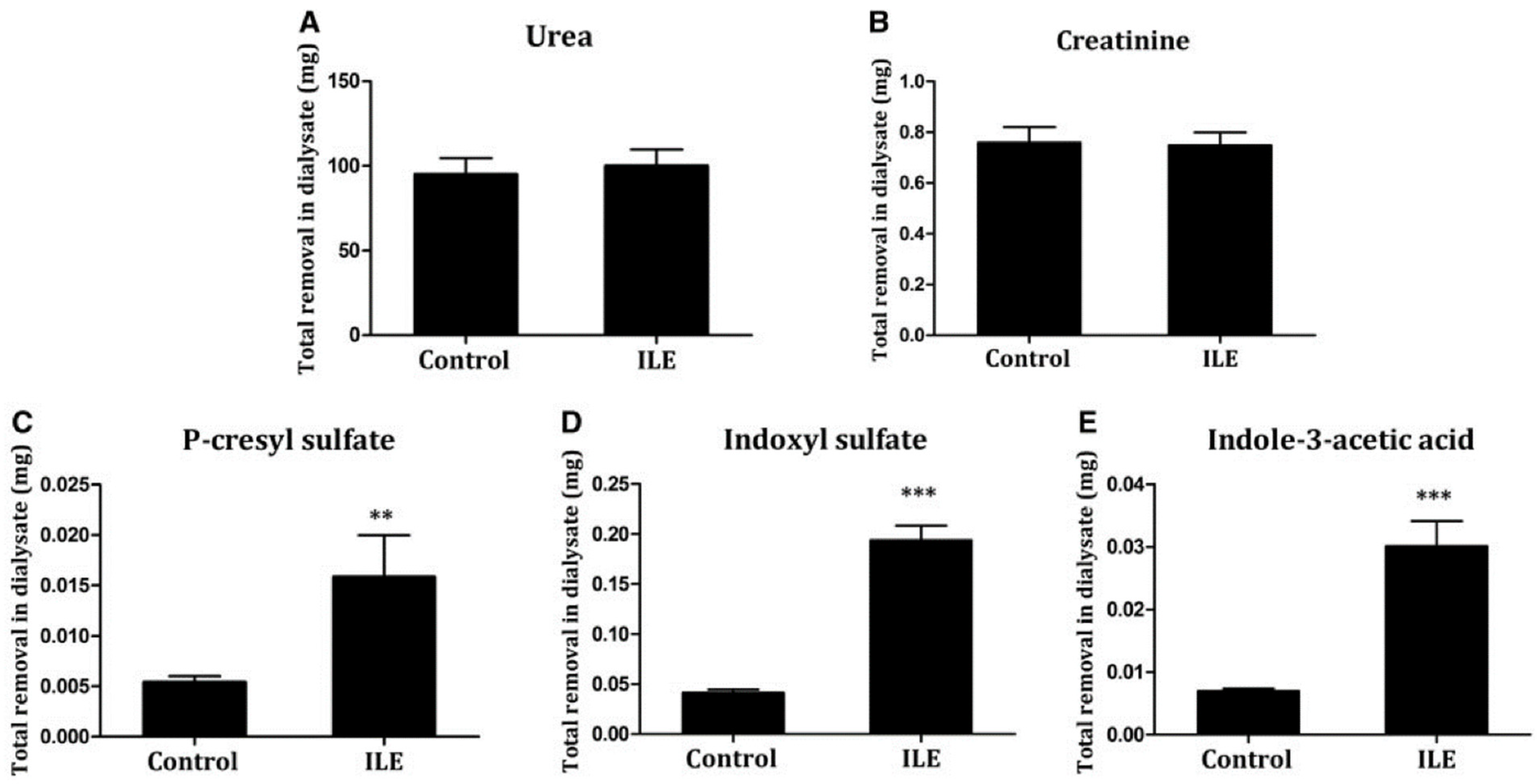



| Study Reference | Study Setting | PBUT(s) Studied | Binding Competitor(s) Used | Study Metric | Study Outcome |
|---|---|---|---|---|---|
| Tao et al. 2015 [13] | In vitro | IS | TRP or docosahexaenoic acid (DHA) infused in extracorporeal circuit at constant rate | Fractional removal in the dialysate | TRP improved IS fractional removal from 10.2% at baseline to 18.5%; DHA improved the IS removal to 27.7% |
| Tao et al. 2016 [17] | Ex vivo | IS, IAA, HA | Ibuprofen + furosemide or TRP infused in extracorporeal circuit at constant rate | Fractional removal in the dialysate | Ibuprofen + furosemide improved IS removal from 6.4% to 18.3% and IAA removal from 16.8% to 34.5%; TRP improved IS and IAA removal to 10.5% and 27.1%, respectively. |
| Li et al. 2019 [18] | Pre-clinical uremic rat model | IS, pCS | Danhong injection or lithospermic acid infused intravenously at constant rate during latter 2 h of 4-h microdialysis. | Removal in first 2 h (without infusion) vs. latter 2 h (with infusion) | IS and pCS removal in dialysate improved by 119.5% and 127.6%, by lithospermic acid, respectively, which made up of 88% and 47%, respectively, of the total displacement effects of IS and pCS introduced by Danhong injection. |
| Maheshwari et al. 2019 [22] | In silico analysis of IS and pCS removal during HD | IS, pCS | TRP or ibuprofen infused into the extracorporeal circuit at constant rate during 4-h HD | Time-averaged concentration (TAC) after 1 month | TRP infusion in every HD session reduced the TAC by 28% for IS and 30% for pCS. |
| Shi et al. 2019 [19] | In vitro | CMPF, IAA, IS, pCS | Free fatty acids infused in extracorporeal circuit at constant rate | Fractional removal in the dialysate | CMPF fractional removal improved to 14.4% vs. no removal at baseline; pCS, IS, and IAA fractional removal from 8%, 11.7%, and 15.7% at baseline to 28%, 35%, and 40%, respectively. |
| Shi et al. 2019 [19] | Pre-clinical uremic rat model | pCS, IS, IAA | Intralipid™ (20%) infused intravenously 30 min before start of dialysis | Total solute removal in spent dialysate | Removal of pCS, IS, and IAA increased approximately 300%, compared to control. |
| Madero et al. 2019 [21] | First-in-man proof-of-concept study in 18 ESKD patients on maintenance hemodialysis | IS, pCS, HA, TRP | Ibuprofen infused at constant rate during 20–40 min of 4-h HD | Dialysate clearance comparison during pre-infusion phase (0–20 min) vs. infusion phase (21–40 min) | Clearance improved from 6.6 mL/min to 20 mL/min for IS, and 4.4 to 14.9 mL/min for pCS; TRP clearance increased moderately. Urea and creatinine clearance were unchanged. |
| Maheshwari et al. 2020 [23] | In silico analysis of drug intoxication treatment | Phenytoin, Carbamazepine | Infusion in extracorporeal circuit at constant rate. For phenytoin, aspirin was infused; for carbamazepine, ibuprofen was infused | Time required to bring patient back into therapeutic concentration range | For phenytoin, constant aspirin infusion reduced the HD time from 460 min to 330 min; for carbamazepine, constant ibuprofen infusion reduced the HD time from 265 min 220 min. |
| Shi et al. 2021 [20] | Pre-clinical uremic rat model | IS, pCS, IAA, HA | Intralipid™ infused intravenously 30 min before start of dialysis; albumin dialysis with bovine serum albumin; Combination of binding competition and albumin dialysis | Total solute removal in spent dialysate | In the Intralipid™ arm, approximately 10-fold increase in IS and pCS removal compared to control arm. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maheshwari, V.; Tao, X.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review. Toxins 2021, 13, 622. https://doi.org/10.3390/toxins13090622
Maheshwari V, Tao X, Thijssen S, Kotanko P. Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review. Toxins. 2021; 13(9):622. https://doi.org/10.3390/toxins13090622
Chicago/Turabian StyleMaheshwari, Vaibhav, Xia Tao, Stephan Thijssen, and Peter Kotanko. 2021. "Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review" Toxins 13, no. 9: 622. https://doi.org/10.3390/toxins13090622
APA StyleMaheshwari, V., Tao, X., Thijssen, S., & Kotanko, P. (2021). Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review. Toxins, 13(9), 622. https://doi.org/10.3390/toxins13090622





