The Reduction of the Combined Effects of Aflatoxin and Ochratoxin A in Piglet Livers and Kidneys by Dietary Antioxidants
Abstract
:1. Introduction
2. Results
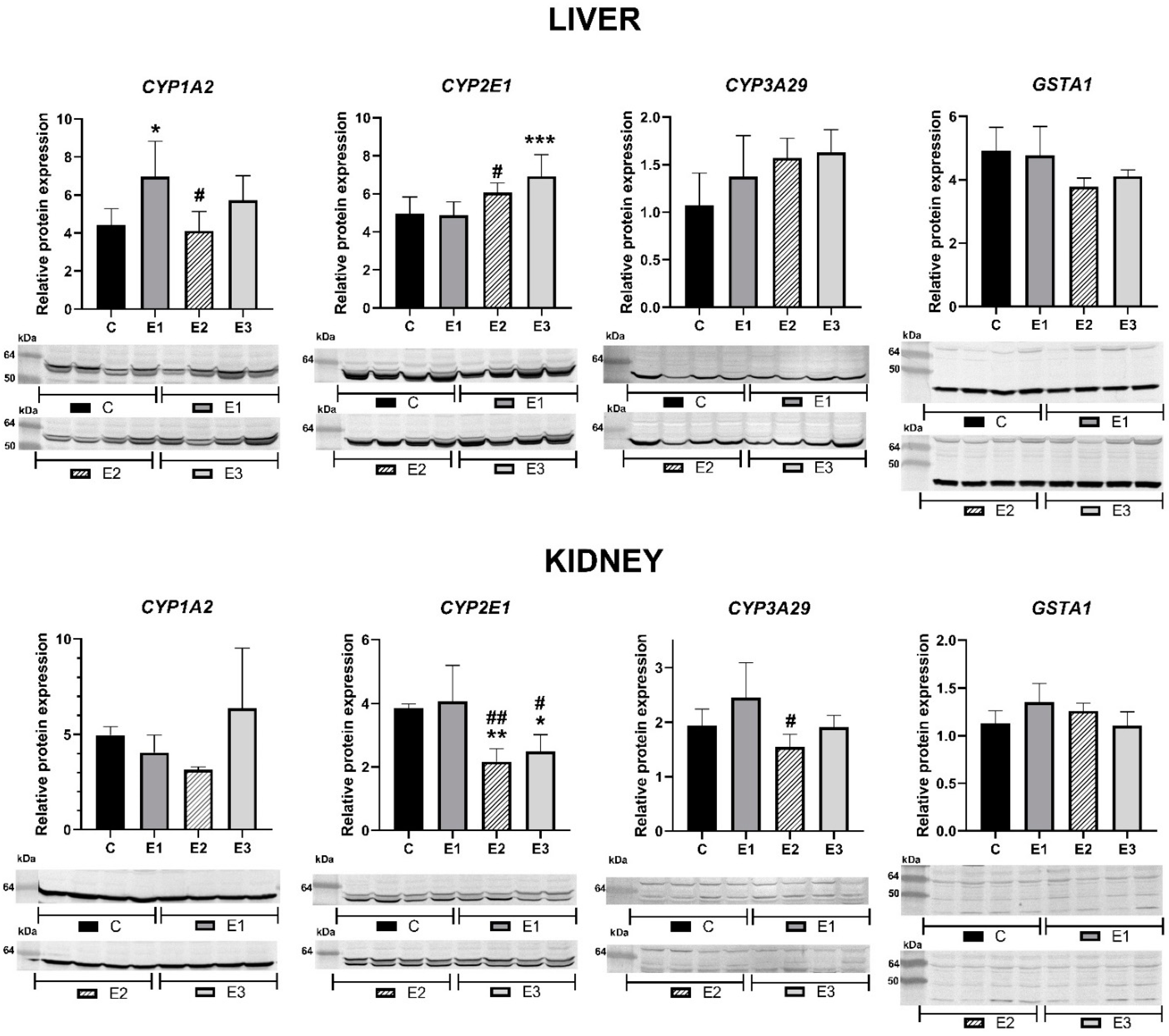
2.1. Relative Protein Expression
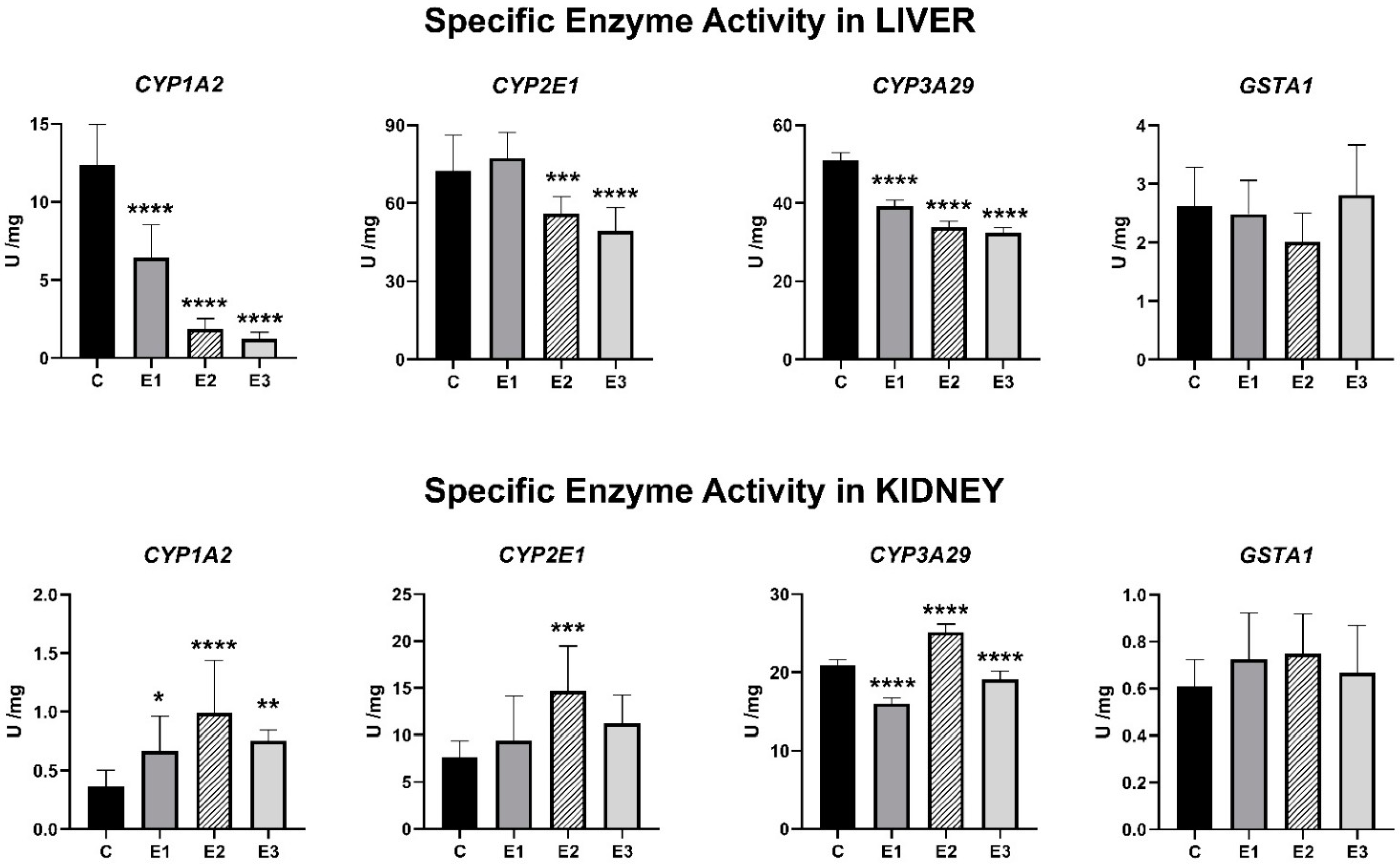
2.2. Enzymatic Activities
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals, Treatment and Sampling
5.2. Isolation of the Microsome Fraction
5.3. Western Blot Analysis
5.4. Enzymatic Activity Assays
5.4.1. CYP1A2
5.4.2. CYP2E1
5.4.3. CYP3A29
5.4.4. GSTA1
5.4.5. Protein Determination
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Aalim, M.M.; Khan, M.Z.; Saleemi, M.K.; He, C.; Khatoon, A.; Gul, S.T. Amelioration of immunosuppressive effects of aflatoxin and ochratoxin A in White Leghorn layers with distillery yeast sludge. Toxin Rev. 2017, 36, 275–281. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Dong, R.; Zhang, Z.; Jia, F.; Yu, H.; Wang, Y. Protective effect of selenomethionine on intestinal injury induced by T- 2 toxin. Res. Veter. Sci. 2020, 132, 439–447. [Google Scholar] [CrossRef]
- Peltomaa, R.; Mickert, M.J.; Brandmeier, J.C.; Hlav, A.; Moreno-bondi, M.C.; Gorris, H.H.; Benito-pe, E. Competitive upconversion-linked immunoassay using peptide mimetics for the detection of the mycotoxin zearalenone. Biosens. Bioelectron. 2020, 170, 112683. [Google Scholar] [CrossRef] [PubMed]
- Koletsi, P.; Schrama, J.; Graat, E.; Wiegertjes, G.; Lyons, P.; Pietsch, C. The Occurrence of Mycotoxins in Raw Materials and Fish Feeds in Europe and the Potential Effects of Deoxynivalenol (DON) on the Health and Growth of Farmed Fish Species—A Review. Toxins 2021, 13, 403. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Coppa, C.C.; De Neeff, D.V.; Corassin, C.H.; Oliveira, C. Mycotoxins in fruits and fruit-based products: Occurrence and methods for decontamination. Toxin Rev. 2019, 38, 263–272. [Google Scholar] [CrossRef]
- Abdolmaleki, K.; Khedri, S.; Alizadeh, L.; Javanmardi, F.; Oliveira, C.A.; Khaneghah, A.M. The mycotoxins in edible oils: An overview of prevalence, concentration, toxicity, detection and decontamination techniques. Trends Food Sci. Technol. 2021, 115, 500–511. [Google Scholar] [CrossRef]
- Carballo, D.; Moltó, J.; Berrada, H.; Ferrer, E. Presence of mycotoxins in ready-to-eat food and subsequent risk assessment. Food Chem. Toxicol. 2018, 121, 558–565. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.; Rivas-Caceres, R.R.; Salem, A.Z. Mycotoxin toxicity and residue in animal products: Prevalence, consumer exposure and reduction strategies—A review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef]
- Heussner, A.; Dietrich, D.; O’Brien, E. In vitro investigation of individual and combined cytotoxic effects of ochratoxin A and other selected mycotoxins on renal cells. Toxicol. Vitr. 2006, 20, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-C.; Lee, S.; Cho, M.-H. The Simplest Flowchart Stating the Mechanisms for Organic Xenobiotics-induced Toxicity: Can it Possibly be Accepted as a “Central Dogma” for Toxic Mechanisms? Toxicol. Res. 2014, 30, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelkonen, O.; Raunio, H. Metabolic activation of toxins: Tissue-specific expression and metabolism in target organs. Environ. Health Perspect. 1997, 105, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.; Gardner, I.; Swales, N. An Introduction to Drug Disposition: The Basic Principles of Absorption, Distribution, Metabolism, and Excretion. Toxicol. Pathol. 1995, 23, 102–114. [Google Scholar] [CrossRef]
- Mulero, J.; Martínez, G.; Oliva, J.; Cermeño, S.; Cayuela, J.M.; Zafrilla, P.; Martínez-Cachá, A.; Barba, A. Phenolic compounds and antioxidant activity of red wine made from grapes treated with different fungicides. Food Chem. 2015, 180, 25–31. [Google Scholar] [CrossRef]
- Jarolim, K.; Del Favero, G.; Pahlke, G.; Dostal, V.; Zimmermann, K.; Heiss, E.; Ellmer, D.; Stark, T.D.; Hofmann, T.; Marko, D. Activation of the Nrf2-ARE pathway by the Alternaria alternata mycotoxins altertoxin I and II. Arch. Toxicol. 2016, 91, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Antonissen, G.; Devreese, M.; De Baere, S.; Martel, A.; Van Immerseel, F.; Croubels, S. Impact of Fusarium mycotoxins on hepatic and intestinal mRNA expression of cytochrome P450 enzymes and drug transporters, and on the pharmacokinetics of oral enrofloxacin in broiler chickens. Food Chem. Toxicol. 2017, 101, 75–83. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2013, 14, e4. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Constable, P.D.; Eppley, R.M.; Waggoner, A.L.; Tumbleson, M.E.; Haschek, W.M. Fumonisin B1 Is Hepatotoxic and Nephrotoxic in Milk-Fed Calves. Toxicol. Sci. 2001, 60, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Kojima, M.; Degawa, M. Biochemical and Biophysical Research Communications Serum androgen level is determined by autosomal dominant inheritance and regulates sex-related CYP genes in pigs. Biochem. Biophys. Res. Commun. 2013, 430, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Lehman-McKeeman, L.D.; Ruepp, S.U. Chapter 2—Biochemical and Molecular Basis of Toxicity. In Fundamentals of Toxicologic Pathology, 3rd ed.; Academic Press: Waltham, MA, USA, 2018; pp. 15–33. [Google Scholar]
- Anzai, N.; Jutabha, P.; Endou, H. Molecular Mechanism of Ochratoxin a Transport in the Kidney. Toxins 2010, 2, 1381–1398. [Google Scholar] [CrossRef] [Green Version]
- Kok-Yong, S.; Lawrence, L. Drug Distribution and Drug Elimination, Basic Pharmacokinetic Concepts and Some Clinical Applications; Ahmed, T.A., Ed.; IntechOpen: London, UK, 2015; Available online: https://www.intechopen.com/chapters/48275 (accessed on 18 August 2021). [CrossRef] [Green Version]
- Arana, M.R.; Tocchetti, G.N.; Rigalli, J.P.; Mottino, A.D.; García, F.; Villanueva, S.S. Hepatic and Intestinal Multidrug Resistance-Associated Protein 2: Transcriptional and Post-transcriptional Regulation by Xenobiotics. In Toxicology New Aspects to This Scientific Conundrum; Soloneski, S., Larramendy, M.L., Eds.; IntechOpen: London, UK, 2016; Available online: https://www.intechopen.com/chapters/51996, (accessed on 18 August 2021).
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
- Szilagyi, J.T.; Gorczyca, L.; Brinker, A.; Buckley, B.; Laskin, J.D.; Aleksunes, L.M. Placental BCRP/ABCG2Transporter Prevents Fetal Exposure to the Estrogenic Mycotoxin Zearalenone. Toxicol. Sci. 2019, 168, 394–404. [Google Scholar] [CrossRef]
- Ullah, H.A.; Durrani, A.Z.; Ijaz, M.; Javeed, A.; Sadique, U.; Hassan, Z.U.; Rahman, A.U.; Shah, M.; Khattak, I. Dietary mycotoxins binders: A strategy to reduce aflatoxin m1 residues and improve milk quality of lactating Beetal goats. J. Consum. Prot. Food Saf. 2016, 11, 305–309. [Google Scholar] [CrossRef]
- Huwig, A.; Freimund, S.; Käppeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Elliott, C.T.; Connolly, L.; Kolawole, O. Potential adverse effects on animal health and performance caused by the addition of mineral adsorbents to feeds to reduce mycotoxin exposure. Mycotoxin Res. 2020, 36, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meissonnier, G.; Laffitte, J.; Raymond, I.; Benoit, E.; Cossalter, A.-M.; Pinton, P.; Bertin, G.; Oswald, I.; Galtier, P. Subclinical doses of T-2 toxin impair acquired immune response and liver cytochrome P450 in pigs. Toxicology 2008, 247, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-H.; Zhang, N.-Y.; Zhu, M.-K.; Zhao, L.; Zhou, J.-C.; Qi, D.-S. Prevention of Aflatoxin B1 Hepatoxicity by Dietary Selenium Is Associated with Inhibition of Cytochrome P450 Isozymes and Up-Regulation of 6 Selenoprotein Genes in Chick Liver. J. Nutr. 2015, 146, 655–661. [Google Scholar] [CrossRef]
- Goossens, J.; De Bock, L.; Osselaere, A.; Verbrugghe, E.; Devreese, M.; Boussery, K.; Van Bocxlaer, J.; De Backer, P.; Croubels, S. The mycotoxin T-2 inhibits hepatic cytochrome P4503A activity in pigs. Food Chem. Toxicol. 2013, 57, 54–56. [Google Scholar] [CrossRef]
- Shang, Y.; Huang, S. Engineering Plant Cytochrome P450s for Enhanced Synthesis of Natural Products: Past Achievements and Future Perspectives. Plant Commun. 2020, 1, 100012. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.H.; Crespi, C.L.; Waxman, D.J.; Ian, P.R.; Elizabeth, S.A. Spectrophotometric Analysis of Human CYP2E1-Catalyzed p-Nitrophenol Hydroxylation. Methods Mol. Biol. 2006, 320, 127–132. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Ekstrand, B.; Zamaratskaia, G. Comparison of cytochrome P450 concentrations and metabolic activities in porcine hepatic microsomes prepared with two different methods. Toxicol. Vitr. 2011, 25, 343–346. [Google Scholar] [CrossRef]
- Popescu, R.; Bulgaru, C.; Untea, A.; Vlassa, M.; Filip, M.; Hermenean, A.; Marin, D.; Țăranu, I.; Georgescu, S.; Dinischiotu, A. The Effectiveness of Dietary Byproduct Antioxidants on Induced CYP Genes Expression and Histological Alteration in Piglets Liver and Kidney Fed with Aflatoxin B1 and Ochratoxin A. Toxins 2021, 13, 148. [Google Scholar] [CrossRef]
- Helke, K.L.; Nelson, K.N.; Sargeant, A.M.; Jacob, B.; McKeag, S.; Haruna, J.; Vemireddi, V.; Greeley, M.; Brocksmith, D.; Navratil, N.; et al. Pigs in Toxicology: Breed Differences in Metabolism and Background Findings. Toxicol. Pathol. 2016, 44, 575–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schook, L.B.; Collares, T.; Darfour-Oduro, K.A.; De, A.K.; Rund, L.A.; Schachtschneider, K.; Seixas, F.K. Unraveling the Swine Genome: Implications for Human Health. Annu. Rev. Anim. Biosci. 2015, 3, 219–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanger, U.M.; Schwab, M. Pharmacology and Therapeutics Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Žlábek, V.; Chen, G.; Madej, A. Modulation of porcine cytochrome P450 enzyme activities by surgical castration and immunocastration. Animal 2009, 3, 1124–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bais, H.P.; Ravishankar, G.A. Cichorium intybus L.—Cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J. Sci. Food Agric. 2001, 81, 467–484. [Google Scholar] [CrossRef]
- Burk, O.; Arnold, K.A.; Geick, A.; Tegude, H.; Eichelbaum, M. A role for constitutive androstane receptor in the regulation of human intestinal MDR1 expression. Biol. Chem. 2005, 386, 503–513. [Google Scholar] [CrossRef]
- Doehmer, J.; Weiss, G.; McGregor, G.P.; Appel, K. Assessment of a dry extract from milk thistle (Silybum marianum) for interference with human liver cytochrome-P450 activities. Toxicol. Vitr. 2011, 25, 21–27. [Google Scholar] [CrossRef]
- Slaughter, R.L.; Edwards, D.J. Recent Advances: The Cytochrome P450 Enzymes. Ann. Pharmacother. 1995, 29, 619–624. [Google Scholar] [CrossRef]
- Teel, R.W.; Huynh, H. Modulation by phytochemicals of cytochrome P450-linked enzyme activity. Cancer Lett. 1998, 133, 135–141. [Google Scholar] [CrossRef]
- Obach, R.S.; Walsky, R.L.; Venkatakrishnan, K.; Gaman, E.A.; Houston, J.B.; Tremaine, L.M. The Utility of in Vitro Cytochrome P450 Inhibition Data in the Prediction of Drug-Drug Interactions. J. Pharmacol. Exp. Ther. 2006, 316, 336–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, T.; Yamazaki, H.; Mimura, M.; Inui, Y.; Guengerich, F.P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994, 270, 414–423. [Google Scholar] [PubMed]
- Zhou, S.-F.; Liu, J.-P.; Chowbay, B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 2009, 41, 89–295. [Google Scholar] [CrossRef] [PubMed]
- Potkin, S.G.; Bera, R.; Gulasekaram, B.; Costa, J.; Hayes, S.; Jin, Y.; Richmond, G.; Carreon, D.; Sitanggan, K.; Gerber, B. Plasma clozapine concentrations predict clinical response in treatment-resistant schizophrenia. J. Clin. Psychiatry 1994, 55, 133–136. [Google Scholar] [PubMed]
- Faber, M.; Jetter, A.; Fuhr, U. Assessment of CYP1A2 Activity in Clinical Practice: Why, How, and When? Basic Clin. Pharmacol. Toxicol. 2005, 97, 125–134. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Brix, T.H.; Kyvik, K.O.; Brøsen, K. The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics 2002, 12, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Winter, S.; Turpeinen, M.; Schwab, M.; Zanger, U.M. Pathway-Targeted Pharmacogenomics of CYP1A2 in Human Liver. Front. Pharmacol. 2010, 1, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueng, Y.-F.; Shimada, T.; Yamazaki, H.; Guengerich, F.P. Oxidation of Aflatoxin B1 by Bacterial Recombinant Human Cytochrome P450 Enzymes. Chem. Res. Toxicol. 1995, 8, 218–225. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, H.J.; Pyo, M.C.; Ryu, D.; Lee, K.-W. Ochratoxin A-Induced Hepatotoxicity through Phase I and Phase II Reactions Regulated by AhR in Liver Cells. Toxins 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, E.P.; Kunze, K.L.; Stapleton, P.L.; Eaton, D.L. The Kinetics of Aflatoxin B1Oxidation by Human cDNA-Expressed and Human Liver Microsomal Cytochromes P450 1A2 and 3A4. Toxicol. Appl. Pharmacol. 1996, 141, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Bojić, M.; Kondža, M.; Rimac, H.; Benković, G.; Maleš, Ž. The Effect of Flavonoid Aglycones on the CYP1A2, CYP2A6, CYP2C8 and CYP2D6 Enzymes Activity. Molecules 2019, 24, 3174. [Google Scholar] [CrossRef] [Green Version]
- Pyo, M.C.; Shin, H.S.; Jeon, G.Y.; Lee, K.-W. Synergistic Interaction of Ochratoxin A and Acrylamide Toxins in Human Kidney and Liver Cells. Biol. Pharm. Bull. 2020, 43, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, C.; Andretta, E.; Romano, V.; Lauritano, C.; Avantaggiato, G.; Schiavone, A.; Jarriyawattanachaikul, W.; Florio, S.; Ciarcia, R.; Damiano, S. Effects of Some New Antioxidants on Apoptosis and ROS Production in AFB1 Treated Chickens. Med. Sci. Forums 2020, 2, 12. [Google Scholar] [CrossRef]
- Zangar, R.C. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. [Google Scholar] [CrossRef]
- Moorthy, B. The CYP1A Subfamily. Issues in Toxicology of Cytochromes P450; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 97–135. [Google Scholar]
- Xun, T.; Lin, Z.; Wang, X.; Zhan, X.; Feng, H.; Gan, D.; Yang, X. Advanced oxidation protein products downregulate CYP1A2 and CYP3A4 expression and activity via the NF-κB-mediated signaling pathway in vitro and in vivo. Lab. Investig. 2021, 101, 1197–1209. [Google Scholar] [CrossRef]
- Jacquemyn, J.; Cascalho, A.C.C.; Goodchild, R.E. The ins and outs of endoplasmic reticulum-controlled lipid biosynthesis. EMBO Rep. 2017, 18, 1905–1921. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of Liposomal Membrane Fluidity by Flavonoids and Isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef]
- Hlavica, P. Challenges in assignment of allosteric effects in cytochrome P450-catalyzed substrate oxidations to structural dynamics in the hemoprotein architecture. J. Inorg. Biochem. 2017, 167, 100–115. [Google Scholar] [CrossRef]
- Caro, A.A.; Cederbaum, A.I. Oxidativestress, Toxicology, Andpharmacology of CYP2E1. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. Mol. Mech. Mutagen. 2005, 569, 101–110. [Google Scholar] [CrossRef]
- Neafsey, P.; Ginsberg, G.; Hattis, D.; Johns, D.O.; Guyton, K.Z.; Sonawane, B. Genetic Polymorphism in CYP2E1: Population Distribution of CYP2E1 Activity. J. Toxicol. Environ. Health Part B 2009, 12, 362–388. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.M.; Roos, P.H.; Thier, R. The cytochrome P-450 isoenzyme CYP2E1 in the biological processing of industrial chemicals: Consequences for occupational and environmental medicine. Int. Arch. Occup. Environ. Health 2003, 76, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Oscarson, M.; Johansson, I.; Yue, Q.Y.; Dahl, M.L.; Tabone, M.; Arincò, S.; Albano, E.; Ingelman-Sundberg, M. Genetic polymorphism of human CYP2E1: Characterization of two variant alleles. Mol. Pharmacol. 1997, 51, 370–376. [Google Scholar]
- Marí, M.; Cederbaum, A.I. CYP2E1 Overexpression in HepG2 Cells Induces Glutathione Synthesis by Transcriptional Activation of γ-Glutamylcysteine Synthetase. J. Biol. Chem. 2000, 275, 15563–15571. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, M.K.; Zamaratskaia, G. Regulation of Porcine Hepatic Cytochrome P450—Implication for Boar Taint. Comput. Struct. Biotechnol. J. 2014, 11, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Willard, R.R.; Shappell, N.W.; Meekin, J.H.; Talbot, N.C.; Caperna, T.J. Cytochrome P450 expression profile of the PICM-19H pig liver cell line: Potential application to rapid liver toxicity assays. Vitr. Cell. Dev. Biol. Anim. 2009, 46, 11–19. [Google Scholar] [CrossRef]
- Howard, J.T.; O’Nan, A.T.; Maltecca, C.; Baynes, R.E.; Ashwell, M. Differential Gene Expression across Breed and Sex in Commercial Pigs Administered Fenbendazole and Flunixin Meglumine. PLoS ONE 2015, 10, e0137830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bee, G.; Silacci, P.; Ampuero-Kragten, S.; Čandek-Potokar, M.; Wealleans, A.; Litten-Brown, J.; Salminen, J.-P.; Mueller-Harvey, I. Hydrolysable tannin-based diet rich in gallotannins has a minimal impact on pig performance but significantly reduces salivary and bulbourethral gland size. Animal 2017, 11, 1617–1625. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, X.; Wang, X.; Jin, X.; Shi, D.; Wang, J.; Bi, D. Cecropin B Represses CYP3A29 Expression through Activation of the TLR2/4-NF-κB/PXR Signaling Pathway. Sci. Rep. 2016, 6, 27876. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, X.; Zhou, X.; Wang, X.; Shi, D.; Xiao, Y.; Bi, D. Pregnane X receptor is required for IFN-α-mediated CYP3A29 expression in pigs. Biochem. Biophys. Res. Commun. 2014, 445, 469–474. [Google Scholar] [CrossRef]
- Yao, M.; Dai, M.; Liu, Z.; Huang, L.; Chen, D.; Wang, Y.; Peng, D.; Wang, X.; Liu, Z.; Yuan, Z. Comparison of the substrate kinetics of pig CYP3A29 with pig liver microsomes and human CYP3A4. Biosci. Rep. 2011, 31, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Taneja, G.; Maity, S.; Jiang, W.; Moorthy, B.; Coarfa, C.; Ghose, R. Transcriptomic profiling identifies novel mechanisms of transcriptional regulation of the cytochrome P450 (Cyp)3a11 gene. Sci. Rep. 2019, 9, 6663. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, J.; Hu, Z.; Ye, W.; Yuan, Q.; Li, M.; Wen, J.; Deng, Y.; Jun, J. Coordinated Transcriptional Regulation of Cytochrome P450 3As by Nuclear Transcription Factor Y and Specificity Protein 1. Mol. Pharmacol. 2019, 95, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Chen, Q.; Liu, X.; Wen, J.; Jiang, J.; Deng, Y. Role of Specificity Protein 1, Hepatocyte Nuclear Factor 1α, and Pregnane X Receptor in the Basal and Rifampicin-Induced Transcriptional Regulation of Porcine Cytochrome P450 3A46. Drug Metab. Dispos. 2015, 43, 1458–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.; Wang, X. Role of vitamin D receptor in the regulation of CYP3A gene expression. Acta Pharm. Sin. B 2019, 9, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Jover, R.; Bort, R.; Gomez-Lechon, M.J.; Castell, J.V. Cytochrome P450 regulation by hepatocyte nuclear factor 4 in human hepatocytes: A study using adenovirus-mediated antisense targeting. Hepatology 2001, 33, 668–675. [Google Scholar] [CrossRef]
- Saito, T.; Takahashi, Y.; Hashimoto, H.; Kamataki, T. Novel Transcriptional Regulation of the Human CYP3A7Gene by Sp1 and Sp3 through Nuclear Factor κB-like Element. J. Biol. Chem. 2001, 276, 38010–38022. [Google Scholar] [CrossRef]
- Kuban, W.; Daniel, W.A. Cytochrome P450 expression and regulation in the brain. Drug Metab. Rev. 2021, 53, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.; Vilarem, M.-J.; Ribeiro, V.; Maurel, P.; Lechner, M.C. Two CCAAT/enhancer binding protein sites in the cytochrome P4503A1 locus. Potencial role in the glucocorticoid response. JBIC J. Biol. Inorg. Chem. 2003, 270, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Guenthner, T.; Gan, L.-S.; Humphreys, W. CYP3A4 Induction by Xenobiotics: Biochemistry, Experimental Methods and Impact on Drug Discovery and Development. Curr. Drug Metab. 2004, 5, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Glanemann, C.; Loos, A.; Gorret, N.; Willis, L.B.; O’Brien, X.M.; Lessard, P.A.; Sinskey, A.J. Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: Implications for DNA microarray analysis. Appl. Microbiol. Biotechnol. 2003, 61, 61–68. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Molecular Cell Biology, 4th ed.; W. H. Freeman: New York, NY, USA, 2000; Section 3.3, Functional Design of Proteins. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21733/ (accessed on 18 August 2021).
- Hassan, I.; Jabir, N.R.; Ahmad, S.; Shah, A.; Tabrez, S. Certain Phase I and II Enzymes as Toxicity Biomarker: An Overview. Water Air Soil Pollut. 2015, 226, 1–8. [Google Scholar] [CrossRef]
- Yagishita, Y.; Uruno, A.; Yamamoto, M. NRF2-Mediated Gene Regulation and Glucose Homeostasis; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128015858. [Google Scholar]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and induction of CYP enzymes in humans: An update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef]
- Kedderis, G. Biotransformation of Toxicants. Compr. Toxicol. 2010, 1–14, 137–151. [Google Scholar]
- Jančová, P.; Siller, P.J.A.M. Phase II Drug Metabolism; Paxton, J., Ed.; IntechOpen: London, UK, 2012; Available online: https://www.intechopen.com/chapters/29241 (accessed on 18 August 2021). [CrossRef] [Green Version]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef]
- Strange, R.C.; Jones, P.W.; Fryer, A.A. Strange bedfellows in the personal computer industry: Technology alliances between ibm and apple. Toxicol. Lett. 2000, 113, 357–363. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Gupta, S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018, 433, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Murcia, H.W.; Diaz, G.J. Protective effect of glutathione S-transferase enzyme activity against aflatoxin B1 in poultry species: Relationship between glutathione S-transferase enzyme kinetic parameters, and resistance to aflatoxin B1. Poult. Sci. 2021, 100, 101235. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.; Tinkey, P.T.; Avritscher, R.; Van Pelt, C.; Eskandari, G.; George, S.K.; Xiao, L.; Cressman, E.; Morris, J.S.; Rashid, A.; et al. Validation of a Preclinical Model of Diethylnitrosamine-Induced Hepatic Neoplasia in Yucatan Miniature Pigs. Oncology 2016, 91, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Surai, P.F. Antioxidants in Poultry Nutrition and Reproduction: An Update. Antioxidants 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tozlovanu, M.; Canadas, D.; Pfohl-Leszkowicz, A.; Frenette, C.; Paugh, R.J.; Manderville, R.A. Glutathione Conjugates of Ochratoxin a as Biomarkers of Exposure / Glutationski Konjugati Okratoksina a Kao Biomarkeri Izloženosti. Arch. Ind. Hyg. Toxicol. 2012, 63, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Bognanno, M.; Galvano, F. Toxicity of Ochratoxin and Its Modulation by Antioxidants: A Review. Toxins 2013, 5, 1742–1766. [Google Scholar] [CrossRef] [Green Version]
- Gekle, M.; Sauvant, C.; Schwerdt, G. Ochratoxin A at nanomolar concentrations: A signal modulator in renal cells. Mol. Nutr. Food Res. 2005, 49, 118–130. [Google Scholar] [CrossRef]
- Boudra, H.; Saivin, S.; Buffiere, C.; Morgavi, D. Short communication: Toxicokinetics of ochratoxin A in dairy ewes and carryover to milk following a single or long-term ingestion of contaminated feed. J. Dairy Sci. 2013, 96, 6690–6696. [Google Scholar] [CrossRef] [Green Version]
- Hanioka, N.; Tatarazako, N.; Jinno, H.; Arizono, K.; Ando, M. Determination of cytochrome P450 1A activities in mammalian liver microsomes by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 399–406. [Google Scholar] [CrossRef]
- Wanwimolruk, S.; Wanwimolruk, P. Characterization of CYP1A enzyme in Adélie penguin liver. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Chen, G.; Lundström, K. Effects of sex, weight, diet and hCG administration on levels of skatole and indole in the liver and hepatic activities of cytochromes P4502E1 and P4502A6 in pigs. Meat Sci. 2006, 72, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sohl, C.D.; Cheng, Q.; Guengerich, F.P. Chromatographic assays of drug oxidation by human cytochrome P450 3A4. Nat. Protoc. 2009, 4, 1252–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Liu, C.; Wang, X.; Ma, H.; Pan, Y.; Huang, L.; Hao, H.; Dai, M.; Yuan, Z. Structure-Function Analysis of Porcine Cytochrome P450 3A29 in the Hydroxylation of T-2 Toxin as Revealed by Docking and Mutagenesis Studies. PLoS ONE 2014, 9, e106769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.G.; Avramescu, S.; Marin, D.E.; Țăranu, I.; Georgescu, S.E.; Dinischiotu, A. The Reduction of the Combined Effects of Aflatoxin and Ochratoxin A in Piglet Livers and Kidneys by Dietary Antioxidants. Toxins 2021, 13, 648. https://doi.org/10.3390/toxins13090648
Popescu RG, Avramescu S, Marin DE, Țăranu I, Georgescu SE, Dinischiotu A. The Reduction of the Combined Effects of Aflatoxin and Ochratoxin A in Piglet Livers and Kidneys by Dietary Antioxidants. Toxins. 2021; 13(9):648. https://doi.org/10.3390/toxins13090648
Chicago/Turabian StylePopescu, Roua Gabriela, Sorin Avramescu, Daniela Eliza Marin, Ionelia Țăranu, Sergiu Emil Georgescu, and Anca Dinischiotu. 2021. "The Reduction of the Combined Effects of Aflatoxin and Ochratoxin A in Piglet Livers and Kidneys by Dietary Antioxidants" Toxins 13, no. 9: 648. https://doi.org/10.3390/toxins13090648
APA StylePopescu, R. G., Avramescu, S., Marin, D. E., Țăranu, I., Georgescu, S. E., & Dinischiotu, A. (2021). The Reduction of the Combined Effects of Aflatoxin and Ochratoxin A in Piglet Livers and Kidneys by Dietary Antioxidants. Toxins, 13(9), 648. https://doi.org/10.3390/toxins13090648








