A Four-Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotypes C and D
Abstract
:1. Introduction
2. Results
2.1. BoNT/C and BoNT/D Sequence Analysis and Modeling
2.2. Generation and Characterization of Human Monoclonal Antibodies
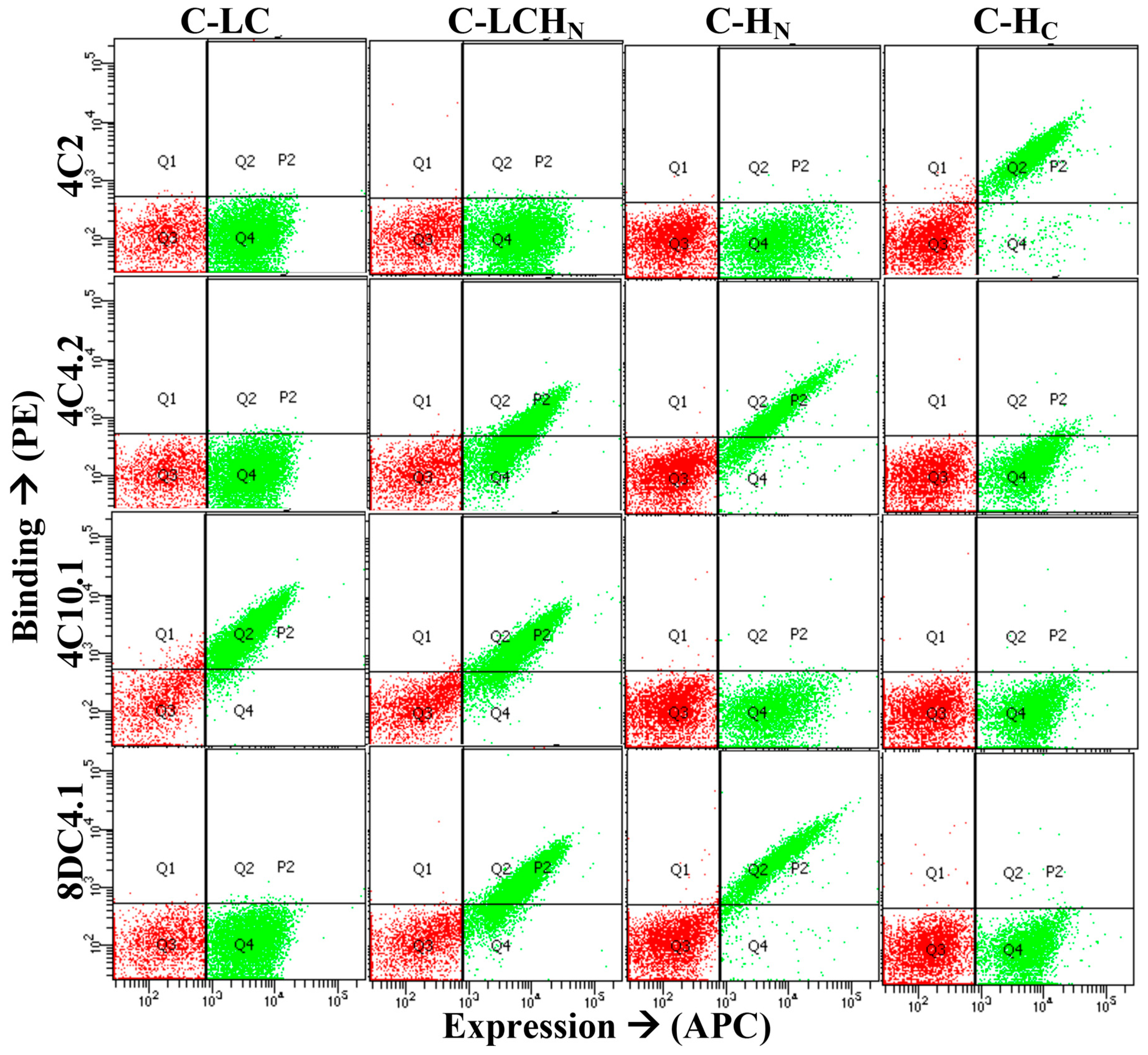
2.3. Epitope Mapping
2.4. Affinity Maturation
2.5. Mouse Neutralization Assays
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics
5.2. Strains, Media, Antibodies, and Toxin
5.3. Yeast-Displayed Human scFv Library Construction and Library Sorting
5.4. Measurement of KD Values of Yeast-Displayed scFv
5.5. Epitope Overlap Analysis
5.6. Affinity Maturation
5.7. Germline Fitting
5.8. Measurement of Solution Phase Affinity at Equilibrium
5.9. Measurement of In Vivo Toxin Neutralization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatheway, C.L. Botulism: The present status of the disease. Curr. Top. Microbiol. Immunol. 1995, 195, 55–75. [Google Scholar]
- Centers for Disease Control and Prevention. Botulism in the United States. In Handbook for Epidemiologists, Clinicians, and Laboratory Workers; Centers for Disease Control and Prevention: Atlanta, GA, USA, 1998; pp. 1899–1996. [Google Scholar]
- Lacy, D.B.; Stevens, R.C. Sequence homology and structural analysis of the clostridial neurotoxins. J. Mol. Biol. 1999, 291, 1091–1104. [Google Scholar] [CrossRef]
- Hill, K.K.; Smith, T.J. Genetic diversity within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. In Botulinum Neurotoxins; Rummel, A., Binz, T., Eds.; Springer: Hanover, Germany, 2013; pp. 1–20. [Google Scholar]
- Takeda, M.; Tsukamoto, K.; Kohda, T.; Matsui, M.; Mukamoto, M.; Kozaki, S. Characterization of the Neurotoxin Produced by Isolates Associated with Avian Botulism. Avian Dis. 2005, 49, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.; Skarin, H.; Knutsson, R.; Blomqvist, G.; Båverud, V. Real-Time PCR for Clostridium botulinum Type c Neurotoxin (BoNTC) Gene, Also Covering a Chimeric C/D Sequence—Application on Outbreaks of Botulism in Poultry. Vet. Microbiol. 2010, 146, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Souillard, R.; Woudstra, C.; Le Maréchal, C.; Dia, M.; Bayon-Auboyer, M.H.; Chemaly, M.; Fach, P.; Le Bouquin, S. Investigation of Clostridium Botulinum in Commercial Poultry Farms in France between 2011 and 2013. JWVPA 2014, 43, 458–464. [Google Scholar]
- Borland, E.; Moryson, C.; Smith, G. Avian Botulism and the High Prevalence of Clostridium Botulinum in the Norflok Broads. Vet. Rec. 1977, 100, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.W.; Hollier, T.J.; Bodinnar, K.D.; Bunn, C.M. An Outbreak of Botulism in Wild Waterbirds in Southern Australia. J. Wildl. Dis. 1985, 21, 347–350. [Google Scholar] [CrossRef]
- Jensen, W.I.; Price, J.I. The global importance of type C botulism in wild birds. In Avian Botulism: An International Perspective; Eklund, M.W., Ed.; Charles C. Thomas: Springfield, IL, USA, 1987; pp. 33–54. [Google Scholar]
- Bruchim, Y.; Steinman, A.; Markovitz, M.; Baneth, G.; Elad, D.; Shpigel, N.Y. Toxicological, Bacteriological and Serological Diagnosis of Botulism in a Dog. Vet. Rec. 2006, 158, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Farrow, B.R.H.; Murrell, W.G.; Revington, M.L.; Stewart, B.J.; Zuber, R.M. Type c Botulism in Young Dogs. Aust. Vet. J. 1983, 60, 374–377. [Google Scholar] [CrossRef]
- Barsanti, J.A.; Walser, M.; Hatheway, C.L.; Bowen, J.M.; Crowell, W. Type C Botulism in American Foxhounds. J. Am. Vet. Med. Assoc. 1978, 172, 809–813. [Google Scholar] [PubMed]
- Lindström, M.; Nevas, M.; Kurki, J.; Sauna-aho, R.; Latvala-Kiesila, A.; Pölönen, I.; Korkeala, H. Type C Botulism due to Toxic Feed Affecting 52,000 Farmed Foxes and Minks in Finland. J. Clin. Microbiol. 2004, 42, 4718–4725. [Google Scholar] [CrossRef] [Green Version]
- Schoenbaum, M.A.; Hall, S.M.; Glock, R.D.; Grant, K.; Jenny, A.L.; Schiefer, T.J.; Sciglibaglio, P.; Whitlock, R.H. An Outbreak of Type c Botulism in 12 Horses and a Mule. J. Am. Vet. Med. Assoc. 2000, 217, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Kinde, H.; Bettey, R.L.; Ardans, A.; Galey, F.D.; Daft, B.M.; Walker, R.L.; Eklund, M.W.; Byrd, J.W. Clostridium botulinum Type-C Intoxication Associated with Consumption of Processed Alfalfa Hay Cubes in Horses. J. Am. Vet. Med. Assoc. 1991, 199, 742–746. [Google Scholar] [PubMed]
- Guizelini, C.C.; Lemos, R.A.A.; de Paula, J.L.P.; Pupin, R.C.; Gomes, D.C.; Barros, C.S.L.; Neves, D.A.; Alcântara, L.O.B.; Silva, R.O.S.; Lobato, F.C.F.; et al. Type C Botulism Outbreak in Feedlot Cattle Fed Contaminated Corn Silage. Anaerobe 2019, 55, 103–106. [Google Scholar] [CrossRef]
- Shnaiderman-Torban, A.; Elad, D.; Kelmer, G.; Avni, G.; Shalit, U.; Steinman, A. An Outbreak of Equine Botulism Type D in Israel. Equine Vet. Educ. 2017, 30, 594–597. [Google Scholar] [CrossRef]
- Smith, G.R.; Milligan, R.A. Clostridium botulinum type D in Britain. Vet. Rec. 1977, 100, 121–122. [Google Scholar] [CrossRef]
- Lindström, M.; Myllykoski, J.; Sivelä, S.; Korkeala, H. Clostridium botulinumin Cattle and Dairy Products. Crit. Rev. Food Sci. Nutr. 2010, 50, 281–304. [Google Scholar] [CrossRef]
- Souillard, R.; Grosjean, D.; Le Gratiet, T.; Poezevara, T.; Rouxel, S.; Balaine, L.; Macé, S.; Martin, L.; Anniballi, F.; Chemaly, M.; et al. Asymptomatic Carriage of C. botulinum Type D/c in Broiler Flocks as the Source of Contamination of a Massive Botulism Outbreak on a Dairy Cattle Farm. Front. Microbiol. 2021, 12, 679377. [Google Scholar] [CrossRef]
- Böhnel, H.; Neufeld, B.; Gessler, F. Botulinum Neurotoxin Type B in Milk from a Cow Affected by Visceral Botulism. Vet. J. 2005, 169, 124–125. [Google Scholar] [CrossRef]
- Dutra, I.S.; Döbereiner, J.; Rosa, I.V.; Souza, L.A.A.; Nonato, M. Surtos de Botulismo Em Bovinos No Brasil Associados à Ingestão de Água Contaminada. Pesqui. Vet. Bras. 2001, 21, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Demarchi, J.; Mourgues, C.; Orio, J.; Prevot, A.R. Existence of Type D Botulism in Man. Bull. Acad. Med. Par. 1958, 142, 580–582. [Google Scholar]
- Prévot, A.R.; Silloc, R.; Quentin, M. Existence En France Du Botulisme Bovin de Type C. Bulletin l’Académie Vétérinaire France 1953, 106, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Lúquez, C.; Dykes, J.K.; Yu, P.A.; Raphael, B.H.; Maslanka, S.E. First Report Worldwide of an Infant Botulism Case due to Clostridium botulinum Type E. J. Clin. Microbiol. 2010, 48, 326–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, K.; Fujii, N.; Tsuzuki, K.; Murakami, T.; Indoh, T.; Yokosawa, N.; Takeshi, K.; Syuto, B.; Oguma, K. The Complete Nucleotide Sequence of the Gene Coding for Botulinum Type C1 Toxin in the C-ST Phage Genome. Biochem. Biophys. Res. Commun. 1990, 171, 1304–1311. [Google Scholar] [CrossRef]
- Eleopra, R.; Tugnoli, V.; Quatrale, R.; Rossetto, O.; Montecucco, C. Different Types of Botulinum Toxin in Humans. Mov. Disord. 2004, 19 (Suppl. 8), S53–S59. [Google Scholar] [CrossRef]
- Kutschenko, A.; Weisemann, J.; Kollewe, K.; Fiedler, T.; Alvermann, S.; Böselt, S.; Escher, C.; Garde, N.; Gingele, S.; Kaehler, S.-B.; et al. Botulinum Neurotoxin Serotype D—A Potential Treatment Alternative for BoNT/a and B Non-Responding Patients. Clin. Neurophysiol. 2019, 130, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.L.M.; LeClaire, R.D. Pathogenesis by aerosol. In Biological Weapons Defense; Springer: Berlin/Heidelberg, Germany, 2005; pp. 65–78. [Google Scholar]
- Fan, Y.; Garcia-Rodriguez, C.; Lou, J.; Wen, W.; Conrad, F.; Zhai, W.; Smith, T.J.; Smith, L.A.; Marks, J.D. A Three Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotype F Subtypes. PLoS ONE 2017, 12, e0174187. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, C.; Razai, A.; Geren, I.N.; Lou, J.; Conrad, F.; Wen, W.-H.; Farr-Jones, S.; Smith, T.J.; Brown, J.L.; Skerry, J.C.; et al. A Three Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotype E Subtypes. Toxins 2018, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.K.; Smith, T.J.; Helma, C.H.; Ticknor, L.O.; Foley, B.T.; Svensson, R.T.; Brown, J.L.; Johnson, E.A.; Smith, L.A.; Okinaka, R.T.; et al. Genetic diversity among Botulinum Neurotoxin-producing clostridial strains. J. Bacteriol. 2007, 189, 818–832. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.J.; Lou, J.; Geren, I.N.; Forsyth, C.M.; Tsai, R.; LaPorte, S.L.; Tepp, W.H.; Bradshaw, M.; Johnson, E.A.; Smith, L.A.; et al. Sequence variation within botulinum neurotoxin serotypes impacts antibody binding and neutralization. Inf. Immun. 2005, 73, 5450–5457. [Google Scholar] [CrossRef] [Green Version]
- Hauser, D.; Eklund, M.W.; Kurazono, H.; Binz, T.; Niemann, H.; Gill, D.M.; Boquet, P.; Popoff, M.R. Nucleotide sequence of Clostridium botulinum C1 neurotoxin. Nucleic Acids Res. 1990, 18, 4924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Inoue, K.; Ikeda, T.; Fujinaga, Y.; Sunagawa, H.; Takeshi, K.; Ohyama, T.; Watanabe, T.; Inoue, K.; Oguma, K. Molecular composition of the 16S toxin produced by a Clostridium botulinum type D strain 1873. Microbiol. Immunol. 1998, 42, 599–605. [Google Scholar] [CrossRef]
- Sunagawa, H.; Ohyama, T.; Watanabe, T.; Inoue, K. The complete amino acid sequence of the Clostridium botulinum type D neurotoxin, deduced by nucleotide sequence analysis of the encoding phage d-16 phi genome. J. Vet. Med. Sci. 1992, 54, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Moriishi, K.; Koura, M.; Abe, N.; Fujii, N.; Fujinaga, Y.; Inoue, K.; Ogumad, K. Mosaic structures of neurotoxins produced from Clostridium botulinum types C and D organisms. Biochim. Biophys. Acta 1996, 1307, 123–126. [Google Scholar] [CrossRef]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef]
- 2017–2018 Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy and Implementation Plan. Available online: https://www.phe.gov/Preparedness/mcm/phemce/Documents/2017-phemce-sip.pdf (accessed on 2 September 2021).
- Cangene Corp. BAT® [Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G)–(Equine)] Sterile Solution for Injection. 2013. Available online: https://www.fda.gov/media/85514/download (accessed on 2 September 2021).
- Fagan, R.P.; Neil, K.P.; Sasich, R.; Luquez, C.; Asaad, H.; Maslanka, S.; Khalil, W. Initial recovery and rebound of type f intestinal colonization botulism after administration of investigational heptavalent botulinum antitoxin. Clin. Infect. Dis. 2011, 53, e125–e128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowakowski, A.; Wang, C.; Powers, D.B.; Amersdorfer, P.; Smith, T.J.; Montgomery, V.A.; Sheridan, R.; Blake, R.; Smith, L.A.; Marks, J.D. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. USA 2002, 99, 11346–11350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Joshi, S.G.; Al-Saleem, F.; Ancharski, D.; Singh, A.; Nasser, Z.; Simpson, L.L. Localization of the sites and characterization of the mechanisms by which anti-light chain antibodies neutralize the actions of the botulinum holotoxin. Vaccine 2009, 27, 2616–2624. [Google Scholar] [CrossRef] [Green Version]
- Gil, L.A.F.; Cunha, C.E.P.D.; Moreira, G.M.S.G.; Salvarani, F.M.; Assis, R.A.; Lobato, F.C.F.; Mendonça, M.; Dellagostin, O.A.; Conceição, F.R. Production and evaluation of a recombinant chimeric vaccine against Clostridium botulinum neurotoxin types C and D. PLoS ONE 2013, 8, e69692. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.P.; Smith, T.J.; Wright, P.M.; Montgomery, V.A.; Meagher, M.M.; Smith, L.A. Protection with recombinant Clostridium botulinum C1 and D binding domain subunit (Hc) vaccines against C and D neurotoxins. Vaccine 2007, 25, 4273–4282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Berntsson, R.P.-A.; Tepp, W.H.; Pitkin, R.M.; Johnson, E.A.; Stenmark, P.; Dong, M. Botulinum neurotoxin D-C uses synaptotagmin I and II as receptors, and human synaptotagmin II is not an effective receptor for type B, D-C and G toxins. J. Cell Sci. 2012, 125 Pt 13, 3233–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, R.; Forsyth, C.M.; LaPorte, S.L.; Geren, I.N.; Smith, L.A.; Marks, J.D. Fine and domain-level epitope mapping of botulinum neurotoxin type A neutralizing antibodies by yeast surface display. J. Mol. Biol. 2007, 365, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rodriguez, C.; Levy, R.; Arndt, J.W.; Forsyth, C.M.; Razai, A.; Lou, J.; Geren, I.; Stevens, R.C.; Marks, J.D. Molecular evolution of antibody cross reactivity for multiple subtypes of type A botulinum neurotoxin. Nat. Biotechnol. 2007, 25, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Geren, I.; Garcia-Rodriguez, C.; Forsyth, C.M.; Wen, W.; Knopp, K.; Brown, J.; Smith, T.; Smith, L.A.; Marks, J.D. Affinity maturation of human botulinum neurotoxin antibodies by light chain shuffling via yeast mating. Protein Eng. Des. Sel. 2010, 23, 311–319. [Google Scholar] [CrossRef]
- Razai, A.; Garcia-Rodriguez, C.; Lou, J.; Geren, I.N.; Forsyth, C.M.; Robles, Y.; Tsai, R.; Smith, T.J.; Smith, L.A.; Siegel, R.W.; et al. Molecular evolution of antibody affinity for sensitive detection of botulinum neurotoxin type A. J. Mol. Biol. 2005, 351, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Dong, J.; Lou, J.; Wen, W.; Conrad, F.; Geren, I.N.; Garcia-Rodriguez, C.; Smith, T.J.; Smith, L.A.; Ho, M.; et al. Monoclonal antibodies that inhibit the proteolytic activity of botulinum neurotoxin serotype/B. Toxins 2015, 7, 3405–3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomic, M.T.; Espinoza, Y.; Martinez, Z.; Pham, K.; Cobb, R.R.; Snow, D.M.; Earnhart, C.G.; Pals, T.; Syar, E.S.; Niemuth, N.; et al. Monoclonal antibody combinations prevent serotype A and serotype B inhalational botulism in a guinea pig model. Toxins 2019, 11, 208. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Geren, I.N.; Dong, J.; Lou, J.; Wen, W.; Conrad, F.; Smith, T.J.; Smith, L.A.; Ho, M.; Pires-Alves, M.; et al. Monoclonal antibodies targeting the alpha-exosite of botulinum neurotoxin serotype/A inhibit catalytic activity. PLoS ONE 2015, 10, e0135306. [Google Scholar] [CrossRef]
- Pellett, S.; Tepp, W.H.; Scherf, J.M.; Pier, C.L.; Johnson, E.A. Activity of botulinum neurotoxin type D (strain 1873) in human neurons. Toxicon 2015, 101, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Snow, D.M.; Riling, K.; Kimbler, A.; Espinoza, Y.; Wong, D.; Pham, K.; Martinez, Z.; Kraus, C.N.; Conrad, F.; Garcia-Rodriguez, C. Safety and Pharmacokinetics of a Four Monoclonal Antibody Combination Against Botulinum C and D Neurotoxins. Antimicrob. Agents Chemother. 2019, 63, e012700-19. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Thompson, A.A.; Fan, Y.; Lou, J.; Conrad, F.; Ho, M.; Pires-Alves, M.; Wilson, B.A.; Stevens, R.C.; Marks, J.D. A single-domain llama antibody potently inhibits the enzymatic activity of botulinum neurotoxin by binding to the non-catalytic alpha-exosite binding region. J. Mol. Biol. 2010, 397, 1106–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.U.; Griffiss, J.M.; McKenzie, R.; Fuchs, E.J.; Jurao, R.A.; An, A.T.; Ahene, A.; Tomic, M.; Hendrix, C.W.; Zenilman, J.M. Safety and pharmacokinetics of XOMA 3AB, a novel mixture of three monoclonal antibodies against botulinum toxin A. Antimicrob. Agents Chemother. 2014, 58, 5047–5053. [Google Scholar] [CrossRef] [Green Version]
- Sheets, M.D.; Amersdorfer, P.; Finnern, R.; Sargent, P.; Lindqvist, E.; Schier, R.; Hemingsen, G.; Wong, C.; Gerhart, J.C.; Marks, J.D. Efficient construction of a large nonimmune phage antibody library: The production of high-affinity human single-chain antibodies to protein antigens. Proc. Natl. Acad. Sci. USA 1998, 95, 6157–6162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudicelli, V.; Brochet, X.; Lefranc, M.-P. IMGT/V-QUEST: IMGT Standardized Analysis of the Immunoglobulin (IG) and T Cell Receptor (TR) Nucleotide Sequences. Cold Spring Harb. Protoc. 2011, 2011, 5633. [Google Scholar] [CrossRef] [PubMed]

| Holotoxin | LC | HN | HC | |
|---|---|---|---|---|
| BoNT/C vs.: | ||||
| BoNT/CD | 76.0% | 96.2% | 93.0% | 41.6% |
| BoNT/DC | 64.7% | 47.1% | 67.7% | 75.8% |
| BoNT/D | 51.2% | 46.8% | 67.9% | 38.9% |
| BoNT/CD vs.: | ||||
| BoNT/DC | 51.9% | 47.5% | 69.4% | 39.1% |
| BoNT/D | 68.7% | 47.3% | 69.2% | 89.8% |
| BoNT/DC vs.: | ||||
| BoNT/D | 76.5% | 98.2% | 95.8% | 40.9% |
| Clone a | Epitope | Yeast-Displayed scFv KD by FACS (×10−9 M−1) | IgG KD (×10−12 M) | ||||
|---|---|---|---|---|---|---|---|
| C | CD | DC | D | C | DC | ||
| 4C4 | HN | 3.0 | + | 10.5 | + | 888 | 597 |
| 4C10 | LC | 1.1 | + | 107 | + | 401 | ND |
| 8DC1 | HN | 11 | + | 5.2 | + | 1800 | 95 |
| 8DC4 | HN | 3.0 | + | 39 | + | 1400 b | 591 b |
| 1C1 | HN | 0.5 | NB | NB | NB | 1.6 | NB |
| 1C2 | ND | 24 | NB | NB | NB | ||
| 1C8 | ND | 42 | NB | >200 | NB | ||
| 87C1 | HN | 2.0 | + | NB | NB | ||
| 87C2 | HN | 7.0 | NB | NB | NB | ||
| 87C78 | LC-HN | 1.0 | + | NB | NB | 0.42 | NB |
| 4C1 | LC | 2.7 | + | NB | NB | ||
| 4C2 | HC | 0.19 | NB | 0.5 | NB | 14.7 | 0.51 |
| 4C3 | LC | 4.7 | + | NB | NB | ||
| 4C5 | LC | 0.14 | + | NB | NB | ||
| 4C7 | ND | 64 | NB | NB | NB | ||
| 4C8 | ND | 51 | NB | NB | NB | ||
| 4C9 | LC | 30 | + | NB | NB | ||
| 8DC2 | HC | 0.2 | NB | 0.5 | NB | 15.7 | 7 |
| 8DC3 | HC | NB | NB | 16.5 | + | 256,000 | 227 |
| 8DC5 | LC | NB | NB | 15 | + | ||
| 8DC6 | LC | NB | NB | 43 | + | ||
| 8DC7 | HC | NB | NB | 16 | + | ||
| 8DC8 | HC | NB | NB | 7.3 | + | ||
| 8DC9 | ND | NB | NB | 7.6 | + | ||
| 8DC10 | LC | NB | NB | 12 | + | ||
| 8DC11 | HC | NB | NB | 20 | + | ||
| 8DC12 | LC | NB | NB | 73 | + | ||
| 8DC13 | LC | NB | NB | 182 | + | ||
| Dissociation Constant, KD (×10−12 M−1) | ||||
|---|---|---|---|---|
| Antibody | BoNT/C | BoNT/CD | BoNT/DC | Binds BoNT/D |
| 4C4 | 888 | ND | 597 | ND |
| 4C4.1 | 570 | 1400 | 58 | 898 |
| 4C4.2 | 35 | 252 | 126 | 254 |
| 4C4.4 | 16 | 16 | 87 | 34 |
| 4C10 | 401 | ND | 107,000 (as scFv) | ND |
| 4C10.1 | 95 | 374 | 7300 | 6000 |
| 4C10.2 | 34 | 0.73 | 892 | 1450 |
| 4C10.5 | 1.1 | 2.43 | 15 | 17 |
| 8DC1 | 1809 | ND | 95 | ND |
| 8DC1.2 | 779 | 426 | 174 | 370 |
| 8DC4 | 3000 (as scFv) | ND | 39,000 (as scFv) | ND |
| 8DC4.1 | 1400 | 591 | 1400 | 1400 |
| 8DC4.4 | 136 | 117 | 146 | 157 |
| 8DC4.5 | 147 | 38 | 25 | 12 |
| LD50 of BoNT/ C | 20 | 200 | 500 | 2500 | 5000 | 10,000 | 20,000 | 40,000 |
|---|---|---|---|---|---|---|---|---|
| mAbs | Mice surviving/10 mice treated | |||||||
| 1C1.1 | 10/10 | 10/10 | 1/10 | |||||
| 4C2 | 6/10 | 0/10 | ||||||
| 4C10 | 6/10 | 0/10 | ||||||
| 1C1.1:4C2 | 10/10 | 10/10 | 7/10 | 2/10 | ||||
| 1C1.1:4C10 | 10/10 | 3/10 | 2/10 | |||||
| 4C2:4C10 | 10/10 | 7/10 | 2/10 | |||||
| 1C1.1:4C2:4C10 | 10/10 | 10/10 | 1/10 | |||||
| 4C4.2:8DC4.1:4C10.2 | 2/10 | 0/10 | ||||||
| 4C2:4C4.2:8DC4.1:4C10.2 | 9/10 | 0/10 | ||||||
| Number of Survivors/Total Mice Treated | ||||
|---|---|---|---|---|
| Toxin Used and Lot | BoNT/C C112913-01 | BoNT/CD CDU 021113 | BoNT/DC D010604-01 | BoNT/D U 021113 |
| Total Antibody Dose per Mouse | ||||
| 50 µg | 10/10 | 10/10 | 10/10 | 4/5 |
| 10 µg | 10/10 | 5/10 | 10/10 | |
| 5.0 µg | 10/10 | 5/10 | 0/10 | |
| 1.0 µg | 0/10 | 0/10 | 0/10 | |
| 0.5 µg | 0/10 | 0/10 | 0/10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Rodriguez, C.; Yan, S.; Geren, I.N.; Knopp, K.A.; Dong, J.; Sun, Z.; Lou, J.; Conrad, F.; Wen, W.-H.; Farr-Jones, S.; et al. A Four-Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotypes C and D. Toxins 2021, 13, 641. https://doi.org/10.3390/toxins13090641
Garcia-Rodriguez C, Yan S, Geren IN, Knopp KA, Dong J, Sun Z, Lou J, Conrad F, Wen W-H, Farr-Jones S, et al. A Four-Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotypes C and D. Toxins. 2021; 13(9):641. https://doi.org/10.3390/toxins13090641
Chicago/Turabian StyleGarcia-Rodriguez, Consuelo, Shude Yan, Isin N. Geren, Kristeene A. Knopp, Jianbo Dong, Zhengda Sun, Jianlong Lou, Fraser Conrad, Wei-Hua Wen, Shauna Farr-Jones, and et al. 2021. "A Four-Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotypes C and D" Toxins 13, no. 9: 641. https://doi.org/10.3390/toxins13090641
APA StyleGarcia-Rodriguez, C., Yan, S., Geren, I. N., Knopp, K. A., Dong, J., Sun, Z., Lou, J., Conrad, F., Wen, W.-H., Farr-Jones, S., Smith, T. J., Brown, J. L., Skerry, J. C., Smith, L. A., & Marks, J. D. (2021). A Four-Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotypes C and D. Toxins, 13(9), 641. https://doi.org/10.3390/toxins13090641







